Abstract
Uterine leiomyomas, also known as fibroids or myomas, are a common benign gynecologic tumor found in women of reproductive age. Though advances have been made in understanding leiomyomas, the etiology and pathogenesis of this disease are not fully characterized. Current evidence supports a role of putative human uterine stem/progenitor cells in the onset of uterine disease such as uterine myomas. In this study, we report that increased expression of CXCL12 in leiomyomas recruits bone marrow-derived cells (BMDCs) that may contribute to leiomyoma growth. Tissue was collected from leiomyomas or control myometrium from women with or without leiomyomas. qRT-PCR analysis showed increased expression of CXCL12 and decreased CXCR4 expression in the leiomyoma and myometrium of women with leiomyoma compared with normal myometrium. Increased CXCL12 protein secretion from cultured myoma cells was confirmed by ELISA. Further, we found that BMDCs migration was increased toward leiomyoma conditioned medium compared with conditioned medium from normal myometrium. CXCR4 antagonist AMD3100 completely blocked this migration. Engraftment of BMDCs significantly increased in myoma of mouse uteri treated with CXCL12 compared with placebo. We conclude that CXCL12 may play a role in leiomyomas growth by attracting bone marrow-derived cells to leiomyoma. Therefore, CXCL12 and its receptors are novel targets for leiomyoma therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uterine leiomyomas, also known as uterine fibroids or uterine myomas, are the most common benign gynecologic tumors found in women of reproductive age [1,2,3,4,5,6,7]. The most frequent symptoms associated with uterine leiomyoma are excessive menstrual bleeding, anemia, and pressure from the pelvic mass itself. [8] Additionally, these tumors contribute to infertility and pregnancy loss and account for 200,000 hysterectomies every year in the USA alone [9,10,11]. Although the specific etiology and pathogenesis of myomas are unknown, numerous advances have been made in understanding the epidemiology, pathology, genetics, and molecular biology of these tumors [12,13,14,15,16].
Major uterine morphological changes occur in response to cyclical hormonal cues from the ovary. Uterine remodeling in response to these stimuli may be related to adult stem (or progenitor) cells residing in the individual’s endometrial and myometrial compartments [14, 15]. The myometrium undergoes significant changes under specific physiological and pathological conditions and similar changes occur in leiomyomas [15]. A leiomyoma is thought to be a benign monoclonal tumor that is derived from a single transformed myometrial smooth muscle cell; however, it is not known what cell type is responsible for tumor growth [14, 15, 17]. The cells that subsequently contribute to their growth likely include stem cells. A further understanding of stem cells, and their interactions with specialized cell populations within leiomyomas, may help in the development of therapies designed to stop leiomyoma growth or eliminate leiomyomas altogether [12,13,14].
Existence of myometrial stem cells (SCs) has been demonstrated by the identification of label-retaining cells in animal models [18] and in the human myometrium [19] by the side population (SP) method. These progenitor stem cells likely give rise to continued tumor growth. Previously, we have shown that engraftment of bone marrow-derived stem cells (BMSCs), including multipotent mesenchymal stem cells, to the endometrium contributes to the physiology of pregnancy [20]. CXCL12 is a chemokine that functions as a chemoattractant of BMSCs to endometrium and endometriosis lesions [21, 22]. Here we identified increased expression of CXCL12 in leiomyomas compared with normal myometrium and demonstrated the ability of this chemokine to recruit BMSCs. Bone marrow-derived cells (BMDCs) may contribute to the growth of uterine leiomyoma.
Materials and Methods
Sample Collection
Uterine tissue specimens were acquired from patients undergoing hysterectomy or abdominal myomectomy for symptomatic uterine fibroids. We collected a total of 48 specimens from 38 subjects: 28 leiomyomas, 10 of which had normal-appearing myometrium surrounding the myoma, and 10 normal myometria from premenopausal subjects without myoma undergoing hysterectomy for pelvic pain or prolapse. None of the subjects was using exogenous hormones. Myomas and myometrium were confirmed histologically. Human Investigations Committee approval was obtained from the Yale University Institutional Review Board.
RNA Isolation
Tissue samples collected from myoma as well as normal myometrium were processed immediately or frozen at − 80 °C in RNAlater solution. The tissue was homogenized in TRIzol (100 mg/1 ml TRIzol) reagent (Invitrogen, Carlsbad, CA), and homogenates were kept on ice for 5 min. 0.2 ml of chloroform was added to each separately, and homogenates were vortexed for 15 s. Samples were then incubated at room temperature for 3 min and centrifuged at 12,000 rpm at 4 °C for 15 min. The aqueous layer was transferred to a fresh tube, and RNA was precipitated by adding 0.5 ml of isopropyl alcohol. All tubes were centrifuged at 10,000 rpm to form RNA pellets, which were then collected, washed with 75% ethanol, and dissolved in RNase-free water. Total RNA was purified using the Qiagen RNeasy Cleanup Kit (Qiagen, Valencia, CA), according to the manufacturer’s protocol and quantified using NanoDrop spectrophotometer. Purified RNA was immediately used for cDNA synthesis. The remainder was stored at − 80 °C until later use.
Quantitative Real-Time Polymerase Chain Reaction Analysis
Purified RNA (25 ng) was reverse-transcribed in 10 μl reaction mixture using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using SYBR Green (Bio-Rad) and optimized in the MyiQ single-color real-time PCR detection system (Bio-Rad). The specificity of the amplified transcript and absence of primer-dimers were confirmed by a melting curve analysis. All products yielded the predicted melting temperature. Gene expression was normalized to the expression of β-actin. Relative mRNA expression for each gene was calculated using the comparative cycle threshold (Ct) method (2-ΔΔCT) [23, 24]. The primers used were the following: CXCL12: forward-AACACTCCAAACTGTGCCCT, reverse-CTCTCACATCTTGAACCTCTTGTT; CXCR4: forward-GCAGAGGAGTTAGCCAAGATGT, reverse-CATTGGGGTAGAAGCGGTCA; β-actin: forward-ATCAAGGAGAAGCTCTGCTACATC, reverse-TCAGACTCGGCTGGAAGAGA; CXCR7: forward-CCTGCCTTGGTCCTAAGTGT, reverse-GGCAAAACCAGCCTCCTTAC. Negative controls consisted of nuclease-free water instead of cDNA template and the reaction mix without reverse transcriptase. Each assay was run twice in triplicates.
Cell Culture
Primary cell cultures were prepared by mincing the tissue collected from myoma, normal myometrium, and normal-appearing myometrium surrounding myoma. Each treatment group included samples cultured independently from at least five unique patients. The finely cut tissue was incubated in HBSS containing HEPES (25 mm), 1% penicillin/streptomycin, collagenase (1 mg/ml, 15 U/mg), and DNase (0.1 mg/ml, 1500 U/mg) for 45 min at 37 °C with agitation and pipetting. During and at the end of the incubation, the tissue was pipetted gently to disperse the cells every 15 min. Endometrial cells were pelleted, washed, and suspended in Ham’s DMEM/F12 (1:1) containing 10% FBS, 1% penicillin/streptomycin, and 1% Amphotericin B. The cells were plated and cultured for an additional 48 h before further experiments were carried out.
CXCL12 Assay from Conditioned Medium by Enzyme-Linked Immunosorbent Assay
The protein concentrations of CXCL12 in the conditioned medium from primary cell cultures were measured by enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, USA) according to the manufacturer’s instructions. Tissue from myoma, normal myometrium, and normal-appearing myometrium surrounding myoma were cultured in DMEM/F12 supplemented with 10% FBS and 1% penicillin and streptomycin in a 6-well plate (2 × 105 cells/well). The supernatants or conditioned media were collected from 48-h old cell cultures and used immediately or frozen at − 80 °C until use.
Migration Assay
The migration of mice bone marrow cells (mBMC) was carried out by transwell migration assay using 8-μm pore size polycarbonate membrane (Millipore, USA). The conditioned medium (600 μl), collected from 48-h old cell cultures from myoma, normal myometrium, and normal myometrium surrounding myoma, was added into the lower chamber, and 200 μl of mBMCs containing (5 × 104/well) cells was placed into the upper insert. The cells in the upper insert were serum starved for 24 h, and treated with or without AMD3100, an antagonist of CXCR4, for 30 min before the migration assay. After 16 h in a humidified CO2 incubator at 37 °C, the non-migrating cells were scraped with a cotton swab from the top of the membrane. The cells migrating across the membrane were fixed, stained, and lysed according to the manufacturer’s instructions. Optical densities were read in triplicates at 560 nm using a Bio-Rad Laboratories (Hercules, CA, USA) plate reader. To determine the relative percentage of migration, results were compared with the 100% migration control. Each experiment was performed three times in duplicate. Results were reported as chemotactic index (CI), that is, cells migrating in response to the conditioned media supernatants divided by cells response to the serum-free DMEM/F12 medium.
Engraftment of BMDCs in Myometrium
Six- to eight-week-old C57BL/6 J female wild-type mice (N = 20) purchased from Charles River Laboratories (Wilmington, MA, USA) and an equal number of mice that expressing GFP produced in the Yale Animal Resources Center (YARC) at Yale School of Medicine were used for this experiment. All animal experiments were conducted in accordance with an approved protocol from the Institutional Animal Care and Use Committee (IACUC) of Yale University. Mice (N = 20) received chemotherapy as previously described [20]. Subsequently, the mice underwent bone marrow transplantation (BMT) using marrow from C57BL/6 GFP-positive donors. Briefly, two doses of 125 mg/Kg of 5-FU (5-fluorouracil) were injected intraperitoneally (i.p.) 6 days and 1 day prior to BMT. BMT was then performed by obtaining unfractionated bone marrow cells (30 × 106) from mice expressing GFP. Bone marrow was flushed from the femurs and tibias of 8- to 10-week GFP-positive donor mice with cold sterile phosphate-buffered saline (PBS). The marrow suspension was filtered through a sterile 70 μm Nitex mesh (Becton, Dickinson and Co.) and then injected in the retro-orbital space of recipient wild female mice. After 4 weeks, mice were evaluated for bone marrow engraftment which we have previously reported to be from 40 to 50% [25]. The animals were divided into two groups (each group N = 10) to receive direct uterine injection of CXCL12 (Recombinant mouse SDF-1α, Gemini-Bio®, West Sacramento, CA, USA) or placebo. BSA 0.1% was used as the diluent for CXCL12 as well as the placebo injection. Three hundred microliters of solution, with or without 2.5 μg of CXCL12, were injected directly into the uterine horns using a 30-gauge needle during a surgical procedure. All mice were euthanized after 6 weeks in diestrus and hysterectomies were performed. Uterine horns were collected, fixed in 4% paraformaldehyde for 24 h, immersed in 70% ethanol, and embedded in paraffin for histological studies.
Histology and Immunohistochemistry
The uterine tissue embedded in paraffin was cut in 5-μm slices and mounted on to the microscopic slides. Routine hematoxylin and eosin (H&E) staining was used for histological analysis. Immunostaining for the presence of GFP cells in the myometrium was carried out using anti-GFP (1:1000; #ab6673, Cambridge, Massachusetts, EUA) primary antibody and rabbit anti-goat secondary antibody (1:200, Vector laboratories, #PK-4005, Burlingame, CA). All slides were identified using codified numbers to avoid bias during immunohistochemistry analysis. Two observers blinded to treatment group counted GFP-positive stained cells in the myometrium. Slides were analyzed and photographed using conventional optical microscopy.
Statistical Analysis
Data from qRT-PCR, ELISA, and migration assay were analyzed by one-way analysis of variance (ANOVA). Cell count from histology and immunohistochemistry (IHC) was analyzed using unpaired t test. A level of p < 0.05 was considered significant. The data is reported as mean ± standard error of the mean (SEM).
Results
Increased Expression of CXCL12 in Women with Myoma
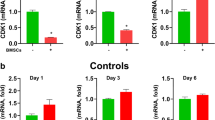
The mean age of subjects was 40 years (range 33–48). The qRT-PCR results showed increased expression of CXCL12 (2.7-fold, p < 0.01) in myomas compared with normal myometrium (control), and a similar increase was observed in the expression of CXCL12 (2.1-fold, p < 0.05) in normal-appearing myometrium surrounding myomas (adjacent) compared with the control (Fig. 1a). In order to confirm the increased expression of CXCL12 protein, we used ELISA to determine CXCL12 protein levels in the conditioned medium of primary cell cultures from myoma, normal myometrium, and normal-appearing myometrium surrounding myoma. The secreted CXCL12 protein concentration significantly was higher in the myoma conditioned media than in the conditioned media of the control (1497 ± 360 versus 305 ± 50 pg/ml, respectively, p < 0.05) (Fig. 1b). Protein levels in conditioned media of the adjacent tissue was increased by 2.2-fold compared with the conditioned media from the control tissue (Fig. 1b).
Increased expression of CXCL12 mRNA and protein. a qRT-PCR results show increased mRNA levels of CXCL12 in myoma and in adjacent myometrium compared with control. b ELISA results show increased protein levels of CXCL12 in cells cultured from myoma and adjacent myometrium. Data is presented as mean ± SEM from two individual experiments and each experiment was performed in triplicate. *Denotes p < 0.05 while **denotes p < 0.01 compared with control
qRT-PCR results showed a 3-fold (p < 0.001) decreased expression of CXCR4 in myoma samples and a 4-fold decrease in adjacent samples compared with control samples (Fig. 2a). In contrast, we observed a significant increase in CXCR7 expression in myoma (1.6-fold, p < 0.05), but not in adjacent samples, compared with control samples (Fig. 2b).
Decreased expression of CXCR4 and increased expression of CXCR7 mRNA levels. a qRT-PCR results showed decreased mRNA levels of CXCR4 in myoma and adjacent myometrium compared with control. b qRT-PCR results showed increased mRNA levels of CXCR4 in myoma and adjacent myometrium compared with control. Data presented as mean ± SEM for data from three individual experiments and each experiment was performed in triplicate. *Denotes p < 0.05 while ****denotes p < 0.0001 compared with control
Effect of Myoma Produced CXCl12 on Migration of mBMC
To determine the effect of CXCL12 and its chemoattractant activity on mBMCs, a migration assay was carried out using the conditioned media from cultured primary myometrial cells from myoma, normal myometrium (control), and normal-appearing myometrium surrounding myoma (adjacent). As shown in Fig. 3, we found that the migration of mBMCs was increased toward myoma conditioned medium than conditioned medium from normal myometrium (p < 0.0001) as well as myometrium adjacent to myomas (p < 0.0001). There is no difference in migration of mBMCs toward conditioned media of control and adjacent. Pre-treatment of mBMCs with AMD3100, a CXCR4 antagonist, significantly reduced their migration toward the conditioned media from control, myoma, and adjacent compared with mBMCs without treatment toward the same conditioned media (p < 0.001). AMD3100 treatment blocked migration of mBMCs toward all three media conditions.
Determination of chemotactic activity of CXCL12. The chemoattractant activity of mouse bone marrow cells (mBMCs) was determined by migration assay. The migration of mBMCs toward the conditioned medium obtained from primary myometrial cells was determined by seeding cells (5 × 104/well) on inserts and measured the migration toward serum-free medium alone (negative control) or conditioned supernatant. Data are shown as a chemotactic index (CI): cells migrating in response to the conditioned supernatants divided by cells migrating to the serum-free medium. Data is mean ± standard error of the mean (SEM) of 5 samples from controls and myoma. Each experiment was carried out three times, each in duplicate. ****Denotes (p < 0.0001) compared with the control. ###Denotes (p < 0.001) compared with adjacent and ####denotes (p < 0.0001) compared with the control and myoma respectively
Increased Uterine Engraftment of BMDCs in CXCL12-Treated Mice
In order to determine of CXCL12 increased engraftment of myometrium in vivo, we treated mice with intrauterine injections of CXCL12 after undergoing transplant with GFP bone marrow. Normal histology of myometrium was confirmed by H&E staining for both groups (placebo and CXCL12) as shown in Fig. 4a, and there was no significant difference in morphology of CXCL12-treated uterus compared with placebo-treated. In IHC analysis, GFP was used as a marker for engrafted BMDCs. IHC results, shown in Fig. 4a, demonstrated increased presence of GFP-positive cells in the myometria of CXCL12-treated mice compared with those of placebo-treated mice. The number of GFP-positive cells in the myometria of the CXCL12 group was greater by 6.1-fold (p < 0.01) compared with that of the placebo-treated group (Fig. 4b.)
Histological confirmation of myometrium and presence of BMDCs in myometrium. a H&E staining showed intact myometrium of uterine samples from mice induced with endometriosis. There was no difference in morphology of the myometria of the CXCL12 treatment group compared with those of the placebo treatment group. b Immunohistochemistry demonstrated an increased presence of GFP-positive cells in the myometria of CXCL12 group compared with those of the placebo group. Histogram shows the number of GFP-positive bone marrow-derived cells in myometrium of placebo vs CXCL12 injected into uteri (*p < 0.01) between CXCL12 treatment and placebo
Discussion
Uterine leiomyomas are the most common gynecological tumors in women, 70–80% of whom will develop at least one leiomyoma by the age of 50 [10]. Up to 30% of women with leiomyoma will experience severe symptoms, including pelvic pain, recurrent pregnancy loss, and excessive uterine bleeding [10]. Thus, leiomyoma presents a large health burden to the population. In the USA, leiomyomas are the most frequent indication for hysterectomy [26], with approximately 200,000 hysterectomies, and additionally, 30,000 myomectomies performed annually to treat leiomyoma [10]. As a result, the economic burden of this disease is over $30 billion per year [27]. The morbidity and socioeconomic costs associated with LM suggest a critical need to better characterize the molecular mechanisms underlying these tumors so that new therapies may be developed.
Current treatments for leiomyoma are intended to primality address the symptom of heavy menstrual bleeding. Typical first line medical therapy is a hormonal contraceptive; while these agents may result in diminished bleeding, in large part due to an endometrial effect, they do not result in regression of the leiomyoma. Hysteroscopic or abdominal myomectomy to remove individual fibroids is the standard surgical treatment for those wishing to preserve the ability to conceive. Hysterectomy is commonly performed when a women has completed childbearing. There are currently no medications available for long-term use in the USA that lead to regression of leiomyoma. Further, even the medications used to treat symptoms are contraceptive or contraindicated in pregnancy; there are no drugs available to improve leiomyoma associated infertility or pregnancy loss. A better understanding of the cellular biology of leiomyoma and the potential to restrict stem cell recruitment may be a useful novel agent to prevent fibroid growth.
The presence of progenitor stem cells in leiomyoma is well-established. The need for vast expansion of the uterus during pregnancy predicts the presence of these cells which was first identified by the side population method [19]. These cells were later isolated from leiomyoma, where they were demonstrated to have low levels of estrogen and progesterone receptor expression [17]. The lack of direct regulation by sex steroids suggested paracrine regulation by neighboring myometrial cells. These progenitor stem cells have been identified as expressing Stro-1/CD44 [28] or CD34/CD49b [29], markers used to isolate and characterize these cells further. The pool of stem cells is also modified by developmental exposure to endocrine disruptors that are known to be associated with the development of leiomyoma [30, 31]. Recent work has demonstrated the importance of progesterone-regulated RANKL gene expression in activating stem cell proliferation and fibroid tumor development [32]. Interestingly, in other tissues, both CXCL12 and RANKL are regulated by estrogens and by a common microRNA [33, 34]. It is likely that common upstream regulators control multiple aspects of leiomyoma growth that coordinate proliferation and the engraftment of new stem cells. Despite identification of resident progenitor myometrial and leiomyoma stem cells, the role of bone marrow-derived stem cells in leiomyoma has not been characterized. In other uterine conditions, we have established a role for bone marrow-derived stem cells; these cells repopulate the endometrium [35], play a role in uterine repair [36,37,38], are necessary for pregnancy [20], and contribute to endometriosis [39]. Here we show that myometrium attracts bone marrow-derived stem cells and that leiomyoma increases migration and recruitment of these cells. It is likely that bone marrow-derived cells have a similar role in myometrium/leiomyoma as that already established in endometrium/endometriosis. Recruitment of stem cells may have potential to repair normal myometrium. Prevention of stem cell engraftment may help to limit leiomyoma growth.
In summary, we demonstrate that myometrium produces the bone marrow stem cell chemoattractant CXCL12. Leiomyoma secretes higher levels of CXCL12 than normal myometrium that likely leads to increased growth through enhanced stem cell engraftment. Blocking CXCL12 has potential as a therapy for leiomyoma.
References
Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004;104(2):393–406.
Bulun SE. Tissue stem cells and uterine physiology and pathology. Semin Reprod Med. 2015;33(5):313–4.
Taylor HS. Fibroids: when should they be removed to improve in vitro fertilization success. Fertil Steril. 2018;109(5):784–5.
Cao T, Jiang Y, Wang Z, Zhang N, al-Hendy A, Mamillapalli R, et al. H19 lncRNA identified as a master regulator of genes that drive uterine leiomyomas. Oncogene. 2019;38(27):5356–66.
Cermik D, Arici A, Taylor HS. Coordinated regulation of HOX gene expression in myometrium and uterine leiomyoma. Fertil Steril. 2002;78(5):979–84.
Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93(6):2027–34.
Sinclair DC, Mastroyannis A, Taylor HS. Leiomyoma simultaneously impair endometrial BMP-2-mediated decidualization and anticoagulant expression through secretion of TGF-beta3. J Clin Endocrinol Metab. 2011;96(2):412–21.
Marsh EE, Bulun SE. Steroid hormones and leiomyomas. Obstet Gynecol Clin N Am. 2006;33(1):59–67.
Parker WH. Uterine myomas: management. Fertil Steril. 2007;88(2):255–71.
Bulun SE. Uterine fibroids. N Engl J Med. 2013;369(14):1344–55.
Doherty L, Mutlu L, Sinclair D, Taylor H. Uterine fibroids: clinical manifestations and contemporary management. Reprod Sci (Thousand Oaks, Calif). 2014;21(9):1067–92.
Segars JH. Uterine fibroid research: a work in progress. Reprod Sci (Thousand Oaks, Calif.). 2014;21(9):1065–6.
Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111(8):1037–54.
Sozen I, Arici A. Cellular biology of myomas: interaction of sex steroids with cytokines and growth factors. Obstet Gynecol Clin N Am. 2006;33(1):41–58.
Blake RE. Leiomyomata uteri: hormonal and molecular determinants of growth. J Natl Med Assoc. 2007;99(10):1170–84.
Ciarmela P, Islam MS, Reis FM, Gray PC, Bloise E, Petraglia F, et al. Growth factors and myometrium: biological effects in uterine fibroid and possible clinical implications. Hum Reprod Update. 2011;17(6):772–90.
Ono M, Qiang W, Serna VA, Yin P, Coon JS 5th, Navarro A, et al. Role of stem cells in human uterine leiomyoma growth. PLoS One. 2012;7(5):e36935.
Szotek PP, Chang HL, Zhang L, Preffer F, Dombkowski D, Donahoe PK, et al. Adult mouse myometrial label-retaining cells divide in response to gonadotropin stimulation. Stem Cells. 2007;25(5):1317–25.
Ono M, Maruyama T, Masuda H, Kajitani T, Nagashima T, Arase T, et al. Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc Natl Acad Sci U S A. 2007;104(47):18700–5.
Tal R, Shaikh S, Pallavi P, Tal A, López-Giráldez F, Lyu F, et al. Adult bone marrow progenitors become decidual cells and contribute to embryo implantation and pregnancy. PLoS Biol. 2019;17(9):e3000421.
Sahin Ersoy G, Zolbin MM, Cosar E, Moridi I, Mamillapalli R, Taylor HS. CXCL12 promotes stem cell recruitment and uterine repair after injury in Asherman’s syndrome. Mol Ther Methods Clin Dev. 2017;4:169–77.
Moridi I, Mamillapalli R, Cosar E, Ersoy GS, Taylor HS. Bone marrow stem cell chemotactic activity is induced by elevated CXCl12 in endometriosis. Reprod Sci (Thousand Oaks, Calif.). 2017;24(4):526–33.
Barr A, Manning D. G Proteins Techniques of Analysis. Boca Raton: CRC Press, Inc.; 1999. p. 227–45.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–8.
Lee B, Du H, Taylor HS. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod. 2009;80(1):79–85.
Drayer SM, Catherino WH. Prevalence, morbidity, and current medical management of uterine leiomyomas. Int J Gynaecol Obstet. 2015;131(2):117–22.
Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e211–9.
Mas A, Nair S, Laknaur A, Simon C, Diamond MP, Al-Hendy A. Stro-1/CD44 as putative human myometrial and fibroid stem cell markers. Fertil Steril. 2015;104(1):225–234.e223.
Yin P, Ono M, Moravek MB, Coon JS 5th, Navarro A, Monsivais D, et al. Human uterine leiomyoma stem/progenitor cells expressing CD34 and CD49b initiate tumors in vivo. J Clin Endocrinol Metab. 2015;100(4):E601–6.
Mas A, Stone L, O'Connor PM, Yang Q, Kleven D, Simon C, et al. Developmental exposure to endocrine disruptors expands murine myometrial stem cell compartment as a prerequisite to leiomyoma tumorigenesis. Stem Cells. 2017;35(3):666–78.
Prusinski Fernung LE, Yang Q, Sakamuro D, Kumari A, Mas A, Al-Hendy A. Endocrine disruptor exposure during development increases incidence of uterine fibroids by altering DNA repair in myometrial stem cells. Biol Reprod. 2018;99(4):735–48.
Liu S, Yin P, Kujawa SA, Coon JS, Okeigwe I, Bulun SE. Progesterone receptor integrates the effects of mutated MED12 and altered DNA methylation to stimulate RANKL expression and stem cell proliferation in uterine leiomyoma. Oncogene. 2019;38(15):2722–35.
Wang X, Mamillapalli R, Mutlu L, Du H, Taylor HS. Chemoattraction of bone marrow-derived stem cells towards human endometrial stromal cells is mediated by estradiol regulated CXCL12 and CXCR4 expression. Stem Cell Res. 2015;15(1):14–22.
Lian WS, Ko JY, Chen YS, Ke HJ, Hsieh CK, Kuo CW, et al. MicroRNA-29a represses osteoclast formation and protects against osteoporosis by regulating PCAF-mediated RANKL and CXCL12. Cell Death Dis. 2019;10(10):705.
Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. Jama. 2004;292(1):81–5.
Simoni M, Taylor HS. Therapeutic strategies involving uterine stem cells in reproductive medicine. Curr Opin Obstet Gynecol. 2018;30(3):209–16.
Liu Y, Tal R, Pluchino N, Mamillapalli R, Taylor HS. Systemic administration of bone marrow-derived cells leads to better uterine engraftment than use of uterine-derived cells or local injection. J Cell Mol Med. 2018;22(1):67–76.
Alawadhi F, Du H, Cakmak H, Taylor HS. Bone marrow-derived stem cell (BMDSC) transplantation improves fertility in a murine model of Asherman’s syndrome. PLoS One. 2014;9(5):e96662.
Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25(8):2082–6.
Funding
This work was supported by NIH U54 HD052668 and R01 HD076422.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moridi, I., Mamillapalli, R., Kodaman, P.H. et al. CXCL12 Attracts Bone Marrow-Derived Cells to Uterine Leiomyomas. Reprod. Sci. 27, 1724–1730 (2020). https://doi.org/10.1007/s43032-020-00166-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00166-x