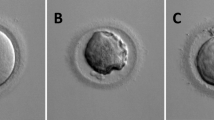
Abstract
It remained unknown whether HDAC6 affected the histone deacetylation of in vitro maturation oocytes and the reprogramming of nuclear transplantation in pig. Our results indicated that HDAC6 specific inhibition did not affect overall HDAC activity and meiosis process, which increased histone H3K9/K14 and H4K8 acetylation of porcine in vitro maturation oocytes and pseudo-pronucleus embryos. HDAC6 inhibition also significantly enhanced the cleavage and blastocyst of nuclear transfer embryos (0.81 ± 0.12 vs. 0.68 ± 0.12 and 0.46 ± 0.19; 0.73 ± 0.13 vs. 0.63 ± 0.18 and 0.40 ± 0.16, P<0.05). The inhibition of HDAC6 significantly enhanced histone H3K9/K14 and H4K8 acetylation, and upregulated the OCT4 and CDX2 expressions (1.83 ± 0.16 vs. 1.00 ± 0.00 %; 2.07 ± 0.09 vs. 1.00 ± 0.00; P<0.05) in porcine SCNT blastocysts. Interestingly, HDAC6 inhibition significantly increased the pseudo-pronucleus volume during somatic cell reprogramming. Thus, HDAC6 was required for porcine histone deacetylation during the in vitro maturation and pseudo-pronucleus stages. HDAC6 inhibition improved the in vitro development of nuclear transfer embryos. HDAC6 may restrict the reprogramming of somatic nuclear transfer by regulating pseudo-pronucleus expansion. We need further research to confirm this in the future.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The abnormal epigenetic reprogramming of nuclear transfer embryo affects the development efficiency of nuclear transfer embryo. The N-terminal acetylation of histones H3 and H4 is an important part of epigenetic modification and nuclear transplantation [1]. The H4K8 acetylation of pseudo-pronucleus is close to that of male and female pronucleus, which upregulates the expression of pluripotent genes and improves the efficiency of embryo development [1, 2]. Histone H4K5 and H3K9/K14 acetylation are involved in the protamine replacement and the regulation of chromosome structure [3, 4]. Increased H3K9 acetylation in donor cells and nuclear transfer embryos enhances the development efficiency and the expression level of pluripotent genes [5]. A large number of histone H3K9 and H4K8 were acetylated in the expression regions of human and mouse pluripotent genes [6,7,8]. However, how histone acetylation of nuclear transfer embryo is erased remains unclear.
Histone acetylation is mediated by histone acetylase and histone deacetylase [9]. Class IIb histone deacetylases are involved in histone deacetylation during in vitro maturation of porcine oocytes [10,11,12]. Global histones show high acetylation in germinal vesicle (GV) oocytes and very low acetylation in Metaphase I (MI) and Metaphase II (MII) oocytes [11, 13, 14]. Histone acetylation is removed by histone deacetylase in the cytoplasm [10]. The granular cells injected into MII oocytes maintain high acetylation levels, but histone acetylation is removed rapidly after the nuclear membrane broken [10]. Increasing H3K9 acetylation of the donor is still erased by histone deacetylase in the cytoplasm [5]. Histone H4K8 and H3K9 acetylation of nuclear transfer embryo is maintained by using broad-spectrum deacetylase inhibitors [2, 15, 16].
Histone deacetylases 6 (HDAC6), a class IIb histone deacetylase with two functional catalytic domains, can shuttle between cytoplasm and nucleus to regulate intracellular signals. With closely related to cancer and neurodegenerative diseases, HDAC6 has become a hot therapeutic target [17]. HDAC6 shows the highest expression level and locates near the chromosome in mouse MII oocytes [12, 18]. The upregulation of HDAC6 expression significantly reduces histone acetylation in K562 cells [19]. HDAC6 knockout mices show high levels of histone acetylation in spermatogenic cells [20]. Overexpression of HDAC6 upregulates related genes of follicular development and increases reproductive capacity in mice [21]. But HDAC6 inhibition hinders chromosome segregation which damages oocyte meiosis and early embryo development [12, 14].
However, it was not addressed whether HDAC6 caused the changes of histone deacetylation in oocyte maturation and reprogramming of nuclear transfer embryos. We used HDAC6 non-specific inhibitor (SAHA), a class I/IIa deacetylase inhibitor (VPA) and HDAC6 specific inhibitor Bufexamac. Our understanding of HDAC6 was required for histone deacetylation of oocyte maturation and nuclear transplantation reprogramming, however, not disrupt meiosis maturation to improve the development of nuclear transfer embryo. We explored the effect of HDAC6 inhibition on histone acetylation, deacetylase activity, embryos development and related genes expression in porcine oocyte maturation and nuclear transplantation.
Materials and Methods
In Vitro Maturation
The process of oocyte in vitro maturation was in accordance with the experimental procedure of previous article [2]. Porcine ovaries came from the local slaughterhouse in Nanning, China. About 40 pig ovaries can produce 100–150 high-quality oocytes. Cumuluse oocyte complexes (COCs) were transferred into the maturation medium (9.8g TCM-199, 10% fetal bovine serum, 10% follicular fluid, 15IU/mL pregnant mare serum gonadotropin and 10IU/mL human chorionic gonadotropin) supplemented with histone deacetylase inhibitors (7.5μmol/L SAHA, S1047; 1mmol/L VPA, S1168; 20μmol/L Bufexamac, S3023; selleck Chemicals) in MI (28h) or MII (44h) oocytes at 38.5°C under 5% CO2 air atmosphere.
HDAC and HDAC6 Activity Analysis
HDAC and HDAC6 activity was detected by using HDAC Activity Fluorometric Assay Kit (K330-100, BioVision) and HDAC6 Activity Assay Kit (K466-100, BioVision) according to the manufacturer’s instructions. The COCs were divided into four groups (control, 7.5μmol/L SAHA, 1mmol/L VPA and 20μmol/L Bufexamac) and two stages (MI and MII). About 100 oocytes were collected from about 40 pig ovaries in each group and each stage. The samples were measured using a microplate fluorometer (Fluoroskan Ascent FL; Thermo Fisher) at Ex/Em 380/490 nm in an end point mode at 37°C. According to calculation scheme of the instructions, sample HDAC and HDAC6 activity was calculated.
Immunofluorescence Staining and Orcein Stain
The process of immunofluorescence staining was in accordance with the experimental procedure of previous article [2]. About 20-30 oocytes and nuclear transfer embryos were preserved in 4% PFA, and permeabilized 1% Triton X. They were incubated overnight in AcH3K14 antibody (monoclonal AcH3K9/K14, ABclonal) or AcH4K8 antibody (monoclonal AcH4K8, Abcam) in 4°C. They were transfered into secondary antibody (monoclonal fluorescein isothiocyanate co-njugated, Sigma) for 1.5h in the darkroom. At last they were transfered into PBS with propidium iodide (30 μg/mL). The oocytes and nuclear transfer embryos were observed by a laser confocal microscope (SP4, Leica). According to previous article, 1% (w/v) orcein was gently pushed to cover fixed oocytes. The nuclear phase of in vitro maturation oocytes were observed under inverted microscope.
Donor Cells’ Treatment and Preparation
The process of primary culture, drug treatment, and immunofluorescence of donor cells was in accordance with the experimental procedure of previous article [5]. The ear skin tissue of local pig (born for 1–2 weeks) was collected from the live pig gene bank in Nanning, China. The ear tissue was cut into pieces and inoculated into culture dish for about 4 h. Ear tissue was cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% FBS at 38.5°C and 5% CO2. About 70–80% confluent donor cells were cryopreserved or used for experiments. Donor cells were seeded into 12-well plates which were treated by histone deacetylase inhibitor for 24 h. Prior to nuclear transfer, histone H3K9/K14 and H4K8 acetylation of donor cells were determined by immunofluorescence. Donor cells were gently blown to suspend, and then cell suspension is transferred into the embryo manipulation medium. Donor cells with intact and smooth appearance were used for nuclear transplantation.
Somatic Cell Nuclear Transfer and Parthenogenetic Activation
The process of nuclear transfer and parthenogenetic activation was in accordance with the experimental procedure of previous article [5]. The process of nuclear transfer was that oocytes were enucleated by a blind aspiration to remove the first polar body with the surrounding. Donor cells were injected into the perivitelline of enucleated oocytes from the original incision. The couplets were activated and fused by an alternating current at 0.08 kV/cm and a continuous current at 2 kV/cm for 30 μs. The process of parthenogenetic activation was that high-quality oocytes were aligned in a straight line and activated by a continuous current at 2 kV/cm for 30 μs. Somatic nuclear transfer (SCNT) was divided into two groups (control and 20μmol/L Bufexamac), and parthenogenetic activation (PA) was divided into four groups (control, 7.5μmol/L SAHA, 1mmol/L VPA and 20μmol/L Bufexamac). The SCNT and PA oocytes were placed in electric activation medium, and activated/fused at the same time. The rates of cleavage, blastocyst, and the total number of nuclei in every blastocyst were recorded. The 1-cell, 2-cells 4-cells, and blastocysts of SCNT and PA embryos were collected.
Quantitative PCR Analysis
The process of reverse transcription and quantitative PCR was in accordance with the experimental procedure of appropriate reference. The gene primers (Table 1) were produced from Sangon Biotech (Shanghai). Five embryos were collected in each group and different stages. Reverse transcription of different embryos (1-cell, 2-cells 4-cells, and blastocysts) was carried out using the SuperScript TM II Reverse Transcriptase kit (Invitrogen). The expression of these genes was detected and recorded by a PCR analyzer (ABI 7500). Each gene was repeated at least for three times. The 18s expression was compared as the endogenous control gene. The relative expression levels of the target genes were determined by using 2–△△CT method.
Statistics Analysis
The data from developmental effect and quantitative PCR were analyzed by Duncan’s multiple comparison. All results were shown using mean and standard deviation. P<0.05 were considered significant difference. The experiment of the embryo development repeated 3-4 times and required 20–25 embryos at a time.
Results
Changes of HDAC and HDAC6 Activity
To measure whether Bufexamac had specific HDAC6 inhibitory effect on porcine in vitro matured oocytes, we analyzed the changes of HDAC and HDAC6 activity treatment with control, SAHA, VPA, and Bufexamac. HDAC activity in Bufexamac group was not different from control and VPA groups, which was significantly higher than SAHA group during MI and MII stages (Fig. 1a, b). On the contrary, HDAC6 activity of the Bufexamac group was not different from that of the SAHA group, but HDAC6 activity of the Bufexamac and SAHA group was lower than that of the control and VPA groups during MI and MII stages (Fig. 1c, d). These informed that HDAC6 was specific inhibited by Bufexamac during porcine oocytes maturation.
Effect of HDAC6 Inhibition on Histone Deacetylation During In Vitro Maturation
The levels of histone H3K9/K14 and H4K8 acetylation were significantly decreased from GV to MI and MII (Fig. 2). It was not reported whether Bufexamac as a HDAC6 specific inhibitor can increase global histone acetylation during porcine oocytes maturation. Our results showed HDAC6 inhibition significantly increased H3K9/K14 and H4K8 acetylation at MI and MII stages (Figs. 3 and 4). SAHA as a class I/II deacetylase inhibitor can significantly enhance histone acetylation. On the contrary, VPA as a class I and class IIa inhibitor had no effect on histone acetylation [10]. Compared with control, SAHA (positive control), VPA (negative control), and Bufexamac, we confirmed that HDAC6 was involved in histone deacetylation of porcine oocyte maturation.
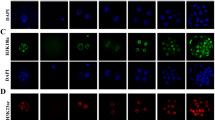
The change of histone acetylation in in vitro maturation of porcine oocytes. Original magnification was 200×. Note: A: AcH3K9/K14 in GV oocytes; B: AcH3K9/K14 in MI oocytes; C: AcH3K9/K14 in MII oocytes; D: AcH4K8 in GV oocytes; E: AcH4K8 in MI oocytes; F: AcH4K8 in MII oocytes; Green: AcH3K9/K14 or AcH4K8; Red: DNA. More than 80% of the pictures have similar situation
The effect of HDAC6 inhibition on AcH3K9/K14 in in vitro maturation of porcine oocytes. Original magnification was 200×. Note: A: Control group in MI stage; B: SAHA group in MI stage; C: VPA group in MI stage; D: Bufexamac group in MI stage; E: Control group in MII stage; F: SAHA group in MII stage; G: VPA group in MII stage; H: Bufexamac group in MII stage; Green: AcH3K9/K14; Red: DNA. More than 80% of the pictures have similar situation
The effect of HDAC6 inhibition on AcH4K8 in in vitro maturation of porcine oocytes. Original magnification was 200×. Note: A: Control group in MI stage; B: SAHA group in MI stage; C: VPA group in MI stage; D: Bufexamac group in MI stage; E: Control group in MII stage; F: SAHA group in MII stage; G: VPA group in MII stage; H: Bufexamac group in MII stage; Green: AcH4K8; Red: DNA. More than 80% of the pictures have similar situation
Effect of HDAC6 Inhibition on In Vitro Maturation of Porcine Oocytes
To detect the effect of HDAC6 inhibition on porcine meiotic maturation, the proportion of MI oocytes in control, VPA, and Bufexamac groups was significantly higher than that of SAHA group for 24h (90.95 ± 1.75 %, 87.50 ± 3.61 % and 90.66 ± 3.45 % vs. 50.00 ± 3.72 %, P<0.05) (Fig. 5a); moreover, they were not different for 48h (92.75 ± 0.94 %, 91.13 ± 3.05 % and 91.66 ± 2.42 % vs. 92.75 ± 0.94 %, P>0.05) (Fig. 5b). It suggest that HDAC6 hibition could damage oocyte meiotic maturation [12, 14], so we evaluated the efficiency of parthenogenetic activation in embryonic development. The cleavage rates of porcine embryos were not significantly different in control, VPA, and Bufexamac groups, which were higher than those of SAHA group (81.85 ± 7.26 %, 74.31 ± 8.54 % and 76.98 ± 10.52 % vs. 63.29 ± 9.58 %, P<0.05) (Fig. 5c). However, the blastocyst rate of porcine embryos in Bufexamac group was higher than that of control, SAHA and VPA groups (51.68 ± 7.47 % vs. 44.06 ± 9.02 %, 42.90 ± 7.47 % and 29.00 ± 8.42 %, P<0.05) (Fig. 5c). Our results indicated HDAC6 inhibition had no significant effect on the in vitro maturation of porcine oocytes.
Effect of HDAC6 Inhibition on Histone Deacetylation of Pseudo-pronucleus
To further confirm that HDAC6 was required for histone deacetylation in the pseudo-pronucleus of nuclear transfer embryo, we compared the effect of HDAC6 inhibition on pseudo-pronucleus deacetylation in three different treatments. With HDAC6 specific inhibition and non-specific inhibition during in vitro maturation, histone H3K9/K14 and H4K8 acetylation was significantly increased in pseudo-pronucleus embryos (Fig. 6). But the donor cells with high levels of histone H3K9/K14 and H4K8 acetylation were rapidly deacetylated by the enucleated oocytes within 4 h (Fig. 7a–h). Interestingly, the radius and area of histone H3K9/K14 and H4K8 acetylation were significantly higher than those of the control group (Fig. 7i, j). As far as we know, this phenomenon had not been reported. These indicated that HDAC6 inhibition enhanced histone H3K9/K14 and H4K8 acetylation of nuclear transfer embryos, which may increase the pseudo-pronucleus volume during nuclear reprogramming.
The effect of HDAC6 inhibition in histone acetylation and nuclear area of pseudo-pronucleus embryos. Original magnification was 200×. Note: A: the increased AcH3K9/K14 of donors treated by SAHA for 72h; B: the decreased AcH3K9/K14 of donors in control group for 72h; C: the donors with high AcH3K9/K14 for nuclear transplantation; D: the donors with low AcH3K9/K14 for nuclear transplantation; E: the increased AcH4K8 of donors treated by SAHA for 72h; F: the decreased AcH4K8 of donors in control group for 72h; G: the donors with high AcH3K9/K14 for nuclear transplantation; H: the donors with low AcH3K9/K14 for nuclear transplantation; green: AcH3K9/K14 or AcH4K8. More than 80% of the pictures have similar situation. I: The change of nuclear radius in Bufexamac or control groups; J: the change of nuclear area in Bufexamac or control groups
Effect of HDAC6 Inhibition on SCNT Embryo
We analyzed whether HDAC6 inhibition during meiotic maturation affected the development of nuclear transfer embryo. HDAC6 inhibition showed a significant increase in the blastocyst rate (25.03 ± 4.94 % vs. 16.37 ± 3.92 %, P<0.05) (Fig. 8a, b, c). The AcH3K9/K14 and AcH4K8 levels of the blastocysts in HDAC6 inhibition group were higher than those of NT-C group (Fig. 9a–d). The expression of OCT4 and CDX2 was significantly upregulated in the blastocysts stage (1.83 ± 0.16 vs. 1.00 ± 0.00 %; 2.07 ± 0.09 vs. 1.00 ± 0.00; P<0.05) (Fig. 9e, f). Our results explained that HDAC6 inhibition could increase histone AcH3K9/K14 and AcH4K8 levels resulting in upregulation of the expression levels of developmental related genes, which can improve the development efficiency of nuclear transfer embryos.
The effect of HDAC6 inhibition in the development efficiency of nuclear transfer embryo. Original magnification was 200×. Note: A: the nuclear transfer blastocysts in NT-Bu group; B: the nuclear transfer blastocysts in NT-Control group; C: the efficiency of polar body, cleavage, and blastocyst in NT-Bu and NT-Control groups; D: the total number of blastocyst cell in NT-Bu and NT-Control groups; the rate of polar body was assessed at 42 h after in vitro maturation; The rates of cleavage and blastocyst were assessed at 24 h and 168 h after nuclear transplantation; the total number of blastocyst cell was stained at 5% hochest33342
The effect of HDAC6 inhibition in histone acetylation and key developmental genes of nuclear transfer blastocyst. Original magnification was 200×. Note: A: the AcH3K9/K14 of nuclear transfer blastocysts in NT-C group; B: the AcH3K9/K14 of nuclear transfer blastocysts in NT-Bu group; C: the AcH4K8 of nuclear transfer blastocysts in NT-C group; D: the AcH4K8 of nuclear transfer blastocysts in NT-Bu group; E: the OCT-4 expression of nuclear transfer blastocysts in NT-Bu and NT-C group; F: the CDX-2 expression of nuclear transfer blastocysts in NT-Bu and NT-C group
Discussion
Histone acetylation was mediated by histone acetylase and histone deacetylase [9]. Class IIb histone deacetylases were involved in histone deacetylation [10, 14], but it was still unknown whether HDAC6 removes global histone acetylation during oocytes in vitro maturation. Bufexamac, a preferential HDAC6 specific inhibitor, may be no effect on other histone deacetylases [22]. We compared the effects of SAHA (positive group), VPA (negative group), and Bufexamac on deacetylase activity. Bufexamac did not affect the overall HDAC activity. Our results suggest that HDAC6 could be specific inhibited by Bufexamac during porcine oocytes maturation. Previous studies reported that broad-spectrum deacetylase inhibitors can significantly improve histone acetylation during in vitro maturation [10]. Our results showed that HDAC6 inhibition significantly upregulated the level of histone acetylation to the level of positive group. Moreover, HDAC6 specific inhibition significantly increased the levels of histone H3K9/K14 and H4K8 acetylation to those of positive group in pseudo-pronucleus embryos. These results confirmed that HDAC6 was required for histone deacetylation during the in vitro maturation and pseudo-pronucleus stages of porcine oocytes.
HDAC6 inhibition significantly damaged oocyte maturation and zygote development in mice [12, 14]. Our results indicated HDAC6 inhibition has no significant effect on the in vitro maturation of porcine oocytes and the development of parthenogenetic activation embryos. The reason why our results were different from previous reports was that mouse oocytes were less tolerant to deacetylase inhibitors [12, 23]. The high concentration of broad-spectrum deacetylase inhibitors can inhibit the process of chromosome division [17, 24], and then damage the early embryo development. However, there was no specific immunofluorescence antibody, which did not show the location changes of HDAC6 in different periods. It was not reported that HDAC6 may be involved in reprogramming of nuclear transfer embryos. In our experiments, HDAC6 inhibition showed a significant increase in the development of somatic nuclear transfer. Our results implied that HDAC6 may directly affect the process of somatic reprogramming. HDAC6 would be an important protein to improve reprogramming efficiency and explore reprogramming mechanism. These results suggest that HDAC6 inhibition did not affect the meiosis of oocytes chromosomes, but promoted the reprogramming of somatic nuclear transfer.
We analyzed the mechanism of HDAC6 inhibition to improve the development efficiency of nuclear transfer embryos. Histone H3K9 and H4K8 acetylation were shown in the expression regions of human and mouse pluripotent related genes [6,7,8]. Increased H3K9 and H4K8 acetylation in donors and cloned embryos improved the development efficiency of nuclear transplantion [2, 25, 26]. In our data, The H3K9/K14 and H4K8 acetylation of the pseudo-pronucleus and blastocysts were significantly enhanced in HDAC6 inhibition group. CDX2, a trophoblast expression gene, represented the standard for post-implantation development [27]. OCT4, a key pluripotency factor, is vital for early embryo development [28]. In this study, the CDX2 and OCT4 transcription of SCNT blastocysts in HDAC inhibition group were higher than that of control group. The results were similar to other findings in mice and pig [29, 30]. Interestingly, the pseudo-pronucleus volume of the Bufexamac group was significantly higher than that of the control group. HDAC6 may be closely related to the pseudo-pronucleus reconstruction of nuclear transplantation, which was the profound mechanism to understand nuclear reprogramming. We proposed that HDAC6 may restrict the reprogramming process of nuclear transplantation by regulating pseudo-pronucleus expansion. In general, HDAC6 inhibition increased histone H3K9/K14 and H4K8 acetylation resulting in upregulating the expression of the key developmental genes, which improved the in vitro development of nuclear transfer embryos.
To summarize, HDAC6 inhibition did not affect overall HDAC activity and meiosis process, but increased histone H3K9/K14 and H4K8 acetylation of porcine in vitro maturation oocytes and pseudo-pronucleus embryos. Interestingly, the radius and area of histone H3K9/K14 and H4K8 acetylation were significantly higher than those of the control group. It also enhanced embryonic H3K9/K14 and H4K8 acetylation, and upregulated the OCT4 and CDX2 expressions in porcine SCNT embryos. Thus our study indicated that HDAC6 was required for porcine histone deacetylation during the in vitro maturation and pseudo-pronucleus stages. Moreover, HDAC6 inhibition upregulated developmental genes expression and improved the in vitro development of nuclear transfer embryos.
References
Agalioti T, Chen GY, Thanos D. Deciphering the transcriptional histone acetylation code for a human gene. Cell. 2002;111(3):381–92.
Sun JM, Cui KQ, Li ZP, Lu XR, Xu ZF, Liu QY, et al. Suberoylanilide hydroxamic acid, a novel histone deacetylase inhibitor, improves the development and acetylation level of miniature porcine handmade cloning embryos. Reprod Domest Anim. 2017;52(5):763–74.
Sobel RE, Cook RG, Perry CA, Annunziato AT, Allis CD. Conservation of deposition-related acetylation sites in newly synthesized histones H3 and H4. Proc Natl Acad Sci. 1995;92:1237–41.
Parthun MR. Hat1: the emerging cellular roles of a type B histone acetyltransferase. Oncogene. 2007;26(37):5319–28.
Sun J, Cui K, Li Z, et al. Histone hyperacetylation may improve the preimplantation development and epigenetic status of cloned embryos. Reprod Biol 2020.
Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A Chromatin Landmark and Transcription Initiation at Most Promoters in Human Cells. Cell. 2007;130(1):77–88.
Liang GN, Lin JCY, Wei VV, Yoo C, Cheng JC, Nguyen CT, et al. Distinct localization of histone H3 acetylation and H3-K4 methylation to the transcription start sites in the human genome. Proc Natl Acad Sci U S A. 2004;101(19):7357–62.
Karmodiya K, Krebs AR, Oulad-Abdelghani M, Kimura H, Tora L. H3K9 and H3K14 acetylation co-occur at many gene regulatory elements, while H3K14ac marks a subset of inactive inducible promoters in mouse embryonic stem cells. BMC Genomics. 2012;13:424.
Gurdon JB, Wilmut I. Nuclear Transfer to Eggs and Oocytes. Cold Spring Harb Perspect Biol. 2011;3(6):a002659–9.
Endo T, Kano K, Naito K. Nuclear Histone Deacetylases Are Not Required for Global Histone Deacetylation During Meiotic Maturation in Porcine Oocytes. Biol Reprod. 2008;78(6):1073–80.
Endo T, Naito K, Aoki F, Kume S, Tojo H. Changes in histone modifications during in vitro maturation of porcine oocytes. Mol Reprod Dev. 2005;71(1):123–8.
Ling L, Hu F, Ying X, Ge J, Wang Q. HDAC6 inhibition disrupts maturational progression and meiotic apparatus assembly in mouse oocytes. Cell Cycle. 2018;17(5):550–6.
Akiyama T, Kim J-M, Nagata M, Aoki F. Regulation of histone acetylation during meiotic maturation in mouse oocytes. Mol Reprod Dev. 2004;69(2):222–7.
Zhou D, Choi Y-J, Kim J-H. Histone deacetylase 6 (HDAC6) is an essential factor for oocyte maturation and asymmetric division in mice. Sci Rep. 2017;7(1).
Wang YS, Xiong XR, An ZX, Wang LJ, Liu J, Quan FS, et al. Production of cloned calves by combination treatment of both donor cells and early cloned embryos with 5-aza-2 '-deoxycytidine and trichostatin A. Theriogenology. 2011;75(5):819–25.
Chawalit S, Nguyen NT, Tseng JK, Lo NW, Tu CF, Ju JC. Trichostatin A and Ascorbic Acid Assist in the Development of Porcine Handmade Cloned Embryos via Different Physiologic Pathways. Reprod Sci. 2012;19(9):976–86.
Seidel C, Schnekenburger M, Dicato M, Diederich M. Histone deacetylase 6 in health and disease. Epigenomics. 2015;7(1):103–18.
Eshun-Wilson L, Zhang R, Portran D, Nachury MV, Toso DB, Löhr T, et al. Effects of alpha-tubulin acetylation on microtubule structure and stability. Proc Natl Acad Sci U S A. 2019;116(21):10366–71.
Liu YJ, Peng LR, Seto E, Huang SM, Qiu Y. Modulation of Histone Deacetylase 6 (HDAC6) Nuclear Import and Tubulin Deacetylase Activity through Acetylation. J Biol Chem. 2012;287(34):29168–74.
Zhang Y, Kwon S, Yamaguchi T, Cubizolles F, Rousseaux S, Kneissel M, et al. Mice Lacking Histone Deacetylase 6 Have Hyperacetylated Tubulin but Are Viable and Develop Normally. Mol Cell Biol. 2008;28(5):1688–701.
Zhang X, Yang J, Wang H, Guo R, Yin Y, Zhang D, et al. Overexpression of Hdac6 extends reproductive lifespan in mice. Protein Cell. 2017;8(5):360–4.
Bantscheff M, Hopf C, Savitski MM, Dittmann A, Grandi P, Michon AM, et al. Chemoproteomics profiling of HDAC inhibitors reveals selective targeting of HDAC complexes. Nat Biotechnol. 2011;29(3):255–65.
Sui L, Huang R, Yu H, Zhang S, Li Z. Inhibition of HDAC6 by tubastatin A disrupts mouse oocyte meiosis via regulating histone modifications and mRNA expression. J Cell Physiol. 2020;235:7030–42.
Kim T-Y, Bang Y-J, Robertson KD. Histone Deacetylase Inhibitors for Cancer Therapy. Epigenetics. 2014;1(1):15–24.
Sangalli JR, de Bem THC, Perecin F, Chiaratti MR, Oliveira LJ, de Araújo RR, et al. Treatment of Nuclear-Donor Cells or Cloned Zygotes with Chromatin-Modifying Agents Increases Histone Acetylation But Does Not Improve Full-Term Development of Cloned Cattle. Cell Rep. 2012;14(3):235–47.
Costa-Borges N, Santalo J, Ibanez E. Comparison between the Effects of Valproic Acid and Trichostatin A on the In Vitro Development, Blastocyst Quality, and Full-Term Development of Mouse Somatic Cell Nuclear Transfer Embryos. Cell Rep. 2010;12(4):437–46.
Wang YS, Su JM, Wang LJ, Xu W, Quan F, Liu J, et al. The Effects of 5-Aza-2 '- Deoxycytidine and Trichostatin A on Gene Expression and DNA Methylation Status in Cloned Bovine Blastocysts. Cell Rep. 2011;13(4):297–306.
Kirchhof N, Carnwath JW, Lemme E, Anastassiadis K, Scholer H, Niemann H. Expression pattern of Oct-4 in preimplantation embryos of different species. Biol Reprod. 2000;63(6):1698–705.
Li XP, Kato Y, Tsuji Y, Tsunoda Y. The effects of trichostatin a on mRNA expression of chromatin structure-, DNA methylation-, and development-related genes in cloned mouse blastocysts. Cloning Stem Cells. 2008;10(1):133–42.
Kumar BM, Jin H-F, Kim J-G, Ock SA, Hong Y, Balasubramanian S, et al. Differential gene expression patterns in porcine nuclear transfer embryos reconstructed with fetal fibroblasts and mesenchymal stem cells. Dev Dyn. 2007;236(2):435–46.
Acknowledgements
These experiments were supported from the China National High Technology Research and Development Program (863) Project (2011AA100607), National Natural Science Foundation Project (31260552 and 31401267), Guangxi Natural Science Foundation (Grant No. 2014GXNSFCB118003), and Guangxi Medical University Youth Science Foundation Project (GXMUYSF201829).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
All experiments were conducted in accordance with the guiding principles of the ethical review committee of Guangxi Medical University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, J., Liu, Q., Lv, L. et al. HDAC6 Is Involved in the Histone Deacetylation of In Vitro Maturation Oocytes and the Reprogramming of Nuclear Transplantation in Pig. Reprod. Sci. 28, 2630–2640 (2021). https://doi.org/10.1007/s43032-021-00533-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00533-2