Abstract
The current study was designed to evaluate the relationship between adenomyosis and its subtypes with endometriotic lesions (ovarian endometrioma (OMAs) and posterior deep infiltrative endometriosis (DIE)), to examine the probability of existence of a common cause of these mysterious diseases, and to evaluate the accuracy, sensitivity, and specificity of both transvaginal ultrasonography (TVS) and MRI in diagnosis of adenomyotic uterus. In this retrospective cross-sectional study, we selected 154 women with coexistence of endometriosis and adenomyosis according to their imaging, intraoperative, or pathological findings who were nominated for laparoscopic surgery. Eighty-six patients with just DIE resection without LH (laparoscopic hysterectomy) (group 1), and 68 patients with LH + DIE resection (group 2). The accuracy, sensitivity, and specificity of ultrasonographic and MRI findings for diagnosing adenomyosis were 72.1%, 77.6%, 40.0% and 49.2%, 41.5%, 90.0% respectively. So, TVS is a more sensitive diagnostic tool for diagnosing adenomyosis. However, MRI was more specific than TVS in the diagnosis of diffuse adenomyosis especially with simultaneous presence of uterine leiomyoma. Regarding the association of different types of adenomyosis (focal and diffuse) with different endometriosis lesions (OMA and posterior compartment DIE), we just found diffuse type of adenomyosis more frequent in the absence of rectal and rectovaginal septum (RVS) DIE (p ≤ 0.05). In addition to the questionable different nature of rectal and RVS DIE lesion, there is no relationship between adenomyosis subtypes and endometriotic lesions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Adenomyosis is an estrogen-dependent disease caused by the proliferation of endometrial glands and stroma, leading to ill-defined lesions within the myometrium [1] and affecting 19.5% of women in their reproductive age [2, 3].
Surrounding myometrium hypertrophy and hyperplasia can lead to significant focal or diffuse uterine enlargement [4]. Recent advances in imaging technique have influenced the diagnosis of adenomyosis. Imaging criteria along with histopathologic findings are now part of the diagnostic trial for the detection of adenomyosis [5].
Based on ultrasonographic and MRI appearance, adenomyosis can be classified into three types: diffuse adenomyosis, focal adenomyosis, and adenomyoma. Adenomyosis is called diffuse when endometrial glands or stroma comprises the myometrium and focal adenomyosis consists of the aggregated foci of stroma and gland in myometrium with additional compensatory hypertrophy of the surrounding myometrium; moreover, it is not similar to adenomyoma that was identified as a loculated or regular oval mass with mixed echogenicity and translesional color flow in the ultrasound imaging [6].
Adenomyosis has received a great deal of research interest over the recent years owing to its strong relationship with endometriosis and even myomatous uterus as reported by recent studies and the importance of their treatment in fertility preservation [7,8,9,10,11].
Clinical presentations of adenomyosis include menorrhagia, metrorrhagia, dysmenorrhea, dyspareunia, chronic pelvic pain, and infertility [12, 13]. These symptoms are non-specific and can be associated with other hormone-dependent pelvic lesions, such as myoma, polyps, deep pelvic endometriosis (DIE), or endometrial hyperplasia [14, 15].
The pathophysiology of this disease is still unknown and multiple theories have been proposed for its incidence in multiparous and nulliparous women:
Tissue Injury and Repair (TIAR)
The estrogen level of the menstrual blood is higher in patients with adenomyotic uterus than in normal healthy individuals. Besides, the response to progesterone decreases in the endometrial stromal and functional cells of the patients with adenomyotic uterus. Therefore, cell proliferation continues even during the secretory phase of the cycle under the effect of local estrogen [7, 16,17,18,19]. This high estrogen level could trigger the oxytocin receptors located in the fundo-corneal raphe of the uterus and induce hyperperistalsis, stress, and strain in the junctional zone. TIAR can play a major role in the development of adenomyosis even in nulliparous women due to hyperestrogenic state, progesterone resistance of eutopic endometrium rather than disease-free endometrium, hyperperistalsis of the junctional zone, and increased cell proliferation.
Continuous stimulation of estrogen and oxytocin receptors could lead to myometrial muscular injury and fibrosis through the increased local concentrations of interleukin 6 (IL-6) and prostaglandin E2 (PGE2) [20, 21].
De Novo Theory
The embryonic pluripotent cells originate from urogenital ridge and are located in the niche between basal endometrial cell and intramyometrial region; they are responsible for the cyclic repair of endomyometrial junction and are able to migrate into the myometrial side. These cells can be transformed into ectopic endometrial tissues in myometrial wall and induce adenomyosis by differentiation into endometrial stroma and glands [17, 22,23,24,25].
The migration and invasion of these multipotential cells (epithelial–mesenchymal transition (EMT)) have also been observed in tissue repair and cancer cell migration procedures, particularly in the hyperestrogenic state [26].
Outside to Inside Invasion Theory
Chaperon and Marcellin hypothesized the migration of ectopic endometrial cells from posterior or anterior compartment endometriotic nodule to the adjacent myometrium [10, 27]. This hypothesis is corroborated by the increase in prevalence of focal adenomyosis in the posterior uterine wall (posterior compartment of pelvis) in patients with DIE (deep infiltrative endometriosis) as diagnosed by MRI.
However, the pathophysiology of adenomyosis is yet to be known and cannot be explained by a mere hypothesis due to the heterogeneous nature of this disease.
Accordingly, the current study was designed to evaluate the accuracy, sensitivity, and specificity of imaging systems in diagnosing adenomyosis. This was done by comparing these procedures with histopathologic findings as a gold standard for the diagnosis of adenomyosis in order to replace surgery with a less invasive and more affordable technique; we also specified the relationship between focal and diffuse types of adenomyosis and two different phenotypes of endometriosis (OMA and DIE). We further studied the possibility for a common origin for both diseases, based on their association. Our secondary goal was to achieve of a novel treatment, especially for young infertile women with adenomyosis/endometriosis.
Materials and Methods
Study Design and Population
A retrospective cross-sectional study was conducted on 3725 patients with clinical symptoms that were indicative of endometriosis over a period of 3 years (March 2015 to March 2018) in two referral Hospitals of Shiraz University of Medical Sciences. The study protocol was approved by the Ethics Committee of Shiraz University of Medical Sciences (code: IR.SUMS.MED.REC.1399.204). Transvaginal (transrectal for virgin women) ultrasonography and/or MRI were performed and endometriosis diagnosis was confirmed. Based on the pain symptom, desire for pregnancy, vital organ involvement, and nonresponse to medical therapy, 919 patients underwent surgery.
We enrolled all endometriotic patients of reproductive age requiring surgery with findings in favor of adenomyosis in ultrasound, MRI, and pathologic or intraoperative findings retrospectively.
To assess the accuracy, sensitivity, and specificity of the imaging techniques for adenomyosis diagnosis, we set the pathology report as the gold standard. Therefore, these data were examined in a group undergoing DIE resection and a concomitant laparoscopic hysterectomy. To diagnose the relationship between endometriosis and adenomyosis and its subtypes, we included all patients suffering from both conditions as confirmed by ultrasound, MRI, pathologic, and intraoperative findings. Hysterectomy was performed on those who had symptoms (dysmenorrhea, dyspareunia, dyschezia, and hypermenorrhea), did not respond to medical treatment, and had no desire to preserve their fertility or their uterus.
Finally, 154 patients were included in our study and categorized into two study groups: endometriosis plus LH (endometriosis + LH) = 68 and endometriosis alone (endometriosis alone) = 86 (Fig. 1).
Preoperative Evaluation
All patients who were referred to our center with a clinical symptom of endometriosis were evaluated by full history including age, parity, body mass index (BMI), and pain symptoms such as dysmenorrhea, dyspareunia, dyschezia, chronic pelvic pain, and AUB, physical examination (vaginal or cul-de-sac tender point and nodularity or palpable adnexal masses) and transvaginal (or transrectal for virgin women) ultrasonography, and/or MRI, which confirmed endometriosis. At the beginning of the study, a written informed consent was obtained from all patients
Transvaginal Ultrasonography (TVS)
All 154 ultrasonography experiments were accomplished by a single expert gynecologist (first author); ultrasonography was carried out using 7.5 MHz probe (Ultrasonix OP machine; British Columbia, Canada or Mindray DC-8Exp ultrasound machine; China, R&D USA) with partially distended bladder and bowel preparation. Ovaries and pelvic compartments (anterior and posterior) were checked for any evidence of OMA and DIEs. Similar to our previous article [28], three or more of the following ultrasonographic features were required for the diagnosis of adenomyosis: globally enlarged uterus (enlarged fundus), asymmetrically enlarged uterus (anterior posterior asymmetry), round cystic area (2–9 mm) within the myometrium, heterogeneous myometrial echotexture and hyperechogenic islands, myometrial hypoechoic linear striations (fan-shaped shadowing), and indistinct and fuzzy transitional zone; diffuse minimal vascularity was detected in ultrasound which did not indicate the normal course of the arcuate and radial arteries. A “question mark” was defined when the uterine corpus was flexed backwards [29,30,31].
According to Exacoustos et al. and also Lazzeri et al. The diagnostic criteria for focal adenomyosis included the following: the uterine contour is often regular, uterine walls are often symmetric, the lesion is ill-defined or well-defined cystic, and it is mostly surrounded by normal myometrium. There may be intramyometrial focal areas of mixed echogenicity, small and large cystic formation in myometrium or subendometrial region, hyperechogenic islands, subendometrial echogenic lines or buds, and sporadic vascularity on color Doppler. In this study, the adenomyosis classification was used according to Lazzerisʼ classification of adenomyosis [32,33,34].
Magnetic Resonance Imaging (MRI)
From 154 patients with clinical and radiological or pathological evidence of adenomyosis, MRI was only performed on 128 by a single expert radiologist. Not all patients were eligible for MRI due to claustrophobia, intrauterine device, tattoos in the area of interest, and ocular prosthesis.
MRI criteria used for adenomyosis diagnosis were increased thickness of junctional zone (JZ), formation of an ill-defined area of low signal intensity on T2-weighted image representing the smooth muscle hyperplasia in association with the heterotopic endometrial tissue, cystic dilatation of gland, and hemorrhagic foci. The uterus may be enlarged with asymmetric outline, especially in the fundus and posterior uterine wall.
JZ thickness ≥ 12 mm is regarded as diagnostic, and JZ < 8mm is considered as exclusion for adenomyosis. JZ from 8–12 mm requires ancillary MRI findings that can support the diagnosis.
Focal adenomyosis is represented with localized intersection of adenomyotic glands, forming a mass-like lesion of adenomyosis, typically situated intramyometrially and in the corpus of the uterus extending directly from junctional zone to anterior or posterior part of the myometrium [14, 35, 36].
Operative Finding and Procedure
Laparoscopic radical excision of DIEs with LH (68 patients) or without LH (86 patients) was performed by one laparoscopic subspecialist (first author). Whole bowel preparation was routinely conducted on all candidates for endometriosis laparoscopic surgery. Preoperative imaging is conducive to a well-planned operation. Video recording during the operation allowed the physicians to review the procedure when required. Posterior compartment DIEs refer to the lesions which involved ovarian fossa, uterosacral ligament, rectal and rectovaginal septum, rectocervical area, and bowel. Disease was staged according to the revised American Society for Reproductive Medicine classification (ASRM) [37]. The objective of the surgery was to excise all DIEs and restore normal pelvic anatomy. The procedure started with OMA by either ablation, cystectomy, or oophorectomy. afterwards, ovarian suspension was performed followed by salpingectomy, ureteric dissection, and pararectal and paravesical dissection according to the location of the nodules. Rectovaginal space dissection was done if rectosigmoid mobilization was required. After that, excision of DIEs was done in the anterior compartments (bladder, uterovesical peritoneum, round ligament) and the posterior and lateral compartments (ureters, periureteric, ovarian fossa, uterosacral ligament, rectocervical area, and vaginal nodules); bowel DIEs were dealt with according to the size and depth of the lesions either by shaving, discoid excision, or segmental bowel resection. LH was done when indicated and all specimens were sent for histopathological confirmation.
Intraoperative diagnosis of adenomyosis was confirmed according to the gross appearance of the uterus. Focal adenomyosis or adenomyoma was defined when the cut surface had a spongy appearance and was darker than the white surface of fibroid. In addition, there was no definite capsule around the adenomyosis. But we occasionally observed small blue spots or cystic spaces representing dilated endometrial gland with bloody content (chocolate colored areas), uterus asymmetry, or global uterine enlargement [38, 39].
Histopathological Evaluation
All surgical specimens including DIEs, tubes, ovaries, or uteri were sent to one pathologist for histological confirmation. Diagnosis of endometriosis was confirmed following hematoxylin and eosin staining and evaluation of glands and stroma. For the pathologic diagnosis of adenomyosis, a cut section was passed from a uterus area with obvious enlarged myometrial thickening or other suspicious areas for adenomyosis according to gross pathologic findings. If typical adenomyosis lesion was not seen, two cut sections were routinely passed from the anterior to the posterior parts of the uterus, which included the endometrial to parametrial surfaces. If in one of these cut sections presenting endometrial stroma and gland were found in the myometrium, junctional zone was also checked in low-power field of microscope (10 power) and more than 3 mm invasion of the endometrium into the myometrium indicated adenomyosis.
Statistical Analysis
Statistical analysis was conducted on the data of a total of 154 patients. Data were entered to SPSS 18 and analysis was done as mean and SD. For the comparison between groups, t test and chi-square test were used. For all statistical analyses, the significance level was set as 0.05 (P value ≤ 0.05).
Results
In the demographic data, patients with DIE surgery alone (without LH) were significantly younger (34.0 ± 6.4) than endometriosis + LH group (44.2 ± 5.7) as hysterectomy was reserved for older patients with no desire for pregnancy. Patients’ BMI was also significantly lower in the first group in comparison with the second group (25.2 ± 4.3 versus 28.0 ± 4.1).
Pain symptoms, such as dysmenorrhea, dyspareunia, dyschezia, and other pain presentations were significantly higher in the first group, while abnormal uterine bleeding menorrhagia and metrorrhagia were significantly higher in the second group (Table 1).
To evaluate the accuracy, sensitivity, and specificity of our imaging system, pathologic confirmation was required as a gold standard for confirming adenomyosis. Consequently, these criteria were only checked in the second group (endometriosis + LH). According to our findings (summarized in Table 2), 63 patients had MRI reports, and all of them (68 = n) had ultrasonography reports prior to surgery. The histopathology was confirmed in 53 patients in the MRI group and 58 patients in the ultrasonography group.
The accuracy of ultrasound in adenomyosis diagnosis was 72.1%, the sensitivity was 77.6%, and its specificity was 40.0%. The accuracy, sensitivity, and specificity of MRI for the diagnosis of adenomyosis in this group were 49.2%, 41.5%, and 90.0%, respectively.
In terms of adenomyosis subtypes in the study population, according to the imaging and operation findings, the following results were obtained:
Ultrasonography was performed on all 154 patients by one gynecology specialist; in addition to ultrasound, 128 patients had MRI findings (63 patients from LH + endometriosis and 65 patients from the endometriosis group).
Our pathologist only reported the presence or absence of adenomyosis and did not specify the subtypes. Therefore, adenomyosis subtypes were only examined on the basis of imaging and intraoperative findings.
MRI findings among 128 patients (n = 128) showed that 26.5% (n = 34) had diffuse adenomyosis, 7.8% (n = 10) had focal adenomyosis, and 65.6% (n = 84) had a normal uterus.
Ultrasonographic findings of all 154 patients showed that 45.5% (n = 70) had diffuse adenomyosis, 27.9% (n = 43) had focal adenomyosis, and 26.6% (n = 41) had no adenomyosis.
Based on the findings related to all cases (n = 154) , 50.6% (n = 78) had diffuse adenomyosis, 5.8% (n = 9) had focal adenomyosis, and 43.5% (n = 67) had no obvious signs of adenomyosis in the uterus. We found no significant relationships between the presence of OMA, uterosacral ligaments DIE (US DIE), rectocervical DIE (RC DIE), and presence or absence of adenomyosis and its subtypes (P > 0.05).
According to the imaging and intraoperative findings, the diffuse type of adenomyosis was found to be more frequent in the absence of rectal and RVS DIE (P ≤ 0.05). However, no specific type of adenomyosis was found in the presence of rectal and RVS DIE.
Although there was no significant relationship between the endometriosis and adenomyosis subtypes, according to MRI and surgery findings (Fig. 2), adenomyotic uterus and focal adenomyosis were observed to be more frequent in the presence of bilateral OMA. However, focal adenomyosis was more prevalent in the surgery findings (n = 5/9) (55.6%) compared with the diffuse type. Nonetheless, both had almost equal prevalence in the MRI findings in the presence of bilateral OMA (focal adenomyosis (n = 4/10) was found in 40.0% of the cases, and diffuse adenomyosis ((n = 13/34) was detected in 38.2%).
In the presence of left uterosacral DIE (± OF DIE), focal adenomyosis was more frequent according to ultrasonographic and surgery findings. 41.9% (n = 18/43) of the cases were observed in ultrasonographic findings and (n = 7/9) 77.7% of the cases were observed in surgery findings.
Similar to US ligament DIE, focal adenomyotic uterus was more frequent in rectocervical DIE according to the imaging and surgery findings (65.1% (n = 28/43) focal adenomyosis in ultrasonographic findings, 70.0% (n = 7/10) in MRI findings, and 66.9% (n = 6/9) in surgery findings).
In terms of cul-de-sac involvement, no obvious difference was observed between the rate of focal and diffuse adenomyosis which are called partial or complete obstruction of the cul-de-sac.
It is possible to detect focal adenomyosis along with the involvement of RC and US ligaments with DIE nodules. However, their relationship is not statistically significant and there was a small number of focal adenomyosis in our sample size.
In the first group, 82 patients had adenomyosis alone and only four patients (4.6%) in the surgical and one patient in the MRI findings had both adenomyosis and leiomyoma. In the second group, 36 patients had concomitant diffuse adenomyosis and leiomyoma (52.9%) which can be justified by the older age of the patients in this group. Of these, 20 (29.41%) patients were diagnosed by ultrasound, 24 (35.2%) patients by MRI, and 23 (33.8%) by intraoperative or pathologic findings.
Discussion
Many authors have suggested a relationship between endometriosis and adenomyosis. They have sought to find a common pathophysiology for their simultaneous presence in the pelvis, since adenomyosis also affects women under 35 years of age. However, its incidence is not higher in women with endometriosis compared with other parous women undergoing hysterectomy for other reasons [40].
The current study was designed to retrospectively follow a cohort of patients who had both endometriosis and adenomyosis diagnosed using the available imaging techniques. They were also confirmed with laparoscopic and histological findings to detect the association between different endometriosis phenotypes and various types of adenomyosis. In this article, adenomyosis was classified into diffuse and focal according to Lazzerisʼ classification [41], and the sensitivity, specificity, and accuracy of different imaging methods were analyzed in the detection of adenomyosis and compared with histological findings.
With respect to demographic data, our findings were similar to Parasar et al. and Riazi et al. They emphasized that endometriosis is a disease that starts in adolescence and active reproductive age with more severity in patients with low BMI [41,42,43,44].
Furthermore, it is well-known that DIEs is the most severe form of endometriosis which can be accompanied by severe pain [45,46,47,48,49]. Similar to our findings, Nelsen et al. reported an overlap, especially in pain symptoms, between adenomyosis and endometriosis. These symptoms include pain, fatigue, bloating, and infertility. On the other hand, abnormal uterine bleeding was more pronounced with adenomyosis [50]. Treatment of the disease, particularly in young women, requires a lifelong management plan to improve the quality of life, fertility and pregnancy outcome in these patients [51].
The relationship between endometriosis and adenomyosis has had different results in the literature review. In earlier studies, the relationship between these two diseases was based on surgical findings. But recent studies with the help of imaging techniques and histopathological confirmation have been able to diagnose the relationship between adenomyosis and endometriosis. They have proposed that a severe form of endometriosis is associated with diffuse adenomyosis and that endometriosis and adenomyosis have closely related pathologies [9, 18, 52, 53].
Our study results showed that the prevalence of adenomyosis was equal to 34.3, 73.3, and 58.8% in the patients with endometriosis according to MRI (the cases had diffuse adenomyosis, and 61.9% had diffuse adenomyosis), ultrasound, and operation findings, respectively. Kunz et al. reported that 79% of the patients with evidence of endometriosis in laparoscopy also had evidence of irregular, thick, and abnormal JZ appearance and peristalsis in MRI [52]. Di Donato et al. reported the prevalence of adenomyosis to be about 21.8% in endometriosis patients. These differences may be attributed to the lack of a single language to describe adenomyosis lesions. This problem has only been partially addressed so far [53].
Because all the patients included in our study were in stages III and IV of endometriosis, it is safe to say that the severe form of endometriosis is strongly associated with the diffuse adenomyosis. This relationship was assessed as a function of the severity of endometriosis according to the revised classification of the American Society for Reproductive Medicine. However, specific local involvement with endometriosis lesions was not necessarily associated with a higher prevalence of adenomyosis.
Recently, Koninckx et al. have raised some questions about the exact relationship between the two diseases in terms of pathology and reviewed the existing studies that are often designed based on just imaging techniques. However, due to extensive variability in the phenotypes of both diseases, they claimed that solid data indicated a limited association. They also underscored the fact that most of the studies in this area are small without any attempts to correlate the heterogeneity of the two diseases with the diagnostic tools used [54].
Regarding the association of different types of adenomyosis (focal and diffuse) with different endometriosis lesions (OMA and posterior compartment DIE), we found no significant relationships between OMA, uterosacral ligaments DIE (US DIE), and rectocervical DIE (RC DIE) and the presence or absence of adenomyosis and its subtypes (P > 0.05). But it was found that diffuse adenomyosis was more frequent in the absence of RVS DIE (P ≤ 0.05). No specific types of adenomyosis were detected in the presence of rectal and RVS DIE.
The focal type of adenomyosis can possibly be detected along with the involvement of RC and US ligaments with DIE nodules. However, these relationships are not significant and there were only a small number of focal adenomyosis in our sample size.
So, do focal and diffuse adenomyosis have different pathophysiologies, behaviors, and origins?
Inoue et al. observed K-RAS mutation in normal endometrium (NE) in the presence of adenomyosis and endometriosis in the pelvis. Interestingly, they reported the highest prevalence of relapsing in patients with K-RAS-positive mutation. On the contrary, they stated that the presence of adenomyosis in multiparous women without endometriosis lesion was attributed to the increased level of cancer-related mutants induced by aging and increased BMI [55].
Khan et al. also mentioned that the estrogen/progesterone receptor (ER/PR) significantly decreased in focal and diffuse adenomyotic lesions compared to other myometrial lesions such as leiomyoma, explaining the failure in response to medication in these patients [56].
To clarify the reason for increased prevalence of diffuse uterine adenomyosis in the absence of rectal and RVS DIE, we can refer to a hypothesis proposed by Donnez and Nisolle 23 years ago. According to their hypothesis, which was proved by histopathologic findings, the multipotential cells located in the cervix or in a cervical adenomyotic nodule can extend outside of the uterus and induce RVS DIE nodules in a tumor-like process. Donnez explained that the pathologic findings of the rectal, vaginal, and bladder nodules were similar to the adenomyotic lesion of uterus in that they contained muscle, gland, and scanty stroma. Similar to the adenomyotic lesion, these nodules had poorly differentiated appearance and hormone-independent nature and were closely related to mesodermal cells with Mullerian origin.
The pathophysiology of these nodules (RVS DIE) is different from other DIE lesions. RVS DIE nodules are likely to be introduced as adenomyotic nodules rather than endometriotic lesions. These implanted foci induce inflammation and fibrosis in the muscularis parts of vagina, rectum, and bladder and create lesions with very similar appearance to adenomyosis [57, 58]. According to these findings, we may be able to differentiate other DIE lesions and OMAs from rectal and RVS DIE nodules in the future.
Contrary to the Chapron et al. study, we found no significant relationship between diffuse and focal adenomyosis and the presence of different types of DIE lesions in posterior compartment, emphasizing that the sample size of focal adenomyosis was very small (n = 10) in our study [10]. Therefore, the hypothesis of posterior compartment endometriosis lesions invading the uterus and causing adenomyosis will be weak.
Inoue et al. reported that in the presence of endometriosis and adenomyosis in the pelvis, there was a high incidence of relapse whether or not the endometriosis surgery or cytoreductive surgery of adenomyosis was done alone. In these cases, it may be advisable to postpone complete surgery to the end of the reproductive age rather than endometriosis surgery, with the aim of increasing the pregnancy rate in this demographic [55, 59].
Therefore, it should be mentioned that each type of adenomyosis and endometriosis lesion may require a specific medical treatment [55,56,57,58].
These two diseases need to be diagnosed based on non-invasive methods for a good treatment strategy. Thus, there is a heated discussion among authors as to the sensitivity, specificity, and accuracy of imaging modalities. Concerning the sensitivity and specificity of conventional imaging techniques in the diagnosis of adenomyosis, some studies have indicated that the sensitivity and specificity of 2D transvaginal scan are comparable with those of MRI, histology, or both, ranging from 75 to 88% and from 67 to 93%, respectively [31, 32]. However, other studies have shown higher rates compared to these figures in the TVS diagnosis of adenomyosis (70–93% sensitivity and specificity and 21–33% adenomyosis prevalence rate) [5, 60, 61].
In their systematic review, Guerriero et al. reported a similar diagnostic performance for both ultrasonography and MRI techniques regarding the diagnosis of adenomyosis [62], which is in line with Nisenblat et al. in their Cochrane review [63]. In contrast to Nisenblat and Guerriero’s results, Bazot et al. (2018) suggested that MRI was more useful than TVS in the diagnosis of adenomyosis [5]. However, in their previous study, ultrasonography was proposed to be as effective as MRI in the diagnosis of adenomyosis, especially in patients without leiomyoma. They only recommended MRI to patients with leiomyoma for the diagnose of adenomyosis [60].
Hanafi and Stamatopoulos reported that MRI was more accurate than ultrasound in the diagnosis of adenomyosis accompanied by myoma, which is in accordance with our study in the presence of leiomyoma and adenomyosis (63.8% rather than 55.5%) [64, 65].
In the present study, the ultrasonographic and MRI findings associated with the diagnosis of adenomyosis in the endometriosis + LH group had accuracy, sensitivity, and specificity of 72.1%, 77.6%, 40.0% and 49.2%, 41.5% 90.0%, respectively.
According to the results obtained from comparing the ultrasound, MRI, and surgery findings, it can be concluded that MRI has a better specificity and ultrasound has a better sensitivity for diagnosis of adenomyosis.
In previous studies, ultrasound was not regarded as a suitable tool for diagnosing adenomyosis. However, with the advances in ultrasound technology such as the use of high-frequency probe and 3D ultrasonography and elastography, evaluation by ultrasound may become the best alternative [66].
It should be pointed out that the exclusion of a large number of patients (86) with DIE excision and the lack of MRI report in some patients might have affected our overall accuracy, sensitivity, and specificity for the diagnosis of adenomyosis by imaging techniques.
Of course, we are capable of enhancing the role of ultrasonography as a low-cost, non-invasive approach with high accuracy and sensitivity in diagnosis of the adenomyosis by applying both 3D ultrasonography and elastography [9, 67].
This study highlights the need for a common language to describe the types of adenomyosis, based on which we can determine the results of our research.
Conclusion
According to our findings, TVS is a reliable first-line diagnostic approach for the diagnosis of adenomyosis, while MRI showed more specificity than TVS.
There is a close relationship between endometriosis and adenomyosis, especially the diffuse type. This relationship was assessed as a function of the severity of the endometriosis. But we found no significant relationship between the presence of OMA, uterosacral ligaments DIE (US DIE), rectocervical DIE (RC DIE), and the presence or absence of adenomyosis and its subtypes (P > 0.05). So, the hypothesis of posterior compartment endometriosis lesions invading the uterus and causing adenomyosis will be weak.
Limitations
A major limitation was lack of common language to describe the types of adenomyosis. Other limitations of this study include the lack of evaluation of patients who have not undergone surgery due to being asymptomatic or responding to medical treatment, as well as the impossibility of sampling for ethical reasons from patients whose uterus was to be preserved, and also, lack of isolation of different subtypes of adenomyosis in pathology reports.
Data Availability
The anonymized data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Rapkin A, Nathan L. Pelvic pain and dysmenorrhoea, adenomyosis. Berek’s and Novak’s gynecology. Chapter 16. 15th ed. Philadelphia: Lippincott Wiliams and Wilkins; 2012.
Dolan M, Hill C, Valea F. Benign gynecologic lesions. Comprehensive gynecology. Chapter 18. 7th ed. Amsterdam: Elsevier; 2017.
West C. Adenomyosis, obstetrics & gynaecology an evidence-based text for the MRCOG. Chapter 78. 3rd ed. Abingdon-on-Thames: Taylor & Francis Group; 2016.
Khaund A, Lumsden M. Benign disease of the uterus, Dewhurst’s textbook of obstetrics & gynaecology. Chapter 54. 8th ed. Hoboken: Wiley-Blackwell; 2012.
Bazot M, Daraï E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril. 2018;109(3):389–97. https://doi.org/10.1016/j.fertnstert.2018.01.024.
Van den Bosch T, Dueholm M, Leone FP, Valentin L, Rasmussen CK, Votino A, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46(3):284–98. https://doi.org/10.1002/uog.14806.
Kunz G, Beil D, Huppert P, Leyendecker G. Structural abnormalities of the uterine wall in women with endometriosis and infertility visualized by vaginal sonography and magnetic resonance imaging. Hum Reprod. 2000;15(1):76–82. https://doi.org/10.1093/humrep/15.1.76.
Yasui T, Hayashi K, Nagai K, Mizunuma H, Kubota T, Lee JS, et al. Risk profiles for endometriosis in Japanese women: results from a repeated survey of self-reports. J Epidemiol. 2015;25(3):194–203. https://doi.org/10.2188/jea.JE20140124.
Leyendecker G, Bilgicyildirim A, Inacker M, Stalf T, Huppert P, Mall G, et al. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch Gynecol Obstet. 2015;291(4):917–32. https://doi.org/10.1007/s00404-014-3437-8.
Chapron C, Tosti C, Marcellin L, Bourdon M, Lafay-Pillet MC, Millischer AE, et al. Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum Reprod. 2017;32(7):1393–401. https://doi.org/10.1093/humrep/dex088.
Vlahos NF, Theodoridis TD, Partsinevelos GA. Myomas and adenomyosis: impact on reproductive outcome. Biomed Res Int. 2017;2017:5926470–14. https://doi.org/10.1155/2017/5926470.
Alabiso G, Alio L, Arena S, Barbasetti di Prun A, Bergamini V, Berlanda N, et al. Adenomyosis: what the patient needs. J Minim Invasive Gynecol. 2016;23(4):476–88. https://doi.org/10.1016/j.jmig.2015.12.017.
Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A)-pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017;40:68–81. https://doi.org/10.1016/j.bpobgyn.2016.09.006.
Agostinho L, Cruz R, Osório F, Alves J, Setúbal A, Guerra A. MRI for adenomyosis: a pictorial review. Insights Imaging. 2017;8(6):549–56. https://doi.org/10.1007/s13244-017-0576-z.
Özkan ZS, Kumbak B, Cilgin H, Simsek M, Turk BA. Coexistence of adenomyosis in women operated for benign gynecological diseases. Gynecol Endocrinol. 2012;28(3):212–5. https://doi.org/10.3109/09513590.2011.593669.
Vannuccini S, Tosti C, Carmona F, Huang SJ, Chapron C, Guo SW, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod BioMed Online. 2017;35(5):592–601. https://doi.org/10.1016/j.rbmo.2017.06.016.
García-Solares J, Donnez J, Donnez O, Dolmans MM. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril. 2018;109(3):371–9. https://doi.org/10.1016/j.fertnstert.2017.12.030.
Leyendecker G, Wildt L. A new concept of endometriosis and adenomyosis: tissue injury and repair (TIAR). Horm Mol Biol Clin Invest. 2011;5(2):125–42. https://doi.org/10.1515/hmbci.2011.002.
Shaked S, Jaffa AJ, Grisaru D, Elad D. Uterine peristalsis-induced stresses within the uterine wall may sprout adenomyosis. Biomech Model Mechanobiol. 2015;14(3):437–44. https://doi.org/10.1007/s10237-014-0614-4.
Mehasseb MK, Panchal R, Taylor AH, Brown L, Bell SC, Habiba M. Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil Steril. 2011;95(7):2228–35, 35.e1. https://doi.org/10.1016/j.fertnstert.2011.02.051.
Jichan N, Xishi L, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in adenomyosis and its rectification by a histone deacetylase inhibitor and a demethylation agent. Reprod Sci. 2010;17(11):995–1005. https://doi.org/10.1177/1933719110377118.
Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22(2):137–63. https://doi.org/10.1093/humupd/dmv051.
Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13(1):87–101. https://doi.org/10.1093/humupd/dml045.
Sobel V, Zhu YS, Imperato-McGinley J. Fetal hormones and sexual differentiation. Obstet Gynecol Clin N Am. 2004;31(4):837–56, x-xi. https://doi.org/10.1016/j.ogc.2004.08.005.
Spencer TE, Hayashi K, Hu J, Carpenter KD. Comparative developmental biology of the mammalian uterus. Curr Top Dev Biol. 2005;68:85–122. https://doi.org/10.1016/s0070-2153(05)68004-0.
Chen YJ, Li HY, Huang CH, Twu NF, Yen MS, Wang PH, et al. Oestrogen-induced epithelial-mesenchymal transition of endometrial epithelial cells contributes to the development of adenomyosis. J Pathol. 2010;222(3):261–70. https://doi.org/10.1002/path.2761.
Marcellin L, Santulli P, Bortolato S, Morin C, Millischer AE, Borghese B, et al. Anterior focal adenomyosis and bladder deep infiltrating endometriosis: is there a link? J Minim Invasive Gynecol. 2018;25(5):896–901. https://doi.org/10.1016/j.jmig.2018.02.002.
Alborzi S, Rasekhi A, Shomali Z, Madadi G, Alborzi M, Kazemi M, et al. Diagnostic accuracy of magnetic resonance imaging, transvaginal, and transrectal ultrasonography in deep infiltrating endometriosis. Medicine (Baltimore). 2018;97(8):e9536. https://doi.org/10.1097/md.0000000000009536.
Andres MP, Borrelli GM, Ribeiro J, Baracat EC, Abrão MS, Kho RM. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25(2):257–64. https://doi.org/10.1016/j.jmig.2017.08.653.
Levy G, Dehaene A, Laurent N, Lernout M, Collinet P, Lucot JP, et al. An update on adenomyosis. Diagn Interv Imaging. 2013;94(1):3–25. https://doi.org/10.1016/j.diii.2012.10.012.
Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16–24. https://doi.org/10.1016/j.bpobgyn.2018.01.013.
Exacoustos C, Zupi E, Piccione E. Ultrasound imaging for ovarian and deep infiltrating endometriosis. Semin Reprod Med. 2017;35(1):5–24. https://doi.org/10.1055/s-0036-1597127.
Exacoustos C, Manganaro L, Zupi E. Imaging for the evaluation of endometriosis and adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2014;28(5):655–81. https://doi.org/10.1016/j.bpobgyn.2014.04.010.
Lazzeri L, Morosetti G, Centini G, Monti G, Zupi E, Piccione E, et al. A sonographic classification of adenomyosis: interobserver reproducibility in the evaluation of type and degree of the myometrial involvement. Fertil Steril. 2018;110(6):1154–61.e3. https://doi.org/10.1016/j.fertnstert.2018.06.031.
Takeuchi M, Matsuzaki K. Adenomyosis: usual and unusual imaging manifestations, pitfalls, and problem-solving MR imaging techniques. Radiographics. 2011;31(1):99–115. https://doi.org/10.1148/rg.311105110.
Gilks CB, Clement PB, Hart WR, Young RH. Uterine adenomyomas excluding atypical polypoid adenomyomas and adenomyomas of endocervical type: a clinicopathologic study of 30 cases of an underemphasized lesion that may cause diagnostic problems with brief consideration of adenomyomas of other female genital tract sites. Int J Gynecol Pathol. 2000;19(3):195–205. https://doi.org/10.1097/00004347-200007000-00001.
Rock JA. The revised American Fertility Society classification of endometriosis: reproducibility of scoring. ZOLADEX Endometriosis Study Group. Fertil Steril. 1995;63(5):1108–10. https://doi.org/10.1016/s0015-0282(16)57556-6.
Robboy S, Mutter G. Robboypathology of female reproductive tract chapter 17 benign gynecological disease (adenomyosis) gross pathology. 2nd ed. London: Churchill Livingstone; 2009.
Stewart E. Uterine adenomyosis, histopathology. UpToDate. Acta Obstet Gynecol Scand 2018; 97:1073. https://www.uptodate.com/contents/uterine-adenomyosis.
Brosens I, Kunz G, Benagiano G. Is adenomyosis the neglected phenotype of an endomyometrial dysfunction syndrome? Gynecol Surg. 2012;9(2):131–7. https://doi.org/10.1007/s10397-011-0723-3.
Parasar P, Ozcan P, Terry KL. Endometriosis: Epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34–41. https://doi.org/10.1007/s13669-017-0187-1.
Riazi H, Tehranian N, Ziaei S, Mohammadi E, Hajizadeh E, Montazeri A. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Womens Health. 2015;15:39. https://doi.org/10.1186/s12905-015-0196-z.
Templeman C, Marshall SF, Ursin G, Horn-Ross PL, Clarke CA, Allen M, et al. Adenomyosis and endometriosis in the California Teachers Study. Fertil Steril. 2008;90(2):415–24. https://doi.org/10.1016/j.fertnstert.2007.06.027.
Schomacker ML, Hansen KE, Ramlau-Hansen CH, Forman A. Is endometriosis associated with irritable bowel syndrome? A cross-sectional study. Eur J Obstet Gynecol Reprod Biol. 2018;231:65–9. https://doi.org/10.1016/j.ejogrb.2018.10.023.
Lazzeri L, Di Giovanni A, Exacoustos C, Tosti C, Pinzauti S, Malzoni M, et al. Preoperative and postoperative clinical and transvaginal ultrasound findings of adenomyosis in patients with deep infiltrating endometriosis. Reprod Sci. 2014;21(8):1027–33. https://doi.org/10.1177/1933719114522520.
Harada T, Ohta I, Endo Y, Sunada H, Noma H, Taniguchi F. SR-16234, a novel selective estrogen receptor modulator for pain symptoms with endometriosis: an open-label clinical trial. Yonago Acta Med. 2017;60(4):227–33. https://doi.org/10.24563/yam.2017.12.003.
Matalliotakis M, Matalliotaki C, Trivli A, Zervou MI, Kalogiannidis I, Tzardi M, et al. Keeping an eye on perimenopausal and postmenopausal endometriosis. Diseases. 2019;7(1). https://doi.org/10.3390/diseases7010029.
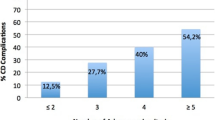
Khazali S, Gorgin A, Mohazzab A, Kargar R, Padmehr R, Shadjoo K, et al. Laparoscopic excision of deeply infiltrating endometriosis: a prospective observational study assessing perioperative complications in 244 patients. Arch Gynecol Obstet. 2019;299(6):1619–26. https://doi.org/10.1007/s00404-019-05144-6.
Cozzolino M, Coccia ME, Lazzeri G, Basile F, Troiano G. Variables associated with endometriosis-related pain: a pilot study using a visual analogue scale. Rev Bras Ginecol Obstet. 2019;41(3):170–5. https://doi.org/10.1055/s-0039-1679879.
Nelsen LM, Lenderking WR, Pokrzywinski R, Balantac Z, Black L, Pokras S, et al. Experience of symptoms and disease impact in patients with adenomyosis. Patient. 2018;11(3):319–28. https://doi.org/10.1007/s40271-017-0284-2.
Vannuccini S, Petraglia F. Recent advances in understanding and managing adenomyosis. F1000Res. 2019;8. https://doi.org/10.12688/f1000research.17242.1.
Kunz G, Beil D, Huppert P, Noe M, Kissler S, Leyendecker G. Adenomyosis in endometriosis--prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum Reprod. 2005;20(8):2309–16. https://doi.org/10.1093/humrep/dei021.
Di Donato N, Montanari G, Benfenati A, Leonardi D, Bertoldo V, Monti G, et al. Prevalence of adenomyosis in women undergoing surgery for endometriosis. Eur J Obstet Gynecol Reprod Biol. 2014;181:289–93. https://doi.org/10.1016/j.ejogrb.2014.08.016.
Koninckx PR, Ussia A, Zupi E, Gomel V. Association of endometriosis and adenomyosis: vast literature but scant conclusive data. J Minim Invasive Gynecol. 2018;25(5):745–8. https://doi.org/10.1016/j.jmig.2018.03.012.
Inoue S, Hirota Y, Ueno T, Fukui Y, Yoshida E, Hayashi T, et al. Uterine adenomyosis is an oligoclonal disorder associated with KRAS mutations. Nat Commun. 2019;10(1):5785. https://doi.org/10.1038/s41467-019-13708-y.
Khan KN, Fujishita A, Koshiba A, Mori T, Kuroboshi H, Ogi H, et al. Biological differences between focal and diffuse adenomyosis and response to hormonal treatment. Reprod BioMed Online. 2019;38(4):634–46. https://doi.org/10.1016/j.rbmo.2018.12.015.
Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68(4):585–96. https://doi.org/10.1016/s0015-0282(97)00191-x.
Donnez J, Spada F, Squifflet J, Nisolle M. Bladder endometriosis must be considered as bladder adenomyosis. Fertil Steril. 2000;74(6):1175–81. https://doi.org/10.1016/s0015-0282(00)01584-3.
Vercellini P, Consonni D, Barbara G, Buggio L, Frattaruolo MP, Somigliana E. Adenomyosis and reproductive performance after surgery for rectovaginal and colorectal endometriosis: a systematic review and meta-analysis. Reprod BioMed Online. 2014;28(6):704–13. https://doi.org/10.1016/j.rbmo.2014.02.006.
Bazot M, Cortez A, Darai E, Rouger J, Chopier J, Antoine JM, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16(11):2427–33. https://doi.org/10.1093/humrep/16.11.2427.
Dueholm M, Lundorf E, Hansen ES, Sørensen JS, Ledertoug S, Olesen F. Magnetic resonance imaging and transvaginal ultrasonography for the diagnosis of adenomyosis. Fertil Steril. 2001;76(3):588–94. https://doi.org/10.1016/s0015-0282(01)01962-8.
Guerriero S, Ajossa S, Orozco R, Perniciano M, Jurado M, Melis GB, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in the rectosigmoid: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2016;47(3):281–9. https://doi.org/10.1002/uog.15662.
Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2(2):Cd009591. https://doi.org/10.1002/14651858.CD009591.pub2.
Hanafi M. Ultrasound diagnosis of adenomyosis, leiomyoma, or combined with histopathological correlation. J Hum Reprod Sci. 2013;6(3):189–93. https://doi.org/10.4103/0974-1208.121421.
Stamatopoulos CP, Mikos T, Grimbizis GF, Dimitriadis AS, Efstratiou I, Stamatopoulos P, et al. Value of magnetic resonance imaging in diagnosis of adenomyosis and myomas of the uterus. J Minim Invasive Gynecol. 2012;19(5):620–6. https://doi.org/10.1016/j.jmig.2012.06.003.
Votino A, Van den Bosch T, Installé AJ, Van Schoubroeck D, Kaijser J, Kacem Y, et al. Optimizing the ultrasound visualization of the endometrial-myometrial junction (EMJ). Facts Views Vis Obgyn. 2015;7(1):60–3.
Exacoustos C, Luciano D, Corbett B, De Felice G, Di Feliciantonio M, Luciano A, et al. The uterine junctional zone: a 3-dimensional ultrasound study of patients with endometriosis. Am J Obstet Gynecol. 2013;209(3):248.e1–7. https://doi.org/10.1016/j.ajog.2013.06.006.
Acknowledgements
The authors would like to thank all staff members from our surgical unit and histopathological unit for their expert assistance in data collecting and interpreting.
Code Availability
Not applicable.
Author information
Authors and Affiliations
Contributions
S.A: Conception and design of study, responsible surgeon or imager
E.A: Data analysis and interpretation, manuscript preparation
F.Kh: Data collection
T.P: Data collection, manuscript preparation
B.A: Manuscript preparation
M.H: Patient recruitment
S.A: Patient selection and collection of patient information in early stage of work, revising the article in making structural changes, reviewing and interpretation of statistics data, English editing
H.R.SH: Statistical analysis
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The protocol of the study was approved by the Ethics Committee of Shiraz University of Medical Sciences (code: IR.SUMS.MED.REC.1399.204). All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to Participate
At the beginning of the study, informed consent was obtained from all patients.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alborzi, S., Askary, E., Khorami, F. et al. A Detailed Study in Adenomyosis and Endometriosis: Evaluation of the Rate of Coexistence Between Uterine Adenomyosis and DIE According to Imaging and Histopathology Findings. Reprod. Sci. 28, 2387–2397 (2021). https://doi.org/10.1007/s43032-021-00527-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00527-0