Abstract
Maternal exposure to dibutyl phthalate (DBP) may result in ovarian dysfunction in female offspring. However, the underlying mechanisms remain elusive. Pregnant Sprague–Dawley rats were intraperitoneally injected with different doses of DBP, estradiol, and corn oil from gestational day 7 until the end of lactation. The reproductive characteristics, mRNA, and protein expression of ovaries for the adult female offspring were compared. KGN cells were cultured in vitro with DBP, estrogen receptor antagonist, or ALK-5 inhibitor. Genes, proteins, estradiol, and progesterone expressed by KGN, cell proliferation, and apoptosis were measured respectively. Maternal perinatal exposure to DBP induced prolonged estrous period, increased secondary follicles, significant decreased mRNA, and protein levels of TGF-β2, TGF-β3, and TGF-βRII in ovaries of the adult female offspring, but none difference for serum levels of sex hormones, ovarian TGF-β1, and estrogen receptor. The mRNA levels of LHR, FSHR, and CYP19a in ovaries were also decreased. DBP might decrease the mRNA of TGF-β2, TGF-β3, and TGF-βR II of KGN. DBP can inhibit the mRNA of CYP19 at 24 h, which might be blocked by the estrogen receptor antagonist, whose effects were attenuated at 48 h. DBP combined with FSH might time-dependently regulate the gene expression of TGF-βR II, inhibitory at 24 h, but stimulative at 48 h, which could be blocked by the ALK5 inhibitor. However, the protein expressed by KGN was not influenced by DBP. DBP stimulated the proliferation of KGN at 24 h, which could be blocked by estrogen receptor antagonist, but attenuated at 48 h. The progesterone in culture medium secreted by KGN was decreased by DBP at 24 h. Maternal perinatal exposure to DBP induced decreased gene expression of TGF-β signaling and functional proteins in ovaries of the adult female offspring. Molecular cross-talk between estrogen receptor and TGF-β signaling pathway may play role in the mechanism of granulosa dysfunction induced by DBP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endocrine-disrupting chemicals (EDCs) are exogenous compounds which can alter hormone biosynthesis, causing adverse effects to human health and their offspring [1]. Phthalate esters are a kind of EDCs, commonly used in products such as plastics, medical equipment, personal care products, and in the coating of some oral medications. Phthalate metabolites have been detected in various body fluids including blood, urine, and follicular fluid [2]. Multigenerational and transgenerational effects on female reproduction are induced by prenatal exposure to the phthalate mixture, such as increasing uterine weight, anogenital distance, and body weight; inducing cystic ovaries; and causing fertility complications in female offspring [3,4,5,6].
Dibutyl phthalate (DBP) is a widely used plasticizer in plastic container and personal care products (which are widely used among women of reproductive age), is rapidly absorbed by oral or skin, then distributed and metabolized to mono-butyl-phthalate [7, 8]. Women of reproductive age have the highest exposure levels of mono-butyl-phthalate than any other age/sex group. DBP use in cosmetics is even banned by several countries (e.g., the European Union, Australia). The estimated daily intake in humans is 7–10 μg/kg/day in the general population and 233 μg/kg/day in patients taking DBP-coated medications [9].
DBP metabolites can reach the ovary and have been measured in the follicular fluid of women (median 2.05 ng/mL) [2]. The ovary is key to female fertility by providing the eggs and sex steroid hormones for fertilization and maintenance of reproductive function, respectively. DBP is capable of disrupting folliculogenesis and the physiology of ovarian granulosa and luteal cell [10]. DBP targets both mature ovarian follicles and luteal cells, and affects steroidogenesis, ovulation, and early pregnancy [11]. It is detrimental to follicle growth and viability, decrease growth of antral follicles, inhibit cell cycle progression, and initiate apoptosis in a dose-specific manner [7].
Perinatal maternal exposure to DBP has been reported to influence the reproductive development and function of female offspring [12]. The embryotoxic effects of DBP on laboratory animals included mainly an increase in fetal resorption and a decrease in live births. Long-term dietary exposure to DBP induced multigenerational effects on body weight and reproductive outcomes in both male and female rats [13]. Maternal perinatal exposure to oral high dose of DBP (10, 100, 600 mg/kg/day) during late pregnancy and breastfeeding had been reported to not affect ovarian development and function in F1 rats, however, may increase serum estradiol and progesterone levels [14]. Perinatal exposure of DBP significantly upregulated the serum estradiol levels in both immature and mature offspring rats [15]. DBP exposure also significantly downregulated the testosterone levels in immature rats and mature male rats, but had no influence on the testosterone levels in mature female rats [15]. Lower doses cause more adverse effects than the highest dose, which is an important fact because of the widespread environmental exposure to DBP [12].
However, the underlying molecular mechanisms of ovarian dysfunction induced by perinatal maternal exposure to DBP have not been clearly elucidated to date. Transforming growth factor β (TGF-β) is a multifunctional cytokine, which regulates cell proliferation, differentiation, and apoptosis of cells in most tissues. After binding of three types of TGF-β with their receptors, Smad2/3 proteins are phosphorylated and form a complex with Smad4. Smad complex translocates to the nucleus and regulates transcriptional expression of downstream genes by binding DNA as a transcription factor. TGF-β signal pathway is critical in regulating granulosa growth and differentiation, steroidogenesis of human ovary, and follicle growth arrest [16, 17]. Some physiologic actions of peptide growth factors are dependent on estrogen receptor (ER), such as TGF [18]. Both the protein kinase A and protein kinase C pathways can elicit ER-dependent transcriptional activation. Regulation of TGF-β by estradiol has been extensively explored in breast, prostate, skin, and lung tissues, and the effects appear to be contextual [19].
DBP shows anti-androgenic effects; however, there are also reports confirming its weak estrogenic effect [12]. DBP may induce the growth of LNCaP prostate cancer by acting on the cross-talk between TGF-β and ER signaling pathways [20]. If DBP can result in ovarian dysfunction by acting on the cross-talk between TGF-β and ER signaling pathways is unknown. In this study, we firstly explore whether perinatal exposure to DBP may promote ovarian dysfunction in adult female offspring through molecular cross-talk between ER signaling and TGF-β signaling pathway.
Material and Methods
Animals and Treatment
All experiments were conducted according to the Guide for the Care and Use of Laboratory Animals of National Research Council and approved by the Ethical Scientific Committee for the Care of Animals at West China Second University Hospital, Sichuan University (No. 2018–007).
Adult female and male Sprague–Dawley rats were purchased and allowed to acclimate to the facility for 2 weeks before use. The rats were maintained in polysulfone cages at Animal Facility of West China Second University Hospital, Sichuan University, under controlled conditions (22 ± 1 °C, 12-h light/dark cycle). Food and water were provided for ad libitum consumption. Groups of two females were mated with one male overnight and the day of the vaginal plug was considered day 0 of gestation. Pregnant females were housed individually with hard wood shavings as bedding and randomly allocated into five groups using a random number table.
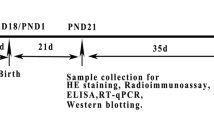
Pregnant rats were treated intraperitoneally (i.p.) with DBP (99.5% pure, Sigma Chemical Co., St. Louis, MO, USA) in corn oil (Sigma, 8001–30-7) at the doses of 33 mg/kg/day, 66 mg/kg/day, and 132 mg/kg/day from gestational day 7 (GD7) throughout post-natal day 21 (PND 21), with estradiol (E2, 20 µg/kg/day) and corn oil (0.3 mL/day) as controls. The DBP dose was chosen based on the previous study describing transgenerational inheritance of reproductive disease with perinatal exposure to DBP [5]. Polycystic ovary and fertility dysfunction were found to be induced by perinatal DBP exposure in female offspring in this study. The exposure period for dams was chosen so as to cover the whole time window of reproductive system development in the rats. The gestational rats were weighted every week during gestation.
At weaning, only female F1 offspring were selected and housed with three individuals per cage up to adult age (PND 90). The weights of F1 offspring were weighted regularly. The vaginal smear of adult female was collected to evaluate the estrous cycle. Then F1 females were anesthetized with isoflurane and killed by carbon dioxide overdose. Fasting blood samples were collected from the heart, separated by centrifugation, and serum samples were stored at − 80℃. The ovaries were collected and weighted. The coefficient of ovaries (mg/g) was calculated by dividing the weight of ovaries to body weights. Left ovary was fixed and dehydrated by formalin and ethanol, embedded in paraffin, serially sectioned (6-mm thickness) throughout the entire ovary and stained by HE method. The section of ovary was used to calculate the number of different stage of follicles. Follicle populations were counted by a pathologist who was blinded to the treatments, at every third section of the entire ovary and were scored as primordial, primary, secondary, antral, or Graafian follicles, based on their morphological appearance, as our previously published literature [21]. The right ovary was stored at − 80 °C for total RNA or protein extraction.
KGN Cells and Exposure Conditions
A stock solution of pure DBP (Sigma-Aldrich), ALK5 inhibitor (A77-01, APEBIO), TGF-β (Novoprotein), follicle-stimulating hormone (FSH, R&D), estrogen receptor antagonist (ERa, Fulvestrant, APEBIO), and E2 (Sigma-Aldrich) was dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich) and diluted in DMEM/F12 (Gibco) to achieve for final working concentrations of 36 µg/mL, 0.5 µg/mL, 50 ng/mL, 100 ng/mL, 0.1 µg/mL, and 0.1 µg/mL in culture medium, respectively.
KGN cell line is an immortalized human granulosa cell line, which has been established from a patient with an invasive ovarian granulosa cell carcinoma. It is a suitable model for understanding the regulation of cell growth and apoptosis, steroidogenesis in human granulosa cells. Thus, it is applied to explore the efficacy of DBP on reproductive function of ovary in present study.
Frozen stocks of KGN cell line were thawed and plated in 10 cm plates (1 × 106 cells/plates). Cells were maintained in DMEM /F12 medium (Gibco) at 37℃ supplemented with 10% FBS (Gibco) and 1% penicillin–streptomycin (Gibco) in a humidified atmosphere of 5% CO2. After 24 h of pre-culture, the attached cells were exposed to different groups of reagents. Control cells were incubated in the culture medium containing an equivalent volume of DMSO. After 24 h or 48 h of exposure, the cells and supernatant were collected for subsequent tests.
The supernatant was stored at − 20℃ for hormonal measurements. The culture wells were washed twice with PBS, lysed with of lysis buffer, stored at − 80℃ for total RNA or protein extraction.
Real-time Polymerase Chain Reaction
Total RNA was extracted from ovarian tissue or KGN cells using TRIzol™ Plus RNA Purification Kit (Invitrogen) according to the manufacturer’s recommendations. The quantity and integrity of total RNA were determined by ultraviolet spectrophotometer and electrophoresis on 1% sepharose gel, respectively. cDNA was synthesized with the ReverTra Ace® qPCR RT Master Mix(Toyobo). Q-PCR was performed using SYBR® Green Realtime PCR Master Mix (Toyobo) and the Applied Biosystems 7500. Polymerase chain reaction for ovarian tissue or KGN cells was carried out over 40 cycles at 95 °C for 20 s, 57 °C for 30 s, and at 95 °C for 15 s, 60 °C for 30 s, respectively. Expression data were normalized using the 2−ΔΔCt method with GAPDH or β-actin as endogenous reference genes for ovarian or KGN samples, respectively. All qPCR reactions were conducted in triplicate. Supplemental Table 1 and supplemental Table 2 list the primers for the genes (Cyp19a, ESR1, Fsh-R, Lh-R, TGF-β1, TGF-β2, TGF-β3, TGF-βR II, GAPDH, β-Actin).
Western Blot Analysis
Antibody against β-actin, P450arom, TGF-β2 and HRP-conjugated secondary antibodies were obtained from Zen-Bioscience Company (Chengdu, Sichuan, China); antibody against ER and TGFβR II from Proteintech Group Inc. (Wuhan, China); and antibody against TGF-β1 and TGF-β3 from Biosntech (Beijing, China).
Tissue and cells were lysed using RIPA Lysis Buffer (Beyotime Inst Biotech, Shanghai, China). The bicinchoninic acid (BCA) protein assay kit (Pierce, IL, USA) was used to determine the protein concentrations. Twenty to forty micrograms of lysate protein was resolved by 10% SDS-PAGE. Proteins were transferred to 0.45 μM polyvinylidene fluoride (PVDF) membranes after electrophoresis. The membranes were incubated with 5% nonfat milk for 90 min at 37 °C, then washed with TBST, and incubated at 37 °C with primary antibodies for 2 h. After a thorough wash with TBST and incubation with respective HRP-conjugated secondary antibodies at 37 °C for 1 h, the membranes were incubated for 2–5 min in enhanced chemiluminescence reagent (Bio-Rad) and were then exposed to film for signal detection. The optical density of target protein was corrected using β-actin and analyzed with Image J (N = 3 in each group).
Enzyme-Linked Immunosorbent Assay
Levels of estradiol and progesterone in the supernatant were measured in duplicate using enzyme-linked immunosorbent assay (ELISA) kits (Jiancheng Nanjing, China), according to the manufacturer’s instructions (N = 3 in each group). The detection limits were 0.5–200 ng/L and 0.05–10 ng/mL for estradiol and progesterone, respectively. The ELISA kit intra-assay coefficient of variation and inter-assay coefficient of variation were < 10% and < 12%, respectively.
Serum estradiol (E2), progesterone (P), testosterone (T), follicle-stimulating hormone (FSH), and luteinizing hormone (LH) were measured in duplicate for 36 female offspring using ELISA kits (CUSABIO BIOTECH, China). The detection limits were 12.5–1000 pg/mL, 0.4–30 ng/mL, 0.13–25.6 ng/mL, 0.13–10 mlU/mL, and 0.3–60mlU/mL respectively. The ELISA kit intra-assay coefficient of variation and inter-assay coefficient of variation were < 10% and < 10%, respectively.
Cell Viability Assay
Cell Counting Kit-8 (CCK-8) (Beyotime, Shanghai, China) and TUNEL (Beyotime) were used to evaluate cell viability. Cells were seeded into a 96-well or 12-well plate at the density of 5 × 104/mL, respectively. Following medium removal after cell adherence, cells were treated with different groups of reagents at 24 h and 48 h. Cell proliferation was performed by adding 10μL of CCK-8 solution. The cell density was determined by measuring the absorbance at 450 nm after 4 h of incubation. Cell apoptosis assays were performed by adding 50 μL of TUNEL solution at 37 °C for 60 min and 500 μL of DAPI (2 μg/mL) for 5 min. Then cells were observed under the fluorescence microscope. All experiments were conducted in three replicates.
Statistical Analysis
All data were presented as the mean and standard deviation (SD). Analysis of variance (ANOVA, one-way or two-way) was used to conduct multiple comparisons among groups and was then followed by Bonferroni post hoc comparisons if equal variances were assumed or Dunnett’s T3 post hoc comparisons if equal variances were not assumed. Statistical analyses were performed using SPSS v.20 software (IBM, Inc.). A P-value < 0.05 was statistically significant.
Results
Animal Experiment In Vivo
The abortion rate of the pregnant F0 rats (N = 33) was higher (7.1% in corn oil; 30% in low DBP; 38.9% in medium DBP; 34.9% in high DPB, P = 0.04) and the number of offspring per litter was fewer (9.9 ± 2.14 in corn oil; 9.1 ± 2.82 in low DBP; 8.2 ± 2.91 in medium DBP; 8.6 ± 2.65 in high DBP, P = 0.03, multiple comparison among four groups) in DBP group than those of corn oil group. The birth weight was not different, but the weight of female offspring began growing heavier from PND7 until adult period (PND90) in all DBP groups than that of corn oil group (P < 0.05). The estrous period was prolonged in DBP group with the dosage-dependent manner (P = 0.034). However, none significant difference was found for the serum sex hormone levels (E2, P, LH, FSH, T) among groups (Table 1). The weight of ovaries in DBP group was heavier than control (P = 0.001) but without difference for the coefficient of ovaries (P = 0.63).
The number of secondary follicles (P = 0.01) in ovaries of female offspring was increased after perinatal exposure to DBP, but none difference for the number of primordial, primary, and corpus luteum (P > 0.05). The number of atretic follicles seems increased in medium and high DBP groups than control group, but without significance (P = 0.05). Meanwhile, the granulosa surrounding the follicles arranged irregularly and loosely in DBP group.
After maternal perinatal exposure to DBP, the mRNA and protein levels of TGF-β2 (P = 0.016; P = 0.028) and TGF-β3 (P = 0.027; P = 0.005) in the ovaries of female adult offspring were significantly decreased, respectively (Figs. 1 and 2). Decreased tendence was also observed for mRNA (P = 0.001) and protein levels (P = 0.054) of TGF-βR II. However, no difference was found for the mRNA and protein levels of TGF-β1 and ER. Meanwhile, the mRNA levels of luteinizing hormone receptor (LHR, P = 0.004), follicle-stimulating hormone receptor (FSHR, P = 0.001), and cytochrome P450 family 19 subfamily A (CYP19a, P = 0.001) were also significantly decreased. The results are illustrated in Figs. 1 and 2.
The mRNA levels of ovary in F1 female offspring rats after perinatal exposure to dibutyl phthalate. F0 pregnant rats were intraperitoneally injected with corn oil, dibutyl phthalate (DBP, 33 or 66 or 132 mg/kg/day), or estradiol (E2, 20 µg/kg/day), respectively. DBP decreased the mRNA levels of TGF-β2, TGF-β3, TGF-βR II, LHR, FSHR, and CYP19a in ovaries of F1 female offspring rats, which were normalized by GAPDH and detected by RT-PCR (N = 50, means ± SD). P value indicated the results of multiple comparisons among five groups by one-way analysis of variance. The letters (a, b c, d) indicated the significant difference in post hoc comparisons when compared to control, low DBP, medium DBP, and high DBP, respectively
The protein levels of ovary in F1 female offspring rats after perinatal exposure to dibutyl phthalate. F0 pregnant rats were intraperitoneally injected with corn oil, dibutyl phthalate (DBP, 33 or 66 or 132 mg/kg/day), or estradiol (E2, 20 µg/kg/day), respectively. DBP decreased the protein levels of TGF-β2 and TGF-β3 in ovaries of F1 female offspring rats which were detected by Western blot (N = 50). (A) Columns show the results of densitometric analysis (means ± SD), while P value indicated the results of multiple comparisons among five groups by one-way analysis of variance. The letters (a, b) indicated the significant difference in post hoc comparisons when compared to control and low DBP, respectively. (B) The bands of Western blot in each group
KGN Experiments In Vitro
Estrogen Receptor
The role of estrogen receptor in the regulating mechanism of DBP on KGN cells is illustrated in Fig. 3. When the KGN was influenced by DBP for 24 h, the mRNA level of CYP19 was significantly decreased when compared to control group (P < 0.05, Fig. 3A). Decreased tendency was seen in the mRNA levels of TGF-β2, TGF-β3, and TGF-βR II but without statistical significance (P > 0.05). No difference was found in the mRNA levels of TGF-β1, ER, and FSHR. However, after the estrogen receptor antagonist (ERa) was added with DBP, the gene expression of ER was decreased (P < 0.05) and the CYP19 seemed increased but without significance, as compared to the DBP group. The expression of other genes was not significantly changed when adding ERa with DBP, as compared to DBP alone. Except for the FSHR mRNA, E2 could significantly decrease the other genes as compared to control.
The role of estrogen receptor in the mechanism of dibutyl phthalate on KGN cells. KGN cells were cultured for 24 and 48 h in five groups (N = 3 in each group): DMEM/F12 (control), dibutyl phthalate (DBP, 36 µg/mL), estrogen receptor antagonist (ERa, 0.1 µg/mL), DBP combined with ERa, estradiol (E2, 0.1 µg/mL). (A) The mRNA levels of CYP19a and estrogen receptor (ER) were decreased by DBP and DBP combined with ERa at 24 h, respectively. Decreased tendency was seen in the mRNA levels of TGF-β2, TGF-β3, and TGF-βR II in DBP group as compared to control. ERa could reverse these inhibitory effects of DBP on gene expression. (B) Protein levels (ER, P450arom, TGF-β2) were not influenced significantly by DBP. Gene expression and protein level were normalized by β-actin, detected by RT-PCR and Western blot, respectively. The letters (a, b, c, d) indicated the significant difference (P < 0.05) in post hoc comparisons (two-way analysis of variance, means ± SD), when compared to control, DBP, DBP combined with ERa, and ERa, respectively
When the KGN was influenced by DBP for 48 h, decreased tendency seemed in the mRNA levels of TGF-β1, TGF-β2, TGF-β3, and TGF-βR II but without statistical significance (P > 0.05, Fig. 3A). No difference was found in the mRNA levels of ER, FSHR, and CYP19. However, after the ERa was added with DBP for 48 h, the gene expression of TGF-β1, TGF-β2, TGF-β3, TGF-βR II, and FSHR was significantly increased, when compared to the control or the DBP group. The expression of ER gene was decreased significantly by DBP combined with ERa when compared to DBP alone, but none change in CYP19. Being inconsistent with the results at 24 h, the inhibitory effects of E2 on gene expression were attenuated at 48 h when compared to the control group.
However, the protein levels of TGF-β2, ER, and P450arom were not influenced significantly by DBP, as compared to the control group or the DBP combined with ERa group (Fig. 3B).
DBP could stimulate the proliferation of KGN especially at the 24-h timepoint, as compared to the control. The estrogen receptor antagonist could block this stimulating efficacy (DBP versus DBP + ERa (P < 0.05), DBP versus ERa (P < 0.05), Fig. 4A). The difference among five groups was significant at 24-h timepoint; however, the effect was attenuated at 48-h timepoint. Furthermore, the apoptosis rates of KGN cells were not different among groups. The levels of progesterone secreted by KGN were decreased by DBP and E2 at 24 h, no matter whether ERa was added or not (Fig. 5A). When compared to control, the levels of progesterone at 48 h were higher in DBP and E2 group, no matter whether the ERa was added with DBP or not.
The proliferation of KGN cells in different groups. P-values of multiple comparisons among five groups were listed (two-way ANOVA). (A) KGN cells were cultured in five groups (N = 3 in each group): DMEM/F12 (control), dibutyl phthalate (DBP, 36 µg/mL), estrogen receptor antagonist (ERa, 0.1 µg/mL), DBP combined with ERa, estradiol (E2, 0.1 µg/mL). P < 0.05 was represented as letters in post hoc comparisons: a (vs. control), b (vs. DBP), c (vs. DBP + ERa). DBP stimulated KGN proliferation at 24-h timepoint, while ERa could block this stimulating efficacy. (B) KGN cells were cultured in five groups (N = 3 in each group) using DMEM/F12 (control), dibutyl phthalate (DBP, 36 µg/mL), follicle-stimulating hormone (FSH, 100 ng/mL), transforming growth factor-β (TGF-β, 50 ng/mL), and ALK5 inhibitor (A77-01, 0.5 µg/mL). P < 0.05 was represented as letters in post hoc comparisons: a (vs. control), b (vs. DBP + FSH), c (vs. TGF-β + FSH), d (vs. A77-01 + FSH). TGF-β + FSH could inhibit the proliferation of KGN at 24 h; A77-01 promoted the proliferation of KGN as compared to TGF-β or DBP. These effects were vanished at 48 h
The levels of progesterone and estradiol in culture medium secreted by KGN in different groups. P-values of multiple comparisons among five groups were listed (two-way ANOVA, N = 3 in each group). The levels of progesterone and estradiol were measured by ELISA method. (A) KGN cells were cultured in five groups: DMEM/F12 (control), dibutyl phthalate (DBP, 36 µg/mL), estrogen receptor antagonist (ERa, 0.1 µg/mL), DBP combined with ERa, estradiol (E2, 0.1 µg/mL). The levels of progesterone were decreased by DBP and E2 at 24 h, but increased at 48 h, no matter whether ERa was added with DBP or not. P < 0.05 was represented as letters in post hoc comparisons: a (vs. control), b (vs. DBP), c (vs. DBP + ERa), d (vs. ERa). (B) KGN cells were cultured in five groups (N = 3 in each group) using DMEM/F12 (control), dibutyl phthalate (DBP, 36 µg/mL), follicle-stimulating hormone (FSH, 100 ng/mL), transforming growth factor-β (TGF-β, 50 ng/mL), and ALK5 inhibitor (A77-01, 0.5 µg/mL). P < 0.05 was represented as letters in post hoc comparisons: a (vs. control), b (vs. DBP + FSH), c (vs. TGF-β + FSH), d (vs. A77-01 + FSH). TGF-β + FSH could decrease the estradiol at 24 h; DBP had no effect on estradiol level at 24 h or 48 h. (C) Progesterone was decreased by DBP + FSH at 24 h, no matter whether A77-01 was added with DBP or not. However, progesterone was increased by DBP + FSH or TGF-β + FSH at 48 h, and the effect of DBP could be inhibited by A77-01
TGF-β Signal Pathway
The role of TGF-β signal pathway in the mechanism of DBP on KGN cells is illustrated in Fig. 6. When the DBP and FSH were both added for 24 h, the mRNA levels of TGF-βR II, FSHR, and CYP19 were significantly decreased (Fig. 6A), however without significant difference for the mRNA and protein of ER (Fig. 6B). Furthermore, only the mRNA level of TGF-βR II has been increased after ALK5 inhibitor (A77-01, which could block the transduction of TGF-β signal pathway) was also added (DBP + FSH versus A77-01 + DBP + FSH group). Meanwhile, as compared to the control group, TGF-β could decrease the mRNA levels of TGF-βR II and FSHR, but increase that of CYP19. ALK5 inhibitor (A77-01) combined with FSH could increase the mRNA level of TGF-βR II, but decrease that of CYP19.
The role of TGF-β signal pathway in the mechanism of DBP on KGN cells. KGN cells were cultured for 24 and 48 h in five groups (N = 3 in each group) using: DMEM/F12 (control), dibutyl phthalate (DBP, 36 µg/mL), follicle-stimulating hormone (FSH, 100 ng/mL), transforming growth factor-β (TGF-β, 50 ng/mL), and ALK5 inhibitor (A77-01, 0.5 µg/mL). P-values of multiple comparisons among five groups were listed (two-way ANOVA). P < 0.05 was represented as letters in post hoc comparisons: a (vs. control), b (vs. DBP + FSH), c (vs. TGF-β + FSH), d (vs. A77-01 + FSH). Gene expression and protein level were normalized by GAPDH and β-actin, detected by RT-PCR and Western blot, respectively. (A) The mRNA level of TGF-βR II was decreased by DBP + FSH, but increased by A77-01 + DBP + FSH at 24 h. TGF-βR II was increased by DBP + FSH, but decreased by A77-01 + DBP + FSH at 48 h. TGF-β + FSH could decrease the mRNA levels of TGF-βR II, increase CYP19 at 24 h; however, A77-01 + FSH had contrary effects. (B) The protein levels of ER and P450arom were not different among groups
After the DBP and FSH were both added for 48 h (Fig. 6), the mRNA levels of TGF-βR II, ER, and FSHR were significantly increased when compared to control, however without significant difference for the mRNA and protein of CYP19 (Fig. 6B). The mRNA levels of TGF-βR II, ER, FSHR, and CYP19 have been decreased after ALK5 inhibitor (A77-01) was added (DBP + FSH versus A77-01 + DBP + FSH group). When compared to control, ALK5 inhibitor (A77-01) combined with FSH could increase the mRNA level of TGF-βR II and ER, but decrease that of CYP19.
When compared to control, TGF-βcombined with FSH for 24 h could inhibit the proliferation of KGN (Fig. 4B). However, ALK-5 inhibitor (A77-01) promoted the proliferation of KGN as compared to TGF-β or DBP. ALK-5 inhibitor (A77-01) could also block the DBP effect and promote the proliferation of KGN (DBP + FSH versus A77-01 + DBP + FSH group; Fig. 4B). However, these effects were vanished at the 48 h. The apoptosis rates of KGN cells were also not different among groups. TGF-β combined with FSH could decrease the estradiol in culture medium at 24 h; however, DBP had no effect on estradiol level at 24 h or 48 h (Fig. 5B). The level of progesterone was decreased by DBP combined with FSH at 24 h, no matter whether A77-01 was added with DBP or not (Fig. 5C). However, progesterone was increased by DBP or TGF-β, combined with FSH at 48 h. The effect of DBP on progesterone at 48 h could be inhibited by A77-01 (DBP + FSH versus A77-01 + DBP + FSH).
Discussion
In present study, the reproductive consequence and the underlying molecular mechanisms of ovarian dysfunction induced by the perinatal maternal exposure to DBP were explored for the adult female offspring. The role of estrogen receptor and TGF-β signal pathway in the molecular mechanism was firstly explored in the ovarian tissue of adult offspring affected by unfavorable intrauterine and lactational environment of DBP.
We demonstrated that the estrous period was prolonged and the number of secondary follicles was increased in female offspring exposed to perinatal DBP, but none significant difference was found for the serum levels of sex hormones. Maternal perinatal exposure to DBP induced decreased mRNA and protein levels of TGFβ2, TGFβ3, and TGFβR II; and decreased mRNA levels of LHR, FSHR, and CYP19a in ovaries of the adult female offspring.
DBP alone in vitro might inhibit the gene expression of TGF-β2, TGF-β3, TGF-βR II, and CYP19 of KGN, and stimulate proliferation of KGN, and these effects might be blocked by estrogen receptor. DBP combined with FSH might time-dependently regulate the gene expression of TGF-βR II, inhibitory at 24 h, but stimulative at 48 h. DBP combined with FSH could inhibit the proliferation of KGN at 24 h. All these effects can be reversed by ALK5 inhibitor, which could block the TGF-β signal pathway. Molecular cross-talk between estrogen receptor signaling and TGF-β signaling pathway may play role in the mechanism of granulosa dysfunction induced by DBP.
Phthalate exposure is of concern because it is shown to be associated with increased risks of infertility and early reproductive senescence [22]. The “Developmental Origins of Health and Adult Disease” (DOHaD) hypothesis proposes that deleterious effects on organogenesis and increased risk of various diseases in adult life may be induced by unfavorable intrauterine environment [23]. Long-term dietary exposure to DBP also induces multigenerational effects on body weight and reproductive outcomes in both male and female rats; however, most previously published studies were focused on the influence of DBP on male reproduction [12, 13]. Furthermore, nearly all published literatures investigated the acute exposure toxicity of DBP on reproduction [12]. The fetus displayed about 50% the levels of DBP observed in the mother. Reduced fetal body weight and number of live fetuses were found; however, the effects of DBP on the reproductive system in F1 females were insignificant [12]. Fewer number of offspring but heavier weight of female adult offspring was found in present study. Especially, the estrous period was prolonged in DBP group with the dosage-dependent manner, longest in the high DBP group. Increased proliferation of granulosa and number of secondary follicles induced by DBP might be the cause of prolonged estrous period. However, the number of atretic follicles seems increased but without significance, which is consistent with the insignificant change of granulosa apoptosis among groups.
Maternal perinatal exposure to DBP induced decreased mRNA levels of LHR, FSHR, and CYP19a in ovaries of the adult female offspring. However, none significant difference was found for the serum levels of sex hormones in vivo or the estradiol level in the culture medium in vitro. Epigenetic influence on the protein expression was speculated by us but need further clarification.
DBP metabolites were also detected in the follicular fluid (1–2 ng/mL) [2]. However, the DBP metabolites or the hormone levels in the follicular fluid of adult female offspring were not investigated in present animal study or previously published studies [5, 11, 14]. These will be further explored by us in future. The ovary is key to female fertility by providing the eggs and sex steroid hormones for fertilization and maintenance of reproductive function, respectively. The influence of perinatal exposure to DBP for the reproductive steroid of offspring was controversial. None difference was found in serum levels of sex hormones for adult female offspring in present study. However, perinatal exposure of DBP was previously reported to significantly upregulate the serum estradiol levels in both immature and mature offspring rats [15]. DBP exposure also significantly downregulated the testosterone levels in immature male and female rats and mature male rats but had no influence on the testosterone levels in mature female rats [15]. Pregnant rats were given intragastric administration of 500 mg/kg body weight DBP daily from GD6 to PND21 in this study. Maternal perinatal exposure to oral high dose of DBP (10, 100, and 600 mg/kg/day) during late pregnancy and breastfeeding was reported to not affect ovarian development and function in F1 rats, however, may increase serum estradiol and progesterone levels [14]. However, exposure to low-dose DBP in rat model maybe more physiological relevance than high-dose DBP. A different metabolic response to low-dose DBP from to high-dose DBP in mother rats might be existed [7]. The effects of a chronic exposure to a mixture of EDCs on the expression of genes and proteins of the steroidogenic pathway and hormonal status were also different from those exposed to single EDC [24]. The animal study on chronic exposure to low dosage of DBP was few, especially for the perinatal maternal exposure.
Mechanistic studies have characterized the mode of action for DBP in the ovary using whole ovaries, antral follicles, and isolated ovarian cells. In vitro exposures to DBP on mouse antral follicles found that DBP at levels comparable to human follicular fluid did not cause growth inhibition or follicular death; whereas, higher concentrations of DBP (≥ 10 μg/mL) caused antral follicle growth inhibition and significant disruptions in cell cycle gene expression and elicited cell cycle arrest followed by follicular death [7]. In rat granulosa cells and preantral follicles, DBP (5–100 μg/mL) interfered with FSH-stimulated expression of Kitl, reduced the expression of Fshr and downstream factors, and reduced steroidogenesis [25]. DBP (10 and 100 μg/mL) selectively decreased estradiol and progesterone secretion accompanied by disrupting expression of mRNAs essential for steroidogenesis (StAR, CYP11A1, and CYP19A1) in human mural granulosa cells [10, 26]. Decreased serum estradiol of mice and increased mRNAs encoding the steroidogenic enzymes Hsd17b1, Cyp17a1, and Cyp19a1 at all concentrations of DBP in vivo (0.01, 0.1, and 1000 mg/kg/day) were reported by Sen et al. and exposure to 0.1 mg/kg/day of DBP also decreased antral follicle numbers [11]. Being consistent with our results, decreased mRNAs and protein levels of LHCGR and FSHR, and decreased of mRNA of CYP19A and ER were also found in ovaries of F1 females exposed to EDCs [27]. The discrepancy among the above results may be attributed to the type of experiment (in vivo vs. in vitro), route of DBP administration (orally, intraperitoneally, vs. addition to the medium), dosage of DBP (low or high), duration of exposure (acute or chronic; single or complex), and different species (human, rat, or mice). The impact of DBP on the expression of steroidogenic genes was focused on a specific target cell population, namely granulosa cells (primary cell or KGN cell line).
When the KGN was incubated by DBP for 24 h, the mRNA level of CYP19 was significantly decreased, the levels of progesterone and E2 in culture medium were decreased; however, the influence was attenuated at 48 h. The hormone endocrinological function was speculated to be inhibited by the DBP, so the levels of progesterone and E2 were decreased in vivo study. This should be verified in animal study in future by us. DBP could stimulate the proliferation of KGN at 24 h, and ERa could block this stimulating efficacy. However, this was also attenuated at 48 h. As compared to the 24 h, the inhibitory effects of E2 on gene expression were also attenuated at 48 h. DBP combined with FSH might also time-dependently regulate the gene expression of TGF-βR II, inhibitory at 24 h, but stimulative at 48 h. All above results suggested that the efficacy of DBP on KGN was significant at 24 h.
The influence of DBP on the TGF-β signal pathway has been reported by few studies in renal tissue and prostate cancer, but not on ovary tissue. Maternal exposure to DBP during pregnancy leads to the accumulation of TGF-β1 in offspring kidneys [28]. Higher expression levels of TGF-β and α-SMA were observed in abnormal renal tissue of adult male offspring which was maternal exposure to DBP and DBP-treated NRK52E cells [29]. DBP significantly reduced the protein expression of p-smad similarly to E2. These regulations caused by DBP were reversed by ICI 182,780, an ER antagonist, indicating that DBP may affect cross-talk between TGF-β and ER signals. DBP may induce the growth of LNCaP prostate cancer by acting on the cross-talk between TGF-β and ER signaling pathways [20].
The role of TGF-β on the effects of DBP for the ovarian tissue was not reported before and firstly explored by us. Decreased levels of TGFβ2, TGFβ3, and TGFβR II were found in ovaries of adult female offspring. DBP in vitro could inhibit the gene expression of TGF-β2, TGF-β3, and TGF-βR II. DBP combined with FSH might time-dependently regulate the gene expression of TGF-βR II. These effects can be blocked by estrogen receptor antagonist or ALK5 inhibitor of the TGF-β signal pathway. Molecular cross-talk between estrogen receptor signaling and TGF-β signaling pathway may play role in the mechanism of ovarian dysfunction induced by DBP.
The strength of present study is to explore the reproductive consequence of perinatal maternal exposure to DBP on the adult female offspring, to explore the environmental deleterious effects on developmental origins of adult reproductive dysfunction. However, our study also has a few limitations. It is still not the animal study exploring the daily chronic exposure to DBP. Whole ovarian tissue was used in the animal experiment in vivo to explore the effect of perinatal exposure to DBP. Therefore, the effect of DBP on granulosa cell, follicle, and hormone level in follicular fluid in vivo was not explored and would be studied furtherly. The mechanism experiment in vitro was relatively acute by exposing KGN to DBP for 24 h and 48 h. Given the widespread exposure of humans to numerous contaminants, the combinatorial effects of multiple chemicals also merit evaluation.
Data Availability
The data and materials could be available by contacting the corresponding author upon reasonable request.
References
Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342.
Du YY, Fang YL, Wang YX, et al. Follicular fluid and urinary concentrations of phthalate metabolites among infertile women and associations with in vitro fertilization parameters. Reprod Toxicol. 2016;61:142–50.
Zhou C, Gao L, Flaws JA. Exposure to an environmentally relevant phthalate mixture causes transgenerational effects on female reproduction in mice. Endocrinology. 2017;158:1739–54.
Zhou C, Gao L, Flaws JA. Prenatal exposure to an environmentally relevant phthalate mixture disrupts reproduction in F1 female mice. Toxicol Appl Pharmacol. 2017;318:49–57.
Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013;8:e55387.
Johansson HK, Jacobsen PR, Hass U, et al. Perinatal exposure to mixtures of endocrine disrupting chemicals reduces female rat follicle reserves and accelerates reproductive aging. Reprod Toxicol. 2016;61:186–94.
Rasmussen LM, Sen N, Vera JC, Liu X, Craig ZR. Effects of in vitro exposure to dibutyl phthalate, mono-butyl phthalate, and acetyl tributyl citrate on ovarian antral follicle growth and viability. Biol Reprod. 2017;96:1105–17.
Hannon PR, Flaws JA. The effects of phthalates on the ovary. Front Endocrinol. 2015;6:8.
Hines C, Hopf N, Deddens J, Silva M, Calafat A. Estimated daily intake of phthalates in occupationally exposed groups. J Expo Sci Environ Epidemiol. 2011;21:133–41.
Adir M, Combelles CMH, Mansur A, et al. Dibutyl phthalate impairs steroidogenesis and a subset of LH-dependent genes in cultured human mural granulosa cell in vitro. Reprod Toxicol. 2017;69:13–8.
Sen N, Liu X, Craig ZR. Short term exposure to di-n-butyl phthalate (DBP) disrupts ovarian function in young CD-1 mice. Reprod Toxicol. 2015;53:15–22.
Czubacka E, Czerczak S, Kupczewska-Dobecka MM. The overview of current evidence on the reproductive toxicity of dibutyl phthalate. Int J Occup Med Environ Health. 2021;34:15–37.
Wine RN, Li LH, Barnes LH, Gulati DK, Chapin RE. Reproductive toxicity of di-n-butylphthalate in a continuous breeding protocol in Sprague-Dawley rats. Environ Health Perspect. 1997;105:102–7.
Xie Z, Wang J, Dai F, et al. Effects of maternal exposure to di-n-butyl phthalate during pregnancy and breastfeeding on ovarian development and function of F1 female rats. Environ Toxicol Pharmacol. 2016;43:38–43.
Li X, Jiang L, Cheng L, Chen H. Dibutyl phthalate-induced neurotoxicity in the brain of immature and mature rat offspring. Brain Dev. 2014;36(8):653–60.
Kristensen SG, Kumar A, Kalra B, Pors SE, Bøtkjær JA, Mamsen LS, et al. Quantitative differences in TGF-beta family members measured in small antral follicle fluids from women with or without PCO. J Clin Endocrinol Metab. 2019;104:6371–84.
Shen H, Wang Y. Activation of TGF-beta1/Smad3 signaling pathway inhibits the development of ovarian follicle in polycystic ovary syndrome by promoting apoptosis of granulosa cells. J Cell Physiol. 2019;234:11976–85.
Ignar-Trowbridge DM, Pimentel M, Teng CT, Korach KS, McLachlan JA. Cross talk between peptide growth factor and estrogen receptor signaling systems. Environ Health Perspect. 1995;103(Suppl 7):35–8.
Smith LC, Moreno S, Robertson L, Robinson S, Gant K, Bryant AJ, et al. Transforming growth factor beta1 targets estrogen receptor signaling in bronchial epithelial cells. Respir Res. 2018;19:160.
Lee HR, Hwang KA, Choi KC. The estrogen receptor signaling pathway activated by phthalates is linked with transforming growth factor-β in the progression of LNCaP prostate cancer models. Int J Oncol. 2014;45:595–602.
Hu Y, Yuan D, Wu Y, Yu L, Xu L, Yue L, et al. Bisphenol A initiates excessive premature activation of primordial follicles in mouse ovaries via the PTEN signaling pathway. Reprod Sci. 2018;25:609–20.
Hauser R, Calafat AM. Phthalates and human health. Occup Environ Med. 2005;62:806–18.
Charles MA, Delpierre C, Bréant B. Developmental origin of health and adult diseases (DOHaD): evolution of a concept over three decades. Med Sci (Paris). 2016;32:15–20.
Buñay J, Larriba E, Patiño-Garcia D, et al. Editor’s highlight: Differential effects of exposure to single versus a mixture of endocrine-disrupting chemicals on steroidogenesis pathway in mouse testes. Toxicol Sci. 2018;161:76–86.
Wang XJ, Xiong GP, Luo XM, et al. Dibutyl phthalate inhibits the effects of follicle-stimulating hormone on rat granulosa cells through down-regulation of follicle-stimulating hormone receptor. Biol Reprod. 2016;94:144.
Adir M, Salmon-Divon M, Combelles CMH, et al. In vitro exposure of human luteinized mural granulosa cells to dibutyl phthalate (DBP) affects global gene expression. Toxicol Sci. 2017;160:180–8.
Patiño-García D, Cruz-Fernandes L, Buñay J, Palomino J, Moreno RD. Reproductive alterations in chronically exposed female mice to environmentally relevant doses of a mixture of phthalates and alkylphenols. Endocrinology. 2018;159:1050–61.
Zhao S, Jiang JT, Li D, Zhu YP, Xia SJ, Han BM. Maternal exposure to di-n-butyl phthalate promotes Snail1-mediated epithelial-mesenchymal transition of renal tubular epithelial cells via upregulation of TGF-β1 during renal fibrosis in rat offspring. Ecotoxicol Environ Saf. 2019;169:266–72.
Sun WL, Zhu YP, Ni XS, et al. Potential involvement of Fgf10/Fgfr2 and androgen receptor (AR) in renal fibrosis in adult male rat offspring subjected to prenatal exposure to di-n-butyl phthalate (DBP). Toxicol Lett. 2018;282:37–42.
Acknowledgements
The authors thank Professor Bin Zhou for sample preservation; Siyu Zhou and Sicong Li for sample collection.
Funding
This work was supported by the Scientific Research Projects of the National Natural Science Fund (21707096) and Technology Support Program of Sichuan Province (2019YFS0422).
Author information
Authors and Affiliations
Contributions
ZJ: Conceptualization, writing original draft, investigation, data curation and analysis, project administration, funding acquisition. ZK: Conceptualization, methodology, validation, funding acquisition. CR: Investigation, data curation and analysis. YM, SX, LX: Investigation, validation. XL: Resources, supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All experiments were conducted according to the Guide for the Care and Use of Laboratory Animals of National Research Council and approved by the Ethical Scientific Committee for the Care of Animals at West China Second University Hospital, Sichuan University (No. 2018–007).
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, J., Zhou, K., Cheng, R. et al. Maternal Perinatal Exposure to Dibutyl Phthalate Promotes Ovarian Dysfunction in Adult Female Offspring via Downregulation of TGF-β2 and TGF-β3. Reprod. Sci. 29, 2401–2413 (2022). https://doi.org/10.1007/s43032-021-00785-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00785-y