Abstract
Most of the findings have focused on the importance of CD4+HLA-G+ and CD8+HLA-G+ regulatory T cells (Treg) during pregnancy. It has been demonstrated that these HLA-G+ T cell subsets could induce maternal immune tolerance against semi-allogenic conceptus during pregnancy. There are only a few experiments regarding the Treg cells in the context of unexplained infertility (UI). Thirty-five participants including 18 primary unexplained infertile and 17 fertile females were enrolled in this study. A total of 3–5 ml blood samples were taken, and peripheral blood mononuclear cells (PBMCs) were separated by using Ficoll. Using a flow cytometer, the frequency of CD4+HLA-G+ and CD8+ HLA-G+ T cells was assessed in the peripheral blood samples of primary unexplained infertile and fertile females. Our results showed that the frequency of CD8+HLA-G+ Treg cells was significantly lower in primary unexplained infertile females than fertile females (P = 0.048). Although the frequency of CD4+HLA-G+ Treg cells in the primary unexplained infertile females was lower than fertile females, the difference was not statistically significant (P = 0.25). Regarding the important role of CD8+HLA-G+ Treg cells during pregnancy and its decrease in females with primary UI, it seems that reduced CD8+ HLA-G+ Treg cells could be a leading immunological factor in the context of infertility. Nevertheless, more researches are needed in this field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Molecular and cellular events including growth factors and cytokine expression lead to embryo implantation, fertility, and successful pregnancy. Implantation failure and subclinical pregnancy loss could result in infertility. The known causes of infertility include fallopian tube and ovulation disorders and endometriosis. Nevertheless, there are some unknown causes which lead to unexplained infertility (UI). It has been documented that nearly 16% of infertility is reported as UI. Female UI is seen in 10–17% of infertile females [1].
Although the precise etiology of UI is unknown yet, it seems that failure in the maternal immune tolerance mechanisms could be important in acceptance (fertility) and rejection of the conceptus (infertility). The maternal immune tolerogenic mechanism needs alterations in innate and adaptive immune responses in the decidua [2]. In this regard, changes in the proportion of adaptive immune cells could break the tolerance and result in infertility, and especially UI [3].
Human leukocyte antigen (HLA)-G is a non-classical class I major histocompatibility complex (MHC) molecule [4] that induces tolerogenic responses in the maternal immune system during pregnancy [5]. Moreover, it has been demonstrated that HLA-G is also expressed by various immune cells including antigen-presenting cells (APCs) and CD4 and CD8 T cells [6]. HLA-G could regulate immune system responses through binding to inhibitory receptors including Ig-like transcript (ILT)-2 and 4, killer cell immunoglobulin-like receptor 2DL4 (KIR2DL4), CD8, and CD4 [7]. The HLA-G molecule plays a key role in fertility and infertility [5]. HLA-G-expressing T lymphocytes such as HLA-G+ CD8+ and HLA-G+ CD4+ T lymphocytes are known as regulatory T cells in the immune system. HLA-G+ CD8+ and HLA-G+ CD4+ T lymphocytes create maternal immune tolerance against conceptus during pregnancy [8]. HLA-G+ regulatory T cells are CD25- and forkhead box P3 (FOXP3)-negative [9]. This study aimed to investigate the frequency of regulatory HLA-G+ T lymphocytes including CD8+ HLA-G+ and CD4+ HLA-G+ T lymphocytes in the peripheral blood of unexplained infertile females.
Materials and Methods
Subjects
Patients were recruited after providing written informed consent at the Fatima Al-Zahra research center for fertility and infertility Babol, Iran. From a total of 35 patients, we identified 18 females with primary UI (case group) and 17 fertile females (control group). Their demographic and clinical parameters are listed in Table 1. The inclusion criteria for the study group were as follows: 21–38 years old, primary infertility (no live birth), regular menstrual cycle (24–35 days), body mass index (BMI) < 26, FSH < 10 mUI/ml, normal thyroid function, prolactin < 25 μg/l, AMH > 0.5 ng/ml, 17-β-estradiol < 50 pg/ml (day 2–3 of the menstrual cycle), normal hysterosalpingogram and laparoscopy results, no pelvic infection, no thrombophilic disorders (lupus anticoagulant, anticardiolipin antibodies, protein C deficiency, protein S deficiency, antithrombin III deficiency, activated protein C resistance, hyperhomocysteinanemia), normal vitamin D, and normal karyotype. The inclusion criteria for the control group were as follows: 21–38 years old, ≥ 1 live birth younger than 2 years old, regular menstrual cycle (24–35 days), BMI < 26, FSH < 10 mUI/ml, normal thyroid function, prolactin < 25 μg/l, AMH > 0.5 ng/ml, 17-β-estradiol < 50 pg/ml (days 2–3 of the menstrual cycle), normal hysterosalpingogram and laparoscopy results, no pelvic infection, no thrombophilic disorders (lupus anticoagulant, anticardiolipin antibodies, protein C deficiency, protein S deficiency, antithrombin III deficiency, activated protein C resistance, hyperhomocysteinanemia), normal vitamin D, and normal karyotype. The control group was recruited from the gynecologist’s office. Women with endometriosis, tubal factor, ovulatory dysfunction, anatomical uterine pathologies, uterine infections, and abnormal male factor (according to the reference values for healthy human semen established in the 2018 World Health Organization guidelines) were excluded by the gynecologist’s diagnosis. The present study was approved by the Ethics Committee of Babol University of Medical Science (number: 1397.008).
Blood Collection and Peripheral Blood Mononuclear Cell Separation
A total of 3–5 ml blood samples were taken. Peripheral blood mononuclear cells (PBMCs) were separated by using the Ficoll Histopaque density gradient (Biowest, Nuaille, France) according to the manufacturer’s directions. As a brief, the peripheral blood was directly added to the Ficoll-Hypaque gradient and was centrifuged at 350×g for 20 min. The middle-phase PBMCs were collected and washed with phosphate-buffered saline (PBS) at 300×g for 7 min. The PBMCs contained monocytes that could interfere with the results. Thus, PBMCs were resuspended in RPMI 1640 and cultured in a cell culture plate (SPL, South Korea). To decrease the number of monocytes, the supernatant was very carefully collected after 1–2 h of incubation. The PBMCs were ready for analysis by using a flow cytometer.
Flow Cytometric Analysis
The PBMCs were labeled with specific antibodies for cell-surface molecules including CD4, CD8, and HLA-G. In more detail, the Human TruStain Fc blocker (Biolegend) was used for blocking FC receptors. In order to conduct cell-surface staining, PE-conjugated anti-HLA-G monoclonal antibodies (mABs) (Clone 87G, Biolegend), FITC-conjugated anti-CD8 mABs (Clone SK1, Biolegend), and APC-conjugated anti-CD4 mABs (Clone RPA-T4, BD Biosciences) were added respectively to the cellular suspension and was incubated at 4 °C for 20 min. Tubes that were incubated only with Human TruStain Fc blocker (unstained) from the same samples served as a negative control. Then the stained cells and unstained cells were then run with FACS Calibur (BD Biosciences), and a minimum of 50,000 events were gated by size (FSC) and granularity (SSC). The frequency of positive cells was calculated from the lymphocyte gate. All analyses were performed by CellQuest (BD Biosciences) and FlowJo 7.6.1 (Tree Star Corporation, Ashland, OR, USA).
Data Analysis and Statistics
Data analyses were performed using the SPSS Statistics 22 (IBM, Armonk, NY) and GraphPad Prism software (version 7). First, the normality check of each parameter was assessed by the Kolmogorov-Smirnov test. The independent sample t test was used for normally distributed data and those with non-normal data were analyzed using the Mann-Whitney U test. A P value of < 0.05 (two-tailed) was considered statistically significant. Data were presented as mean ± SD for normally distributed data and median (interquartile range (IQR)) for non-normal data.
Results
Demographic and Clinical Characteristics of the Two Study Groups
The demographic and clinical characteristics of primary unexplained infertile and fertile females are shown in Table 1.
Frequencies of HLA-G+ Regulatory T Lymphocytes
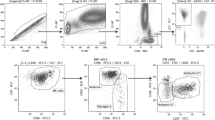
We used three-color flow cytometry to define the frequencies of HLA-G+ regulatory T lymphocyte subsets. Gating strategy and flow cytometry analysis are represented in Fig. 1 according to the two subsets of regulatory T lymphocytes. Our results demonstrated that the mean percentage of CD8+ cells in females with primary UI (9.39) was lower than fertile subjects (10.62) (Fig. 1 B.2 and 1 B.3), but the difference was not significant (P value = 0.51). Although the mean percentage of CD4+ cells in females with primary UI (31.4) was lower than fertile subjects (38.5) (Fig. 1 D.2 and 1 D.3), the difference was not significant (P value = 0.11). Moreover, the percentage of CD8+ HLA-G+ T lymphocytes was determined in the peripheral blood of females with primary UI and fertile females. As shown in Fig. 1 C and 1 F, the mean percentage of this population in females with primary UI and fertile females is 0.26 ± 0.12 and 0.54 ± 0.14 respectively, and the difference was significant (P value = 0.048). Although the mean percentage of CD4+ HLA-G+ T lymphocyte (Fig. 1 E and 1 G) in females with primary UI (0.38 ± 0.04) was lower than fertile subjects (0.58 ± 0.17), the difference was not significant (P value = 0.25). Moreover, the mean percentage of HLA-G+ T lymphocytes (sum of the CD8+HLA-G+ and CD4+HLA-G+ T lymphocytes) was lower in females with primary UI (0.64 ± 0.03) than that in fertile ones (1.13 ± 0.1), but the difference was not significant (P value = 0.11) (Fig. 1 H). As shown in Fig. 2 B.2 and 2 B.3 and Fig. 2 C, our results demonstrated that the mean percentage of HLA-G+ lymphocytes was lower in females with primary UI (1.02) than that in fertile females (1.2).
Flow cytometric analysis and the gating strategy used for HLA-G+ Treg cell detection in the peripheral blood of primary UI (n = 18) and fertile females (n = 17). For the gating strategy, lymphocytes were gated based on the forward and side scatter parameters in A.1) primary UI and A.2) fertile females respectively; then B.2, B.3) CD8 lymphocyte frequency was determined in the pool of lymphocytes in primary UI and fertile females respectively compared with B.1) negative control, C) a histogram of area, in which cells are CD8 positive, was created in order to determine the intensity of HLA-G expression in primary UI and fertile females in comparison with negative control; then D.2 and D.3) CD4 lymphocyte frequency was determined in the pool of lymphocytes in primary UI and fertile females respectively compared with D.1) negative control; E) a histogram of area in which cells are CD4 positive was created in order to determine the intensity of HLA-G expression in primary UI and fertile females in comparison with the negative control. Summary plots of CD8+ HLA-G+, CD4+ HLA-G+, and HLA-G+ T lymphocyte frequencies in the peripheral blood of primary UI and fertile females; F) fertile females had a higher frequency of CD8+ HLA-G+ T lymphocytes in the peripheral blood compared to females with primary UI; G) while the difference between the frequency of CD4+ HLA-G+ T lymphocyte in primary UI and fertile females was not significant, the difference was not significant (P value = 0.25); H) HLA-G+ T lymphocyte frequency (sum of the CD8+ HLA-G+ and CD4+ HLA-G+ T lymphocytes) in primary UI, and fertile females was not different significantly. Graphs show mean ± SD. An independent sample t test was used for data analysis. A P value of < 0.05 was considered statistically significant
Flow cytometric analysis and the gating strategy used for HLA-G+ lymphocyte detection in the peripheral blood of primary UI and fertile females. Lymphocytes were gated based on the forward and side scatter in A.1) primary UI and A.2) fertile females parameters respectively. Then, B.2 and B.3) HLA-G+ lymphocyte frequency was determined in the pool of lymphocytes compared with B.1) the negative control. C) Summary plots of HLA-G+ lymphocyte frequency in primary UI and fertile females were not different significantly. Graphs show mean ± SD. An independent sample t test was used for data analysis. A P value of < 0.05 was considered statistically significant
Discussion
In the present study, we assessed the frequencies of CD4+HLA-G+ and CD8+ HLA-G+ regulatory T lymphocytes in the context of normal pregnancy and UI.
CD8+ CD28− suppressor T cells have been the focus of many studies and their essential role in pregnancy. The differentiation of the CD8+CD28− suppressor T cells is induced on the first trimester of gestation by trophoblastic cells in the decidua [10, 11]. The frequency of CD8+ CD28− suppressor T cells is decreased in females with preeclampsia (PE) in comparison with healthy pregnant females that may result in insufficient control of inflammatory responses in PE [12]. The frequency of CD8+CD28− suppressor T cells is decreased in females with UI compared with that in fertile females [13]. Similar to CD8+ CD28− T lymphocyte, CD8+ HLA-G+ T lymphocyte has a strong regulatory impact on maternal immune responses during pregnancy.
The produced HLA-G molecule by CD8+HLA-G+ T lymphocyte could induce the generation of Tregs, IL-10 production, and inhibition of effector functions of NK and CD8+ T cells and dendritic cell maturation [7, 14, 15].
Regarding the frequency of CD8+ HLA-G+ T lymphocyte in the peripheral blood of preeclamptic patients, some studies [8, 12] demonstrated that this cell subset was significantly higher in the healthy pregnant females compared to non-pregnant and preeclamptic females. In this regard, we investigated these cells in the unexplained infertile females for the first time. Our results indicated that fertile females had a higher level of CD8+HLA-G+ Treg cells in the peripheral blood compared to primary unexplained infertile females. These data suggest CD8+ HLA-G+ regulatory T cell as a distinct regulatory T cell subset that is involved in the pathogenesis of UI.
Most of the findings have focused on the importance of CD4+ CD25+ and CD4+ CD25− regulatory T cells (Treg) during pregnancy. However, there are conflicting data in the literature with studies suggesting no change [16, 17], increased [18, 19], or even lower Treg numbers [20] in preeclamptic females when compared with healthy subjects or non-pregnant women. Similar to CD4+ Treg cells, CD4+ HLA-G+ regulatory T cells were found to be crucial for the maintenance of tolerance in previous studies [6, 8, 21]. Recently Amodio et al. suggested that CD4+HLA-G+ T lymphocytes are present in the fetal-maternal interface during the first trimester of pregnancy [21].
CD4+ HLA-G+ T lymphocytes have an immunosuppressive function as described for CD8+HLA-G+ T lymphocyte. Besides, Hsu et al. reported that the frequency of CD4+HLA-G+ T cells was significantly lower in preeclamptic patients compared to healthy pregnant women [6]. Also, Zare et al. showed that despite the lower frequency of CD4+HLA-G+ Treg cell in the peripheral blood of preeclamptic females compared with that of fertile females, the difference was not significant [8]. In line with Zare et al., we detected lower frequency of CD4+HLA-G+ Treg cell in primary unexplained infertile females than fertile females, although the difference was not statistically significant. Regarding the significant decrease of CD8+ HLA-G+ T cell and non-significant decrease of CD4+HLA-G+ T cells in unexplained infertile females compared with fertile, it is concluded that CD8+HLA-G+ T lymphocyte could play a key role in the maintenance of immune tolerance in human pregnancy.
Our findings show that the mean difference between HLA-G+ lymphocytes and HLA-G+ T lymphocytes in the peripheral blood of primary unexplained infertile females could be attributed to the presence of other HLA-G+ immune lymphocytes (B cells, etc.) that are not evaluated in the present study.
We acknowledge that our study had limitations, including a small sample size and lack of functional analysis of HLA-G+ T cells. Future studies with larger sample size are needed. Moreover, the assessment of the regulatory T/T stimulatory cells ratio will reveal the relationship between the imbalance of T cell subsets and UI.
References
Ehsani M, Mohammadnia-Afrouzi M, Mirzakhani M, Esmaeilzadeh S, Shahbazi M. Female unexplained infertility: a disease with imbalanced adaptive immunity. J Hum Reprod Sci. 2019;12:274–82.
Jasper MJ, Tremellen KP, Robertson SA. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol Hum Reprod. 2006;12:301–8.
Ozkan ZS, Deveci D, Kumbak B, Simsek M, Ilhan F, Sekercioglu S, et al. What is the impact of Th1/Th2 ratio, SOCS3, IL17, and IL35 levels in unexplained infertility? J Reprod Immunol. 2014;103:53–8.
Carosella ED, Favier B, Rouas-Freiss N, Moreau P, Lemaoult J. Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood. 2008;111:4862–70.
Abediankenari S, Farzad F, Rahmani Z, Hashemi-Soteh MB. HLA-G5 and G7 isoforms in pregnant women. Iran J Allergy Asthm. 2015;14:217–21.
Hsu P, Santner-Nanan B, Joung S, Peek MJ, Nanan R. Expansion of CD4(+) HLA-G(+) T cell in human pregnancy is impaired in pre-eclampsia. Am J Reprod Immunol. 2014;71:217–28.
Carosella ED, Gregori S, LeMaoult J. The tolerogenic interplay(s) among HLA-G, myeloid APCs, and regulatory cells. Blood. 2011;118:6499–505.
Zare M, Namavar Jahromi B, Gharesi-Fard B. Analysis of the frequencies and functions of CD4(+)CD25(+)CD127(low/neg), CD4(+)HLA-G(+), and CD8(+)HLA-G(+) regulatory T cells in pre-eclampsia. J Reprod Immunol. 2019;133:43–51.
Feger U, Tolosa E, Huang YH, Waschbisch A, Biedermann T, Melms A, et al. HLA-G expression defines a novel regulatory T-cell subset present in human peripheral blood and sites of inflammation. Blood. 2007;110:568–77.
Shao L, Jacobs AR, Johnson VV, Mayer L. Activation of CD8+ regulatory T cells by human placental trophoblasts. J Immunol. 2005;174:7539–47.
Tilburgs T, Roelen DL, van der Mast BJ, van Schip JJ, Kleijburg C, de Groot-Swings GM, et al. Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(−) T-cells in decidua and maternal blood during human pregnancy. Placenta. 2006;27(Suppl A):S47–53.
Vianna P, Mondadori AG, Bauer ME, Dornfeld D, Chies JA. HLA-G and CD8+ regulatory T cells in the inflammatory environment of pre-eclampsia. Reproduction. 2016;152:741–51.
Hill JA, Faris HMP, Schiff I, Anderson DJ. Characterization of leukocyte subpopulations in the peritoneal fluid of women with endometriosis. Fertil Steril. 1988;50:216–22.
Steinborn A, Rebmann V, Scharf A, Sohn C, Grosse-Wilde H. Placental abruption is associated with decreased maternal plasma levels of soluble HLA-G. J Clin Immunol. 2003;23:307–14.
LeMaoult J, Krawice-Radanne I, Dausset J, Carosella ED. HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc Natl Acad Sci U S A. 2004;101:7064–9.
Hu D, Chen Y, Zhang W, Wang H, Wang Z, Dong M. Alteration of peripheral CD4+CD25+ regulatory T lymphocytes in pregnancy and pre-eclampsia. Acta Obstet Gynecol Scand. 2008;87:190–4.
Paeschke S, Chen F, Horn N, Fotopoulou C, Zambon-Bertoja A, Sollwedel A, et al. Pre-eclampsia is not associated with changes in the levels of regulatory T cells in peripheral blood. Am J Reprod Immunol. 2005;54:384–9.
Sasaki Y, Darmochwal-Kolarz D, Suzuki D, Sakai M, Ito M, Shima T, et al. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin Exp Immunol. 2007;149:139–45.
Prins JR, Boelens HM, Heimweg J, Van der Heide S, Dubois AE, Van Oosterhout AJ, et al. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy. 2009;28:300–11.
Toldi G, Saito S, Shima T, Halmos A, Veresh Z, Vasarhelyi B Jr, et al. The frequency of peripheral blood CD4+ CD25high FoxP3+ and CD4+ CD25- FoxP3+ regulatory T cells in normal pregnancy and pre-eclampsia. Am J Reprod Immunol. 2012;68:175–80.
Amodio G, Mugione A, Sanchez AM, Vigano P, Candiani M, Somigliana E, et al. HLA-G expressing DC-10 and CD4(+) T cells accumulate in human decidua during pregnancy. Hum Immunol. 2013;74:406–11.
Funding
This study was financially supported by grantsfrom Babol University of Medical Sciences and Gerash University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
The guarantor of integrity of entire study: MS; study concepts/study design: MS, MM, SE; data acquisition or data analysis/interpretation: all authors; manuscript drafting or manuscript revision for important intellectual content: MS, MM, ME; approval of the final version of the submitted manuscript: all authors.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ehsani, M., Mohammadnia-Afrouzi, M., Esmaeilzadeh, S. et al. Decreased Frequency of CD8+HLA-G+ T Cell in the Peripheral Blood of Primary Unexplained Infertile Females. Reprod. Sci. 28, 1939–1944 (2021). https://doi.org/10.1007/s43032-020-00431-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00431-z