Abstract
Prenatal testosterone (T) excess, partly via androgenic programming, enhances follicular recruitment/persistence in sheep as in women with polycystic ovarian syndrome (PCOS). Decreased anti-Mullerian hormone (AMH) in early growing and increased AMH in antral follicles may underlie enhanced recruitment and persistence, respectively. Changes in AMH may be mediated by steroidogenic factor 1 (SF1), an enhancer of AMH, and dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1 (DAX1), that antagonizes SF1. Another mediator could be forkhead box 03 (FOXO3) which regulates follicular recruitment/atresia. To test if androgen-programmed changes in SF1, DAX1, and FOXO3 proteins contribute to follicular defects in prenatal T-treated sheep, ovaries from control, prenatal T-, and dihydrotestosterone (DHT)-treated (days 30–90 of gestation) animals at fetal day (FD) 90, FD140, and 1 and 2 years-of-age were studied. Prenatal T increased DAX1 in granulosa cells of primordial through large preantral and theca cells of large preantral follicles at FD140 and increased SF1 in the granulosa cells of preantral and antral and theca cells of large preantral follicle at 2 years-of-age. Prenatal T increased FOXO3 only in theca cells of preantral (FD140) and antral (2 years-of-age) follicles. Prenatal DHT increased DAX1 in granulosa cells from small preantral follicles at FD140 while increasing SF1 in granulosa cells from antral follicles at 1 year-of-age. These age-dependent changes in DAX1/SF1 partly via androgen-programming are consistent with changes in AMH and may contribute to the enhanced follicular recruitment/persistence, and multifollicular phenotype of prenatal T-treated females and may be of translational relevance to PCOS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovarian syndrome (PCOS), an infertility disorder affecting women of reproductive age, is characterized by oligo-/anovulation, clinical and biochemical signs of hyperandrogenism, and multifollicular ovarian morphology [1,2,3]. The etiopathology of PCOS is still not fully understood but appears to stem from gene and environment interaction. [4]. Studies with human disease models such as congenital adrenal hyperplasia (CAH) and animal models of PCOS phenotype provide support for its developmental origin [5,6,7]. Because PCOS features only become apparent during adolescence or adulthood, studies in animal models of prenatal testosterone (T) excess such as sheep that are precocial with developmental timeline similar to human are useful in understanding the developmental origin of PCOS [8].
Prenatal T excess in the sheep induces oligo-/anovulation, functional hyperandrogenism, and multifollicular ovarian morphology, similar to that observed in women with PCOS [9]. Ovarian disruptions observed in PCOS women and the prenatal T-treated sheep include reduction in primordial and increase in primary and antral follicular number [10,11,12,13]. The multifollicular ovarian morphology may therefore stem from enhanced follicular recruitment and/or antral follicular persistence. Studies with prenatal dihydrotestosterone (DHT) (a non-aromatizable androgen) treatment suggest that enhancement of follicular recruitment [14] but not persistence [12] is mediated by androgenic actions of T.
The follicular activation process through which primordial follicles get recruited to enter the growth phase is controlled by various factors of oocyte, ovarian somatic cell, and extra-ovarian origin that either promote or inhibit the process [15]. Among these, anti-Mullerian hormone (AMH) is known to inhibit the activation process [16] and has been identified as a biomarker in women with PCOS [17]. The findings of lower expression of AMH in primordial and primary follicles in ovaries from PCOS women [18] and primordial, primary, and preantral follicles of the sheep model of PCOS phenotype [19, 20] support the notion that reduced inhibitory action of AMH may be involved in the enhanced activation/recruitment process. The observations of an increase in primordial follicular activation in AMH knockout mouse [21] and reduction following AMH treatment of neonatal mouse ovary in vitro [22] are supportive of this premise. In addition, androgens may increase follicular activation through stimulation of AKT signaling–dependent inhibition of transcription factor forkhead box O3 (FOXO3) protein [23]. This is corroborated by the observation of premature follicular activation in FOXO3 knockout mice [24].
While reduced AMH in early-stage follicles may contribute to increased recruitment, increase in AMH and consequent reduction in FSH sensitivity may underlie the follicular arrest and persistence, [17] features evidenced in antral follicles of PCOS women and prenatal T-treated sheep [25, 26]. That AMH reduces FSH sensitivity of the follicle is supported by the observations that (1) AMH treatment inhibits FSH-induced preantral follicle growth [27] and (2) FSH-dependent selection of small antral follicles is increased in AMH knockout mouse [28]. While these studies highlight the importance of AMH in the development of the multifollicular ovarian phenotype in prenatal T-treated sheep and PCOS women, the mechanisms by which changes in AMH are mediated are not known. The granulosa cell expression of AMH is regulated by various oocyte- [29] and somatic cell–secreted factors [30]. Steroidogenic factor 1 (SF1; official gene name NR5A1), a member of the nuclear receptor superfamily, is expressed in the ovine granulosa cells of all follicular types [31] and involved in the regulation of steroidogenic genes in the cytochrome P450 family as well as AMH [32]. SF1 binding sites are present in the AMH promoter [33]. The main regulator of SF1 is DAX1 (product of the dosage-sensitive sex reversal, adrenal hypoplasia congenital critical region on the X chromosome gene 1; official symbol NROB1), which represses the expression of SF1 target genes [34]. These observations indicate that during the process of ovarian development, SF1 and DAX1 tightly regulate AMH expression.
Resistance to atresia and consequent follicular persistence in PCOS women and prenatal T-treated sheep may also stem from increased activation of phosphatidylinositol 3-kinase (PI3K)-AKT–dependent cell signaling pathway, a central player in promoting cell survival [35] The anti-apoptotic effects of PI3K-AKT-dependent signaling involves inhibition of the transcription factor FOXO3 that enhances pro-apoptotic gene expression [36]. A reduction in FOXO3 in antral follicles may therefore reduce follicular atresia and contribute to the follicular peristance evidenced in PCOS women and prenatal T-treated sheep.
Using prenatal T-treated sheep as a model, this study tested the hypothesis that disruption in SF1/DAX1 and/or FOXO3 may contribute to enhanced follicular activation and persistence in the prenatal T-treated sheep. Additionally, because T can be aromatized to estrogen, to distinguish if the programming effects of T were mediated via androgenic or estrogenic actions of T, the findings of prenatal T-treated sheep were compared with those of prenatal DHT-treated sheep.
Methods
Animal housing, general husbandry, and diets of breeder sheep and lamb used have been described previously [37]. Animal care and procedures were performed under the approval of the Institutional Animal Care and Use Committee of the University of Michigan consistent with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Prenatal Treatments
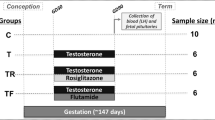
Prenatal T- and DHT-treated animals were generated by intramuscular administration of 100 mg T propionate (1.2 mg/kg; Sigma-Aldrich, St. Louis, MO) and 100 mg of DHT propionate (Steraloids) in 2 mL of cottonseed oil (Sigma-Aldrich), respectively, to pregnant ewes twice weekly from days 30 to 90 of gestation. Ovaries were collected at four different ages, fetal days 90 (FD90) and 140 (FD140), and at postnatal 10 months (end of first breeding season; year 1), and 21 months-of-age (end of second breeding season; year 2). Only one female offspring from each mother was included in the study. As such, the number represented also reflects number of mothers used in this study. The number of animals for FD140, year 1, and year 2 time points was 4 per treatment group, while at FD90, the number of animals in control and prenatal T groups was 4 and prenatal DHT group was 5. There were insufficient DHT-treated females born to include a year 2 prenatal DHT-treated group. Developmental changes in ovarian follicular distribution determined by ovarian morphometry and changes in expression of apoptotic factors, steroid receptors, steroidogenic enzymes, and ovarian VEGF and AMH patterns from the cohort used in this study have been previously published [14, 20, 38,39,40,41,42].
Ovary Collection
Fetal ovaries were collected after dams were euthanized by administration of a barbiturate overdose (Fatal Plus, Vortech, Dearborn, MI). Ovaries from 10- and 21-month-old female sheep were collected during a presumptive follicular phase (18 h post the second injection) after synchronization with prostaglandin F2α (10 mg, intramuscular; Lutalyse, Pfizer, Florham Park, NJ), given twice, 11 days apart. Euthanasia, ovarian collection, and processing details have been reported previously [14].
Immunohistochemistry
To avoid follicular overlap, two sections from each ovary (one in the first one-third and second one in the two-thirds into the ovary) were used. To prevent bias and avoid technical differences during multiple immunohistochemical runs, slides from each developmental time point and treatment groups as well as positive and negative controls were included in each immunostaining series of 20 slides. The homology between the target peptide of the antibody and the corresponding ovine protein was > 95% for all antibodies as determined using the Basic Local Alignment Search Tool (BLAST software; http://www.ncbi.nlm.nih.gov/BLAST). Detection of protein was by the streptavidin-biotin immunoperoxidase method [38, 39]. Briefly, following deparaffinization, antigen was retrieved by microwaving in 10 mM sodium citrate buffer (pH 6.0). Endogenous peroxidase activity was inhibited with 3% (v/v) H2O2 in methanol, and non-specific binding blocked with 10% (v/v) normal goat serum in PBS. The primary antibodies (Abcam, Cambridge, MA) used against SF1 (mouse polyclonal antibody, ab52928), DAX1 (rabbit polyclonal, ab60144), or FOXO3 (rabbit polyclonal, ab70315) were incubated at 1:100 dilution for 18 h at 4 °C and then for 30 min at room temperature with biotinylated secondary antibodies (Cell Marque, Rocklin, CA, USA). The antigens were visualized using the CytoScan HRP Detection System with 3,3-diaminobenzidine (DAB; Liquid DAB-Plus Substrate Kit; Invitrogen; development time 3–4 min) as the chromogen. To verify immunoreaction specificity, adjacent control sections were subjected to the same immunohistochemical method, replacing primary antibodies with rabbit and mouse non-immune sera (Fig. 1).
Specificity of the antibodies for SF1, DAX1, and FOXO3 by Western blot (left panel) and immunohistochemistry without and with primary antibody (middle panels). The boxes in the with primary antibody panel indicate the area that is captured and analyzed by applying the mask using Image Pro analyzer (right panel)
Image Analysis
Four to fifteen follicles from each follicular class from all stages of development were analyzed. Only follicles that were healthy without any signs of atresia (pyknotic nucleus and loss of cell adhesion in the granulosa layer) were included for analysis. Follicle classes were distinguished using criteria previously established [39] and included primordial, primary, small preantral, large preantral, and antral follicles. For antral follicles, granulosa cells from antrum to theca were evaluated to avoid subjectivity and differences in protein location. Also, for each ovary, 10 random images of stromal tissue in the cortical area were analyzed, avoiding any vascular or follicular structures.
Microscopic images were digitized with a color video camera Nikon DS-Fi2 mounted on a conventional light microscope Nikon Eclipse Ci-L Ni (Tokyo, Japan), with a plane apochromatic objective (× 40 magnification). The image analysis was performed using the Image Pro-Plus 3.0.1 (Media Cybernetics, Rockville, MD) as described previously [39] using a well-validated densitometrical methodology [43]. The average density calculated as percentage of immunopositive area for brown stain (produced by the immunoperoxide reaction) relative to total area was obtained through color segmentation analysis (Fig. 1). The percentage of immunopositive area was calculated separately for each stromal and follicular compartment (granulosa, theca interna, and theca externa for antral follicles, granulosa and theca cell layers for large preantral follicles, and granulosa cell layer for small preantral, primary, and primordial follicles).
Statistical Analyses
The percentage of immunopositive area of each follicular compartment, follicle, or stroma was first measured and mean for each of these was computed and used as a measure from each animal. For all analyses, animal was the experimental unit. Student’s t test, ANOVA, and correlation analysis were carried out using Prism software (version 7.0, GraphPad Software, La Jolla, CA). Changes in the granulosa cell layer across follicular stages from the FD140, year 1, and year 2 control animals and between control and prenatal treatment groups at FD90 and FD140 and postpubertal year 1 were compared by ANOVA, followed by Tukey’s post hoc test. A Student’s t test was used when comparison was restricted to primordial and primary follicles in FD90, antral follicles in year 1 and year 2, and between control and prenatal T-treated group at year 2. A p < 0.05 value was considered significant.
Results
Antibody Specificity
Results from Western blot analyses of ovarian tissue homogenate, immunohistochemical recognition of the ovarian section in the presence and absence of the primary antibody, and image analysis (capture and mask) of SF1, DAX1, and FOXO3 are summarized in Fig. 1. Western blot analysis revealed positive bands of appropriate sizes for each of the proteins. Specificity of the antibody is evident from the absence of staining when incubated without primary antibodies. The immunohistochemical stain was captured by applying a mask for the brown stain produced by immunoperoxide reaction with DAB using the Image Pro Plus software.
Developmental Changes in SF1, DAX, and FOXO3 Proteins
Immunohistochemical localization and expression levels of SF1, DAX1, and FOXO3 in different follicular stages at each time point from control animals are shown in Fig. 2. All three proteins were detectable in all follicular classes and in the stromal cells at all ages. There were no differences in granulosa cell SF1 expression across follicular stages in FD90, FD140, and year 2 animals (Fig. 2). In contrast, SF1 expression was lower in granulosa cells of large preantral and antral compared with primary follicles in year 1 animals (Fig. 2). DAX1 was higher in primary compared with primordial follicles at FD90 and primary compared with all other follicle classes in year 1 (Fig. 2). No differences in DAX1 were evident between follicular classes at FD140 and year 2. In contrast to SF1 and DAX1, granulosa cell expression of FOXO3 did not differ across follicular stages at all the time points studied (Fig. 2).
Left panels, photomicrographs showing the immunolocalization of SF1, DAX1, and FOXO3 in the primordial, primary, preantral, and antral follicles from control animals at year 2 age. Right panels, mean ± SEM of follicular stage–dependent and stromal changes in relative protein expression from control sheep at FD90, FD140, year 1, and year 2. Different superscripts indicate significant differences within the given age group as determined by one-way ANOVA followed by Tukey’s multiple comparison test. NP indicates these follicular classes are not present in these fetal ages
In addition, the following age-dependent changes were observed: (1) higher expression of SF1 in granulosa cells from primordial and primary follicles at FD140, year 1, and year 2 groups compared with FD90; (2) progressive increase in DAX1 protein expression in granulosa cells from primordial follicles from FD90 to year 1; (3) higher DAX1 expression in primary and large preantral follicular granulosa cells in years 1 and 2 compared with the two fetal time points; and (4) progressive age-dependent increase in DAX1 expression in granulosa cells of small preantral follicles and the stroma (Fig. 2; statistical differences not incorporated in figure to retain clarity of stage-specific changes).
Effect of Prenatal T-Treatment on Expression of SF1, DAX1, and FOXO3
Prenatal T- or DHT-treatment had no effect on SF1, DAX1, and FOXO3 protein expression in the granulosa cells of primordial and primary follicles or stromal cells at FD90 (data not shown). At FD140, barring reduced levels of SF1 in theca cells from large preantral follicles, no other effect of prenatal T-treatment was observed in granulosa cells from all follicle classes or stromal cells (Figs. 3 and 4). As opposed to lack of effect on SF1, prenatal T-treatment increased DAX1 expression in granulosa cells of primordial, primary, and small and large preantral and theca cells of large preantral follicles (Figs. 3 and 4). In contrast, the prenatal DHT-treatment-induced increase in granulosa cell DAX1 protein was restricted to small preantral follicles (Figs. 3 and 4). Neither prenatal treatment had any effect on DAX1 protein content in the stromal cells at FD140. FOXO3 protein content was only found to be higher in the theca cells from small and large preantral follicles by prenatal T-treatment (Fig. 4).
Mean ± SEM of relative protein expression of SF1, DAX1, and FOXO3 in the granulosa cells (GC) from primordial, primary, and preantral and theca cells (TC) from preantral follicular stages and stromal cells from control, prenatal T-, and prenatal DHT-treated FD140 ovaries. Four animals from each treatment group were studied. Asterisks indicate significant differences from control as determined by ANOVA followed by Tukey’s post hoc analysis
The immunohistochemical localization of SF1/DAX1/FOXO3 and results of densitometric analysis of the immunopositive area in ovaries from control, prenatal T- and DHT-treated year 1 animals are shown in Figs. 5 and 6. Year 2 results are shown in Table 1. At year 1, prenatal T-treatment increased granulosa cell expression of SF1 in small preantral, large preantral, and antral but not in primordial or primary follicles. Prenatal T also increased SF1 expression in theca cells of large preantral but not in antral follicular theca cells or stromal cells. However, the increase in SF1 expression in prenatal DHT-treated females was only evident in the granulosa cells at the antral stage. In contrast to SF1, no change in DAX1 or FOXO3 protein content was evident with either prenatal treatments in any of the follicular classes or cell types or stromal cells. As opposed to the impact of prenatal T on SF1 expression in year 1, prenatal T-treatment had no effect in year 2 on either SF1 or DAX1 protein content in the different follicular classes examined or in the stromal cells while increasing FOXO3 in the theca interna cells of antral follicles (Table 1).
Mean ± SEM of relative protein expression measured as percentage of immunopositive area for SF1, DAX1, and FOXO3 in the granulosa (GC) and theca cells (TC) from different follicular stages and stromal cells from control, prenatal T-, and prenatal DHT-treated from year 1 age sheep ovaries. Ti, theca interna; Te, theca externa. Four animals from each treatment group were studied. Asterisks indicate significant differences from control as determined by ANOVA followed by Tukey’s post hoc analysis
Correlation analysis of relative protein levels of SF1 and DAX1 in Fig. 7 revealed that SF1 and DAX1 were significantly correlated during both early (primordial and primary) and late (preantral and antral) follicular stages in control animals while this relationship was lost in animals that received either prenatal T- or DHT-treatment.
Discussion
The findings from this study show that both SF1 and DAX1 are expressed in the ovarian granulosa, theca, and stromal cells, and are impacted by prenatal T excess, in a follicular stage– and age-specific manner. Increased SF1 expression in postpubertal preantral and antral follicles and DAX1 in fetal primordial, primary, and preantral follicles likely underlie the prenatal T-induced changes in AMH expression, namely increased expression of AMH in antral follicles at postpubertal age [20] and reduced expression in non-growing and early transitional follicles (< 50% cuboidal granulosa cells) in fetal age, [19] respectively. The directionality of prenatal androgen excess–mediated changes in SF1 and DAX1 suggests that they are likely targets via which changes in AMH expression and consequent increases in follicular recruitment and arrest are facilitated. These findings, coupled with lack of changes in FOXO3 protein, suggest that disruptions in SF1/DAX1-dependent AMH expression may account for the follicular developmental defects observed in the prenatal T-treated sheep.
Developmental Changes in SF1, DAX1, and FOXO3 Expression
The findings from this study of SF1 and DAX1 detection are consistent with reported findings in the sheep [31] and rodent ovary, [44] respectively. However, this study is the first to report follicular stage–dependent changes in both SF1 and DAX1 expression. To our knowledge, this is also the first report that FOXO3 is detectable in all follicular stages from fetal and adult ovaries in the sheep. The lack of changes in FOXO3 expression with follicular development in sheep contrasts with the observation in pigs where staining for FOXO3 was stronger in secondary compared with primordial follicles [45].
Consistent with the age-dependent increase in SF1 observed in sheep ovaries, in the mice SF1 transcripts increased in fetal ovary during late- compared with mid-gestation [46] and with age during postnatal period [47]. The contribution for this age-dependent regulation of SF1 gene in the ovary is not known. In both mice and sheep fetuses, SF1 expression is present in the bipotential gonad and disappears in the ovary after sexual differentiation [46, 48], suggesting a potential steroidal regulation of SF1 expression. Induction of SF1 expression by estrogen analogue tamoxifen activation of membrane-bound estrogen receptor G protein–coupled estrogen receptor (GPER) in the endometrial cells [49] supports a role for estradiol in regulation of SF1 expression. The increase in estradiol occurring developmentally from (1) steroidogenically active fetal ovaries [50], (2) during the onset of puberty [51], (3) increase in antral follicular number after birth through the postpubertal period [52], and (4) cyclic changes during the postpubertal ages [53] support the role for estradiol-mediated age-dependent changes in SF1 expression.
Like SF1, the age-dependent regulation of DAX1 is not known. As insulin stimulates DAX1 expression in MA-10 mouse Leydig cell lines [54], developmental changes in insulin action may account for age-related changes in DAX1 expression. Low levels of insulin in fetal stage and higher levels in lambs and adult sheep [55, 56] may account for low DAX1 in fetal stages and higher DAX1 in postpubertal and adult ages. These observations suggest that the endocrine changes associated with puberty may contribute to the changes in SF1 and DAX1 expression in the ovary, a premise that needs further exploration.
Impact of Prenatal Androgen Excess on SF1, DAX1, and FOXO3 Expression
The reduction in SF1 content in the theca cells of large preantral follicles from prenatal T- but not DHT-treated sheep suggests that this reduction is likely mediated by estrogenic actions arising from aromatization of T to estradiol [57]. These findings are consistent with the observations of reduced SF1 in rat fetal testicular interstitial cells (theca cell equivalent in the males) following synthetic estrogen treatment [58]. As such, the increased SF1 content in the antral granulosa cells of prenatal DHT-treatment may stem from metabolism of DHT to 3β-diol, an estrogen receptor β agonist [59]. Alternatively, T induced recruitment of co-factors such as aryl hydrocarbon receptor and/or stimulated extracellular regulated kinases which enhance T’s transcriptional activity but does not appear to occur with DHT-treatment [60] and may underlie the difference in T versus DHT action on SF1 expression.
The lack of effect of prenatal T on SF1 expression at year 2 as opposed to year 1 may reflect the anovulatory status of the prenatal T-treated animals [61] and change in the balance of androgen and estrogen receptor expression in the year 2 ovaries [39]. During achievement of follicular dominance, repression in androgen receptor expression and increase in estrogen receptor beta expression [62] facilitate the follicular dependency on estrogen [63, 64]. The increased expression of androgen receptor and altered ratio of estrogen receptors alpha and beta at this age [39] indicate that prenatal T-treatment leads to increased androgen and reduced estrogenic action and a subsequent block in SF1 increase.
At FD140, DAX1 unlike SF1 was increased by prenatal T in the granulosa cells of all follicle types detectable and in the theca cells of the large preantral follicles, while DHT only increased DAX1 in the granulosa cells of small preantral follicles. This increase in DAX1 is also consistent with androgen-induced increase in DAX1 in ovaries from rainbow trout [65] and the frog [66]. These data as well as the observation that both prenatal T- and DHT-treatment induced selective increase in androgen receptor in the granulosa cells of FD140 sheep ovaries [39] suggest that the induction of DAX1 expression occurs in an androgen-dependent manner in the fetal ovary.
The effect of prenatal T- and DHT-treatment on ovarian FOXO3 expression is not known. The results from this study showing that prenatal T- but not DHT-treatment increased FOXO3 expression in the theca cells of preantral follicles at FD140 and theca externa of antral follicles at year 2 indicate that estrogenic actions of T or T-dependent recruitment of additional co-factors or stimulation of kinases may be responsible. As FOXO3 is associated with increased expression of pro-apoptotic genes in the follicles [36], the increased FOXO3 in antral follicular theca cells is not consistent with the decreased pro-apoptotic proteins BCL2 associated X (BAX) and caspase 3 in these cells [41]. It therefore appears that in prenatal T-treated sheep, FOXO3 may not be a major contributor to the observed increase in follicular recruitment or antral follicle arrest.
Relationship Between SF1, DAX1, and AMH
DAX1 is identified as target of SF1’s transcription activity in the mouse testes with multiple SF1 binding sites on the DAX1 promoter [67]. Consistent with this, a significant correlation exists between SF1 and DAX1 expression in the sheep ovary from control animals (Fig. 7). Absence of such association in prenatal T- or DHT-treated animals suggests that prenatal T- or DHT-treatment disrupts transcriptional regulation of DAX1 by SF1 protein. The absence of transcriptional relationship between SF1 and DAX1 may be a function of changes in androgen, estrogen, and insulin as prenatal T- or DHT-treatment disrupts the intra-follicular estrogen to androgen ratio [68], and peripheral insulin levels [69].
The presence of two binding elements for SF1 on the AMH promoter [70] and requirement of SF1 binding sites for AMH expression in sheep granulosa cells [33] provide evidence that SF1 transcriptionally mediates AMH expression. The fact that DAX1 functions by antagonizing the transactivation of SF1 and therefore represses SF1 target genes [34] indicates that increases in DAX1 negatively affect AMH expression. This relationship between SF1 and DAX1 in regulation of AMH has been clearly demonstrated in testicular cells [30]. Consistent with this, the directionality of prenatal T-induced changes in expression of AMH protein [19, 20] appears to parallel the changes in prenatal T-induced increase in DAX1 and SF1, respectively (Fig. 8). The increase in DAX1 protein in primordial, primary, and preantral follicles in ovaries from prenatal T-treated sheep evident from the present study parallels the lower AMH observed in these follicular types from prenatal T-treated sheep [19, 20]. Similarly, the increased SF1 in the granulosa cells of antral follicles evident during year 1 in the present study matches the increased AMH in the granulosa cells of antral follicles at the same developmental time point [20]. These findings indicate that the SF1 to DAX1 ratio may underlie the changes in granulosa cell AMH content. As both SF1 and DAX1 are co-expressed in multiple organs including ovary [71], a close functional relationship between the two can be expected. However, as DAX1 represses SF1 transactivation [34], the ratio between the two proteins may dictate the transcriptional activation or repression of the SF1 target genes. This relationship has been shown to dictate the SF1-mediated steroidogenic gene expression in the adrenocortical cell lines [72]. The pattern of changes in SF1 and DAX1 (this study), and AMH [19, 20] in prenatal T-treated sheep ovaries also suggests that the ratio of SF1 and DAX1 may influence the AMH expression. A lower SF1 to DAX1 ratio in early growing follicles may govern a lower expression of AMH, while the higher ratio in antral follicles may lead to increase in AMH expression (Fig. 8). These findings suggest that disruptions in the ratio of SF1 and DAX1 may underlie PCOS ovarian phenotype in prenatal T-treated female sheep.
Schematic showing the relationship between changes in ovarian SF1, DAX 1, and FOXO3 expression with previously published changes in AMH at FD14019 and year 1 (20 the same cohort of animals as used in this study) in prenatal T-treated animals as a function of ovarian follicular recruitment and persistence
Translational Significance
As prenatal T-treated female sheep manifest reproductive defects similar to those observed in PCOS women [6], the findings from this study of prenatal T-induced changes in SF1 and DAX1, and lack of changes in FOXO3 are of translational value. Although there is currently no evidence of genetic defects in SF1 and DAX1 in PCOS women [73], they may contribute to the development of PCOS due to their role in regulating steroidogenic genes [74] and AMH expression [30]. As PCOS is a condition associated with hyperandrogenism and elevated AMH [75, 76] and the importance of SF1 and DAX1 in inducing the hyperandrogenic state in PCOS women is recognized [77], it is surprising that changes in SF1 or DAX1 in PCOS ovaries have not been fully characterized. In one study, theca cells from PCOS women were found to have increased activity of promoter containing SF1 binding sites [78] indicative of a potential increase in SF1 expression also in PCOS ovaries. The changes in FOXO3 protein in PCOS women ovaries under basal states are not known but higher levels of FOXO3 mRNA and protein are found in granulosa cells derived from PCOS women undergoing gonadotropin stimulation for in vitro fertilization [79]. Whether gonadotropin stimulation would similarly increase FOXO3 in prenatal T-treated animals remains to be determined.
A limitation to consider is whether the changes in the protein content of SF1 and DAX1 assessed in this study are also reflected at their transcriptional activity. While we see a corresponding increase in AMH with increase in SF1 in postpubertal ovaries from prenatal T-treated animals [20], there was no corresponding increase in steroidogenic genes aromatase or 17 alpha hydroxylase in this model [40]. Whether SF1 or DAX1 changes are associated with differential transcriptional activity on the steroidogenic genes versus AMH needs further exploration. Nevertheless, the changes observed in SF1 and DAX1 reflect the changes in AMH and can potentially explain the increased follicular recruitment. Together with increased AMH, and lack of changes in FOXO3, our findings may help to explain the follicular arrest seen in both prenatal T-treated sheep and PCOS women.
References
Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487–525.
Conway G, Dewailly D, Diamanti-Kandarakis E, et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. European journal of endocrinology / European Federation of Endocrine Societies 2014;171(4):P1–29.
Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057.
Puttabyatappa M, Cardoso RC, Padmanabhan V. Effect of maternal PCOS and PCOS-like phenotype on the offspring’s health. Mol Cell Endocrinol. 2016;435:29–39.
Abbott DH, Barnett DK, Levine JE, et al. Endocrine antecedents of polycystic ovary syndrome in fetal and infant prenatally androgenized female rhesus monkeys. Biol Reprod. 2008;79(1):154–63.
Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373(1–2):8–20.
Maliqueo M, Benrick A, Stener-Victorin E. Rodent models of polycystic ovary syndrome: phenotypic presentation, pathophysiology, and the effects of different interventions. Semin Reprod Med. 2014;32(3):183–93.
Padmanabhan V, Veiga-Lopez A. Reproduction symposium: developmental programming of reproductive and metabolic health. J Anim Sci. 2014;92(8):3199–210.
Cardoso RC, Puttabyatappa M, Padmanabhan V. Steroidogenic versus metabolic programming of reproductive neuroendocrine, ovarian and metabolic dysfunctions. Neuroendocrinology. 2015;102(3):226–37.
Dewailly D, Lujan ME, Carmina E, Cedars MI, Laven J, Norman RJ, et al. Definition and significance of polycystic ovarian morphology: a task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2014;20(3):334–52.
Maciel GA, Baracat EC, Benda JA, et al. Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89(11):5321–7.
Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology. 2007;148(7):3532–40.
Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146(7):3185–93.
Smith P, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve, and ovarian morphology in sheep. Biol Reprod. 2009;80(4):726–36.
Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11(5):461–71.
Gougeon A. Human ovarian follicular development: from activation of resting follicles to preovulatory maturation. Ann Endocrinol. 2010;71(3):132–43.
Dumont A, Robin G, Catteau-Jonard S, Dewailly D. Role of anti-Mullerian hormone in pathophysiology, diagnosis and treatment of polycystic ovary syndrome: a review. Reprod Biol Endocrinol. 2015;13:137.
Stubbs SA, Hardy K, Da Silva-Buttkus P, et al. Anti-Mullerian hormone protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. J Clin Endocrinol Metab. 2005;90(10):5536–43.
Bull L, Stubbs S, Birch R, et al. Reduced expression of anti-Mullerian hormone (AMH) protein in the androgenised sheep ovary. 2004.
Veiga-Lopez A, Ye W, Padmanabhan V. Developmental programming: prenatal testosterone excess disrupts anti-Müllerian hormone expression in preantral and antral follicles. Fertil Steril. 2012;97(3):748–56.
Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140(12):5789–96.
Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, et al. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143(3):1076–84.
Lebbe M, Woodruff TK. Involvement of androgens in ovarian health and disease. Mol Hum Reprod. 2013;19(12):828–37.
Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301(5630):215–8.
Puttabyatappa M, Padmanabhan V. Ovarian and extra-ovarian mediators in the development of polycystic ovary syndrome. J Mol Endocrinol. 2018.
Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14(4):367–78.
Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, et al. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142(11):4891–9.
van Houten EL, Themmen AP, Visser JA. Anti-Mullerian hormone (AMH): regulator and marker of ovarian function. Ann Endocrinol. 2010;71(3):191–7.
Salmon NA, Handyside AH, Joyce IM. Oocyte regulation of anti-Mullerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev Biol. 2004;266(1):201–8.
Rey R, Lukas-Croisier C, Lasala C, Bedecarras P. AMH/MIS: what we know already about the gene, the protein and its regulation. Mol Cell Endocrinol. 2003;211(1–2):21–31.
Logan KA, Juengel JL, McNatty KP. Onset of steroidogenic enzyme gene expression during ovarian follicular development in sheep. Biol Reprod. 2002;66(4):906–16.
Mizutani T, Kawabe S, Ishikane S, Imamichi Y, Umezawa A, Miyamoto K. Identification of novel steroidogenic factor 1 (SF-1)-target genes and components of the SF-1 nuclear complex. Mol Cell Endocrinol. 2015;408:133–7.
Estienne A, Pierre A, di Clemente N, Picard JY, Jarrier P, Mansanet C, et al. Anti-Mullerian hormone regulation by the bone morphogenetic proteins in the sheep ovary: deciphering a direct regulatory pathway. Endocrinology. 2015;156(1):301–13.
Iyer AK, McCabe ER. Molecular mechanisms of DAX1 action. Mol Genet Metab. 2004;83(1–2):60–73.
Li T, Mo H, Chen W, Li L, Xiao Y, Zhang J, et al. Role of the PI3K-Akt signaling pathway in the pathogenesis of polycystic ovary syndrome. Reprod Sci. 2017;24(5):646–55.
Brosens JJ, Wilson MS, Lam EW. FOXO transcription factors: from cell fate decisions to regulation of human female reproduction. Adv Exp Med Biol. 2009;665:227–41.
Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects; partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology. 2006;147(4):1997–2007.
Ortega HH, Rey F, Velazquez MM, Padmanabhan V. Developmental programming: effect of prenatal steroid excess on intraovarian components of insulin signaling pathway and related proteins in sheep. Biol Reprod. 2010;82(6):1065–75.
Ortega HH, Salvetti NR, Padmanabhan V. Developmental programming: prenatal androgen excess disrupts ovarian steroid receptor balance. Reproduction. 2009;137(5):865–77.
Padmanabhan V, Salvetti NR, Matiller V, Ortega HH. Developmental programming: prenatal steroid excess disrupts key members of intraovarian steroidogenic pathway in sheep. Endocrinology. 2014;155(9):3649–60.
Salvetti NR, Ortega HH, Veiga-Lopez A, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on ovarian cell proliferation and apoptotic factors in sheep. Biol Reprod. 2012;87(1):22 21-10.
Ortega HH, Veiga-Lopez A, Sreedharan S, del Lujan Velazquez MM, Salvetti NR, Padmanabhan V. Developmental programming: does prenatal steroid excess disrupt the ovarian VEGF system in sheep? Biol Reprod. 2015;93(3):58.
Lejeune M, Jaen J, Pons L, et al. Quantification of diverse subcellular immunohistochemical markers with clinicobiological relevancies: validation of a new computer-assisted image analysis procedure. J Anat. 2008;212(6):868–78.
Richards JS, Russell DL, Ochsner S, et al. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res. 2002;57:195–220.
Ding W, Wang W, Zhou B, Zhang W, Huang P, Shi F, et al. Formation of primordial follicles and immunolocalization of PTEN, PKB and FOXO3A proteins in the ovaries of fetal and neonatal pigs. J Reprod Dev. 2010;56(1):162–8.
Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol. 1994;8(5):654–62.
Hanley NA, Ikeda Y, Luo X, Parker KL. Steroidogenic factor 1 (SF-1) is essential for ovarian development and function. Mol Cell Endocrinol. 2000;163(1–2):27–32.
Valenzuela N, Neuwald JL, Literman R. Transcriptional evolution underlying vertebrate sexual development. Dev Dyn. 2013;242(4):307–19.
Lin BC, Suzawa M, Blind RD, et al. Stimulating the GPR30 estrogen receptor with a novel tamoxifen analogue activates SF-1 and promotes endometrial cell proliferation. Cancer Res. 2009;69(13):5415–23.
Quirke LD, Juengel JL, Tisdall DJ, Lun S, Heath DA, McNatty KP. Ontogeny of steroidogenesis in the fetal sheep gonad. Biol Reprod. 2001;65(1):216–28.
Ryan KD, Goodman RL, Karsch FJ, Legan SJ, Foster DL. Patterns of circulating gonadotropins and ovarian steroids during the first periovulatory period in the developing sheep. Biol Reprod. 1991;45(3):471–7.
Rawlings NC, Evans AC, Honaramooz A, Bartlewski PM. Antral follicle growth and endocrine changes in prepubertal cattle, sheep and goats. Anim Reprod Sci. 2003;78(3–4):259–70.
Baird DT. Pulsatile secretion of LH and ovarian estradiol during the follicular phase of the sheep estrous cycle. Biol Reprod. 1978;18(3):359–64.
Ahn SW, Gang GT, Kim YD, Ahn RS, Harris RA, Lee CH, et al. Insulin directly regulates steroidogenesis via induction of the orphan nuclear receptor DAX-1 in testicular Leydig cells. J Biol Chem. 2013;288(22):15937–46.
Aldoretta PW, Carver TD, Hay WW Jr. Maturation of glucose-stimulated insulin secretion in fetal sheep. Biol Neonate. 1998;73(6):375–86.
Bassett JM, Alexander G. Insulin, growth hormone and corticosteroids in neonatal lambs. Normal concentrations and the effects of gold. Biol Neonate. 1971;17(1):112–25.
Padmanabhan V, Veiga-Lopez A. Developmental origin of reproductive and metabolic dysfunctions: androgenic versus estrogenic reprogramming. Semin Reprod Med. 2011;29(3):173–86.
Majdic G, Sharpe RM, Saunders PT. Maternal oestrogen/xenoestrogen exposure alters expression of steroidogenic factor-1 (SF-1/Ad4BP) in the fetal rat testis. Mol Cell Endocrinol. 1997;127(1):91–8.
Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm Behav. 2008;53(5):741–52.
Wu Y, Baumgarten SC, Zhou P, Stocco C. Testosterone-dependent interaction between androgen receptor and aryl hydrocarbon receptor induces liver receptor homolog 1 expression in rat granulosa cells. Mol Cell Biol. 2013;33(15):2817–28.
Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144(4):1426–34.
Cheng G, Weihua Z, Makinen S, et al. A role for the androgen receptor in follicular atresia of estrogen receptor beta knockout mouse ovary. Biol Reprod. 2002;66(1):77–84.
Britt KL, Findlay JK. Estrogen actions in the ovary revisited. J Endocrinol. 2002;175(2):269–76.
Drummond AE. The role of steroids in follicular growth. Reprod Biol Endocrinol. 2006;4:16.
Baron D, Houlgatte R, Fostier A, Guiguen Y. Expression profiling of candidate genes during ovary-to-testis trans-differentiation in rainbow trout masculinized by androgens. Gen Comp Endocrinol. 2008;156(2):369–78.
Sugita J, Takase M, Nakamura M. Expression of Dax-1 during gonadal development of the frog. Gene. 2001;280(1–2):67–74.
Hoyle C, Narvaez V, Alldus G, Lovell-Badge R, Swain A. Dax1 expression is dependent on steroidogenic factor 1 in the developing gonad. Mol Endocrinol. 2002;16(4):747–56.
Veiga-Lopez A, Moeller J, Abbott DH, Padmanabhan V. Developmental programming: rescuing disruptions in preovulatory follicle growth and steroidogenesis from prenatal testosterone disruption. J Ovarian Res. 2016;9(1):39.
Padmanabhan V, Veiga-Lopez A, Abbott D, Recabarren S, Herkimer C. Developmental programming: impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology. 2010;151(2):595–605.
Shen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA. Nuclear receptor steroidogenic factor 1 regulates the mullerian inhibiting substance gene: a link to the sex determination cascade. Cell. 1994;77(5):651–61.
Ikeda Y, Takeda Y, Shikayama T, Mukai T, Hisano S, Morohashi KI. Comparative localization of Dax-1 and Ad4BP/SF-1 during development of the hypothalamic-pituitary-gonadal axis suggests their closely related and distinct functions. Dev Dyn. 2001;220(4):363–76.
Ragazzon B, Lefrancois-Martinez AM, Val P, et al. Adrenocorticotropin-dependent changes in SF-1/DAX-1 ratio influence steroidogenic genes expression in a novel model of glucocorticoid-producing adrenocortical cell lines derived from targeted tumorigenesis. Endocrinology. 2006;147(4):1805–18.
Goodarzi MO. Genetics of PCOS. The Polycystic Ovary Syndrome: Current Concepts on Pathogenesis and Clinical Care 2007;27:29.
Bakke M, Zhao L, Hanley NA, Parker KL. SF-1: a critical mediator of steroidogenesis. Mol Cell Endocrinol. 2001;171(1–2):5–7.
Garg D, Tal R. The role of AMH in the pathophysiology of polycystic ovarian syndrome. Reprod BioMed Online. 2016;33(1):15–28.
Wickenheisser JK, McAllister JM. Ovarian steroidogenic abnormalities in PCOS. The Polycystic Ovary Syndrome: Current Concepts On Pathogenesis And Clinical Care: Springer; 2007:69–84.
Calvo RM, Asuncion M, Telleria D, Sancho J, San Millan JL, Escobar-Morreale HF. Screening for mutations in the steroidogenic acute regulatory protein and steroidogenic factor-1 genes, and in CYP11A and dosage-sensitive sex reversal-adrenal hypoplasia gene on the X chromosome, gene-1 (DAX-1), in hyperandrogenic hirsute women. J Clin Endocrinol Metab. 2001;86(4):1746–9.
Wickenheisser JK, Quinn PG, Nelson VL, Legro RS, Strauss JF 3rd, McAllister JM. Differential activity of the cytochrome P450 17alpha-hydroxylase and steroidogenic acute regulatory protein gene promoters in normal and polycystic ovary syndrome theca cells. J Clin Endocrinol Metab. 2000;85(6):2304–11.
Mikaeili S, Rashidi BH, Safa M, Najafi A, Sobhani A, Asadi E, et al. Altered FoxO3 expression and apoptosis in granulosa cells of women with polycystic ovary syndrome. Arch Gynecol Obstet. 2016;294(1):185–92.
Acknowledgments
We are grateful to Mr. Douglas Doop for his expert animal care, facility management, and help with generation of the experimental lambs; Drs. Mohan Manikkam, Teresa Steckler, and Almudena Veiga-Lopez, Ms. Olga Astapova, Ms. Carol Herkimer, and Mr. James Lee for their assistance with prenatal steroid treatment and help during collection of ovaries or with sectioning ovaries; and Dr. Angela S Kelley for reading and editing the manuscript.
Funding
This work was financially supported by National Institutes of Health grant P01 HD44232.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal care and procedures were performed under the approval of the Institutional Animal Care and Use Committee of the University of Michigan consistent with the National Research Council’s Guide for the Care and Use of Laboratory Animals
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Puttabyatappa, M., Matiller, V., Stassi, A.F. et al. Developmental Programming: Prenatal Testosterone Excess on Ovarian SF1/DAX1/FOXO3. Reprod. Sci. 27, 342–354 (2020). https://doi.org/10.1007/s43032-019-00029-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-019-00029-0