Abstract
Determining the contexts of emission and information content of vocal signals can yield insights into the function of different call types, and remains an important step towards understanding the diversification of mammalian vocal repertoires. In this study, we used infra-red video cameras and remote audio recorders to document seasonal and contextual variation in male European badger (Meles meles) churr production over a 24-month period, and acoustic analysis based on source-filter theory to examine whether churr acoustic structure varies according to the caller’s arousal state and identity. Our behavioural observations revealed that male churrs are produced almost exclusively during the breeding season. Further contextual analysis showed that males emit churrs during close-range interactions with female conspecifics, often during copulation attempts, and churr directly into sett entrances. In addition, males involved in close-range social interactions delivered churrs with more call units per second than those vocalising without other conspecifics in close proximity. Discriminant function analysis also revealed that male churrs are individually distinctive, and confirmed that the formants (vocal tract resonances) contribute the most to caller identity. These findings indicate that badger churrs are sexual calls with the potential to signal male arousal state and identity in reproductive contexts. They also add to an increasing body of literature on the importance of formants for identity cueing in nonhuman mammals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Documenting seasonal variation in vocal behaviour and the contexts in which vocal signals are produced can provide important insights into the functional relevance of different call types and their underlying motivational basis. For example, exclusive use of a specific vocalisation during the breeding season (Clutton-Brock and Albon 1979; Ellis et al. 2011) or when males are actively courting and/or copulating with females (Grady and Hoogland 1986; Manno et al. 2007) is consistent with a sexual role, while the production of vocalisations when other conspecifics are not in close vicinity may indicate a contact promoting function (Buesching et al. 1998; Frommolt et al. 2003; Harrington and Mech 1979; McComb et al. 2000). In addition, vocal distinctiveness is likely to be adaptive for animals that live in social groups (Charrier et al. 2001; Insley 2000; McComb et al. 2000), and particularly for territorial social species that often need to discriminate between familiar individuals and strangers (Hardouin et al. 2006; Harrington and Mech 1979).
In the United Kingdom, European badgers (Meles meles) live in social groups of up to 25 individuals (Neal and Cheeseman 1996) within well-defined ranges that are centred on a communal den (or sett) (Macdonald et al. 2015). Olfaction is thought to be a key sensory modality for moderating social interactions (Buesching and Macdonald 2004; Buesching et al. 2003; Fell et al. 2006) and demarcating territories in this species (Buesching and Jordan 2019; Buesching et al. 2002a; Davies et al. 1988; Kruuk et al. 1984; Macdonald et al. 2015; Roper et al. 1986; Tinnesand et al. 2015); however, the European badger also has a diverse vocal repertoire that is likely to be important for mediating close-range interactions (Wong et al. 1999). Adult badgers hiss, snarl, bark, growl and kecker during agonistic interactions, and produce a range of chitters, yelps and purrs in affiliative social contexts (Wong et al. 1999). In addition, males are reported to ‘churr’ when sexually aroused (Wong et al. 1999). Male churrs consist of discrete call units that convey an “oily, bubbling” quality to the calls (Christian 1993; Wong et al. 1999). The behavioural context that male badgers produce churrs implies a sexual function; however, whether call production varies according to season and social context remains an open question. It has also been suggested that male churrs could facilitate individual recognition and/or the transfer of information on male quality in mate choice contexts (Wong et al. 1999). Despite this contention, it is not known whether male churrs, or any of the European badger’s vocalisations, encode information about the caller of potential relevance to other conspecifics.
The source-filter theory (Fant 1960) allows researchers to make informed predictions about which acoustic characteristics could potentially provide receivers with reliable information about the caller, because it explicitly links vocal signal production mechanisms to the acoustic output (Taylor et al. 2016). This theory states that mammal vocal signals are produced when air expelled from the lungs is converted to acoustic energy by the larynx, termed the source (Fant 1960). The rate at which the vocal folds in the larynx open and close determines the fundamental frequency (F0) of the vocalisation and the supra-laryngeal vocal tract acts as a spectral filter, selectively enhancing certain frequencies called formants (Titze 1994). The geometric shape of the vocal tract determines the frequency values of the formants, with longer vocal tracts producing lower, more closely spaced formants (Fitch 1997; Reby and McComb 2003).
Source-related features of nonhuman mammal vocalisations are often dynamically varied to signal short-term changes in motivational state. For instance, previous work on nonhuman mammals has shown that highly aroused callers tend to produce longer duration calls with higher F0 (Briefer et al. 2015; Rendall 2003; Soltis et al. 2005; Stoeger et al. 2011, 2012). In addition, while inter-individual differences in laryngeal and vocal tract morphology make both source- and filter-related acoustic characteristics likely to yield information on a given caller’s identity, several recent studies have emphasised the importance of formants as cues to individual identity in nonhuman mammals (Charlton et al. 2009, 2011; Charlton 2014; Furuyama et al. 2016; McComb et al. 2003; Reby et al. 2006; Rendall 2003; Townsend et al. 2014; Vannoni and McElligott 2007). Badger churrs contain discrete pulses with energy across a broad frequency range (Wong et al. 1999), making them well suited for highlighting inter-individual differences in formant frequency pattern (Charlton et al. 2013; Fitch and Hauser 1995). Furthermore, because the formant pattern of male badger churrs should directly reflect individual differences in vocal tract length and shape, it is likely that formants will provide reliable information about the caller’s identity in the European badger, as they do in other mammals (Taylor et al. 2016).
The goals of this study were (1) to document seasonal and contextual variation in the production of male European badger churrs, (2) to investigate whether the acoustic structure of churrs differs when males are involved in close-range interactions (high arousal) as opposed to vocalising alone (low arousal), (3) to determine whether male badger churrs are individually distinctive, and (4) to investigate the relative importance of different acoustic features for coding individuality. Based on the premise that male churrs are sexual calls (Wong et al. 1999), we expected churrs to be exclusively produced during the breeding season. We had no strong a priori prediction for the effect of behavioural context on male churr production, however, we did expect that churrs produced during close range interactions would be characterised by longer duration and higher F0, simply due to the heightened arousal state associated with this context. Finally, we also predicted that male churrs would be individually distinctive, and that the formant frequencies would contribute the most to individual identity (as found for other mammals: Taylor et al. 2016).
Methods
Study site, animals and handling procedures
This study was conducted on a free ranging and intensively studied population of European badgers at Wytham Woods, Oxfordshire, England (51:46:26 N; 1:19:19 W), (for more details about study site and population see Macdonald et al. 2015; Savill et al. 2010) between June 2016 and June 2018. Acoustic and behavioural data were collected for adult males spread across five social groups. As a part of an ongoing population study, all badgers at this site are trapped regularly under Home Office license PPL 30/3379 and Natural England licence 2019–38863-SCI-SCI. Captured animals are sedated using 0.2 ml/kg body weight ketamine hydrochloride (‘Ketamidor’; Chanelle Vet (UK) Ltd, Freemans House, 127A High Street, Hungerford, Berkshire, UK, RG17 0DL) for measuring and sampling, and given a permanent unique tattoo at first capture. For the purpose of this study, we used a measuring tape to measure the distance from the apex of the thyroid cartilage (which roughly corresponds to the position of the vocal folds in the larynx) to the lips for 27 sedated adult males, to establish an approximate vocal tract length (VTL) for our study population that would allow us to predict the expected number of formants in a given frequency range. All residents at the five focal setts received a unique fur-clip (Stewart and Macdonald 1997) to enable visual identification of individuals in video recordings that could then also be linked to audio recordings.
This study followed the ASAB/ABS guidelines for the use of animals in research, and was approved by the University of Oxford’s Natural England license 2014-5710-SCI-SCI, a Home Office license PPL 30/2385, and University College Dublin’s Animal Research Ethics Committee (AREC-E-16-15-Charlton).
Capture of acoustic data
Motion-detector-activated Crenova RD1000 infra-red video cameras (Crenova, USA) and Song Meter SM4 recorders (Wildlife Acoustics, Inc, Maynard, USA) were time synced and used to capture video and acoustic data, respectively. The video recordings (20 s/trigger event) were captured at full HD 1080p and 15 frames per second, which typically permitted vocalising animals to be identified from their fur-clipping patterns (Wong et al. 1999). The audio recorders were used to capture uncompressed recordings of male churrs (sampling rate: 16 kHz, amplitude resolution: 16 bits) that were then linked to the vocalising animals identified in the video recordings. The Song Meter SM4 recorders were placed at the centre of the setts, where most vocal activity was predicted to occur, and approximately 1 m from the ground. The recordings were transferred from SD cards to an Apple Macintosh Macbook computer, normalized to 100% peak amplitude and saved as WAV files (16 kHz sampling rate and 16 bits amplitude resolution). To minimise inter-observer variation, all behavioural and acoustic analyses were carried out by the lead author.
Definition of behavioural contexts
Badgers within 1 m of one another (roughly two body lengths apart) were considered to be involved in a close-range social interaction, and categorised as belonging to the social context (Fig. 1a). Focal animals were defined as solitary when they were the only individual observed in the video recording (Fig. 1b). We also noted whether focal animals were vocalising into the sett entrance or actively mounting/attempting to copulate with a female conspecific (Fig. 1d).
Behavioural contexts of male churring. Churrs were observed in social contexts (a) and when males were solitary (b). solitary churrs were often delivered into sett entrances (c) and males produced churrs during copulation attempts (d). The arrow in panel c points to the sett entrance. See methods for definitions of the behavioural contexts
Acoustic analyses
The audio processing was conducted using Praat v5.1.32 (www.praat.org). Recordings were initially segmented into separate vocalisations using the edit window and labelling facility in Praat and saved as individual sound files (.wav). Churrs could be distinguished from other badger vocalisations and background noises by viewing narrow band spectrograms (FFT method; window length 0.03 s; time steps = 250; frequency steps = 1000; Gaussian window shape; dynamic range = 45 dB) of the audio sequences captured by the SM4 recorders. A total of 126 recordings of male churrs with accompanying video footage of the behavioural context of call production were collected. Before conducting the acoustic analysis, we selected the best 10 recordings, with the highest signal to noise ratio, for each of 12 individuals. This gave us a total of 120 male churrs for the acoustic analysis; 46 churrs were produced in social contexts and 74 were produced in solitary contexts.
The mean ± SD vocal tract length (VTL) measured from 27 male badgers from our study population was 11.0 cm ± 3.33 (range: 10.5–11.8 cm). Because male churrs are delivered with a closed or partially closed mouth (Wong et al. 1999), the vocal tract could then be modelled as an 11.0 cm linear tube closed (or open) at both ends (i.e. a half-wave resonator: Titze 1994). Using this vocal tract model, the expected position of the first formant can be calculated using the following equation: F1 = c/2*VTL, in which c is the approximate speed of sound in the mammalian vocal tract (350 m/s) (Titze 1994). This gives us a predicted F1 value of = 1591 Hz. Formants F2–F5 are then predicted to occur at 3182 Hz (F2 = 2*F1), 4773 Hz (F3 = 3*F1), 6364 Hz (F4 = 4*F1), 7955 Hz (F5 = 5*F1), respectively. Initial inspection of spectrograms confirmed that five frequency bands exist below 8000 Hz that could represent formants (Fig. 2). As a result, the analysis was set to track and measure five formants in the frequency range 0–8000 Hz. Linear predictive coding (LPC; ‘To Formants (Burg)’ command in Praat) was used to measure the frequency values of the first five formant candidates using the following analysis parameters: time step: 0.01 s; window analysis: 0.03 s; maximum formant value: 6000–8000 Hz; maximum number of formants: 5; pre-emphasis: 50 Hz. To check if Praat was accurately tracking the formants, the outputs were compared with visual inspections of relevant spectrograms and power spectrums (using cepstral smoothing: 200 Hz). The average formant spacing (∆F) was then estimated using a regression method in which each formant value was plotted against its expected value (this method is covered in more detail by Reby and McComb 2003). Because F5 was often poorly defined and could not be consistently measured in all churrs, it was not included in the ∆F calculation or statistical analyses. The number of pulses per second (hereafter F0) was measured using the voice report facility in Praat with the following parameters: search range = 50–150 Hz, time step = 0.01, voicing threshold = 0.3. The Praat voice report facility provides the mean time period between pulses, from which F0 is calculated. In addition, we measured the number of call units per second and overall duration of the call directly from the waveform.
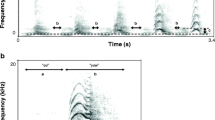
Acoustic structure of male European badger churrs. A waveform (a) and spectrogram (b) of two male churrs from a single male are presented. Male churrs consist of separate call units with each unit having clear pulses. c Five formants (labelled F1–5) can also be detected in the spectral acoustic structure. a Panel c depicts a close up of five call units: the third unit has five pulses denoted by arrows. Spectrogram settings: FFT method, window size = 0.01, Gaussian window shape, dynamic range = 45 dB
To further verify whether the spectral peaks derived from the LPC analysis were formants, we estimated the vocal tract length of one of the males in the analysis (M1663) using the following equation: eVTL = c/2ΔF where eVTL = vocal tract length, c is the approximate speed of sound in the mammalian vocal tract (350 m/s), and ΔF = formant spacing (Hz) (Titze 1994) to compare with the measured VTL for this individual. The measured VTL was 11.5 cm and the estimated VTL derived from the formant spacing was 11.1 cm. The close correspondence between the anatomically verified and estimated VTL, and the uneven spacing of these frequency components confirm that they are very likely to be formants (Fitch 2002).
Statistical analyses
We used general linear mixed models (GLMMs) fitted with maximum-likelihood estimation in R studio v1.1.463 (nlme package) to determine whether the acoustic structure of male churrs differed according to behavioural context. The acoustic measures were normally distributed (Shapiro–Wilk: > 0.05) and scatter plots were used to confirm homoscedasticity. For each GLMM, the mean acoustic values for each subject in the different contexts were entered as dependant variables, the context (social or solitary) was entered as a fixed factor, and the identity of the caller was entered as a random factor to control for uneven subject participation in the dataset. To determine whether churrs are individually distinctive, we then used IBM SPSS version 20 to conduct a discriminant function analysis (DFA) with subject identity as the group identifier, and the acoustic measures (duration, F0, call units per second, F1, F2, F3, F4, ∆F) entered as discriminant variables. For the DFA, both the reclassification and the more conservative leave-one-out cross-validation procedure were applied. In addition, to ensure the robustness of the classification, we pooled the results from 1000 bootstrap samples and used bias-corrected and accelerated confidence intervals (using the ‘Bootstrap…’ option in SPSS). The statistical significance of correct classification of individual callers across all subjects was obtained using the Chi square statistic (X2). The significance level was set at alpha = 0.05.
Results
Acoustic structure of male badger churrs
Figure 2 illustrates the acoustic structure of male European badger churrs. The mean ± SD duration of male churrs was 1.9 ± 0.7 s, and ranged between 0.7 and 3.7 s. Mean ± SD F0 was 84.8 Hz, ranging between 56.7 and 109.5 Hz, and call units per second ranged between 10.9 and 14.3, with a mean ± SD of 12.9 ± 1.0 per second, which corresponds well with the mean of 13.8 call units per second previously reported by Wong et al. (1999). Four observable formants could be consistently measured in the frequency range 0–8000 Hz (Fig. 2). Mean ± SD for F1–F4 and ∆F are as follows: F1 = 1876.8 ± 266.9 Hz; F2 = 2909.2 ± 252.7 Hz; F3 = 4604.4 ± 326.5 Hz; F4 = 6129.4 ± 276.8 Hz; ∆F = 1534.2 ± 63.3 Hz. The formants were static across the male churr call, indicating that very little articulation occurs during vocal production (Fig. 2).
Seasonal and contextual variation in churr production
Our behavioural observations revealed clear seasonal differences in churr production. Males produced churrs almost exclusively during the breeding season (Jan–Mar), with a marked decrease in churr production in March when compared to January and February (Fig. 3). Only two incidences of churring occurred outside of the breeding season, one in May and one in November (Fig. 3). The contextual analyses revealed that 60 male churrs were produced in social contexts (i.e. during close-range interactions with other conspecifics) and 66 churrs were delivered by solitary males (Fig. 3). In addition, 28% (17/60) of social churrs were produced when males attempted to copulate with females, and 27% (16/60) of solitary churrs were emitted directly into sett entrances.
Contextual and inter-individual differences in the acoustic structure of male churrs
The number of call units per second in male churrs significantly increased during close-range social interactions when compared to solitary contexts (F1, 11 = 166.04, P = 0.049) (Fig. 4). None of the other acoustic features differed significantly according to behavioural context (duration: F1, 11 = 0.59, P = 0.584; F0: F1, 11 = 9.25, P = 0.202; F1: F1, 11 = 0.06, P = 0.843; F2: F1, 11 = 12.74, P = 0.174; F3: F1, 11 = 1.17, P = 0.476; F4: F1, 11 = 4.15, P = 0.291; ∆F: F1, 11 = 1.02, P = 0.497) (Fig. 4). The acoustic structure of male churrs also varied according to the identity of callers, with 94.2% of churrs correctly classified to the 12 individual males. This classification level is statistically significant (X211 = 884.8, P < 0.001). When a more conservative leave-one-out cross validation was applied, the accuracy of classification to individual fell marginally to 83.3% but remained statistically significant (X211 = 674.5, P < 0.001). The univariate analysis showed that all the acoustic measures except call units per second differed significantly between individuals (Table 1). The structure matrix generated by the multivariate DFA confirmed that the main contributors to individual vocal distinctiveness were the formants and ∆F (Table 2). Table 1 also provides the variance explained by each of the discriminant factors and the loading of the acoustic measures on these factors.
Discussion
The results of this study show that male European badgers churr almost exclusively during the breeding season, which strongly indicates that these calls are linked to reproduction. The contextual analysis also revealed that males emit churrs during close-range interactions with female conspecifics, including copulation attempts, and often churr directly into sett entrances. Accordingly, we suggest that male European badgers use churrs to provide assurance to female mating partners of a nonaggressive intent, so that copulation can occur without aggressive escalation. The observation that males often churr into sett entrances suggests that these calls are also used to initiate contact with receptive females during the breeding season. While the precise function of male badger churrs will need to be established using playback experiments, the findings of the current study indicate that these calls are important for promoting close-range contact between the sexes to facilitate reproduction.
The prediction that churr duration and F0 would increase during close-range social interactions, due to heightened arousal state, was not supported. We did, however, find that the call units per second in male churrs increased during close-range interactions, which indicates that this may provide a cue to the caller’s arousal state. More highly aroused males could also have higher testosterone levels, which are linked to sperm quality and hence, fertilisation capacity in mammals (Minter and DeLiberto 2008). Vocal cues to testosterone-mediated arousal state may, therefore, be important in female mate choice contexts. Although female European badgers are induced ovulators (Yamaguchi et al. 2006) that are highly promiscuous (Dugdale et al. 2011), more highly aroused, high testosterone males could be most likely to trigger ovulation and ultimately impregnate females. As a result, it would prove adaptive for males to signal their high arousal state using churr vocalisations and for females to attend to this information. Future studies should test these predictions.
We also found that male churrs were individually distinctive, with 94% of calls correctly assigned to individual callers. Although individual vocal distinctiveness is documented in a wide range of mammals (Blumstein and Munos 2005; Reby et al. 1998, 1999, 2006; Rendall 2003; Semple 2001; Soltis et al. 2005), relatively few studies have revealed individual vocal distinctiveness in the Mustelidae. Work to date has only shown that highly social species, such as giant otters, Pteronura brasiliensis (Mumm and Knornschild 2014), Asian small-clawed otters, Aonyx cinerea (Lemasson et al. 2014) and Californian sea otters, Enhydra lutris (McShane et al. 1995) have individually distinctive vocalisations, although all social otter species appear to rely heavily on vocalisations for intra-specific information exchange and group cohesion (reviewed in Buesching and Stankowich 2017). Yet, none of these studies used a source-filter theory approach to identify formants and consider their potential role in identity cueing. Because formant frequencies and spacing are explicitly linked to the shape and size of the vocal tract, which should vary between individuals, they are expected to be individually distinctive. Consistent with our predictions, we found that the formants and ∆F of male badger churrs were highly individualised. The results of the current study, therefore, provide the first indication that formants are individually distinctive components of Mustelid vocalisations, as they are in humans and other nonhuman mammals (Bachorowski and Owren 1999; Owren et al. 1997; Reby et al. 2006; Rendall 2003).
The pulsatile quality of badger churrs is ideal for the auditory discrimination of formant frequencies because each of the discrete pulses contains energy across a broad frequency range, making it likely that individual differences in formant pattern are emphasised (Fitch 1997, 2002; Owren and Rendall 2001). Male vocal distinctiveness may also have fitness benefits for female badgers in mate choice contexts (East and Hofer 1991; Reby et al. 2001), complementing the well-reported olfactory distinctiveness of badger anal (Noonan et al. 2020) and subcaudal (Buesching et al. 2002a, b) gland secretions, where we posit that females could use acoustic cues to select less familiar, potentially more heterozygous males as mating partners (Radwan et al. 2008; Schwensow et al. 2008). Badger cubs sired by more heterozygous males are most likely to survive their first year (Annavi et al. 2014), and interbreeding between neighbouring groups occurs (Evans et al. 1989), with around 50% of cubs sired by non social group members in high-density populations (Macdonald et al. 2015). Accordingly, females could use churrs alongside olfactory cues to identify and preferentially mate with unfamiliar males, and in doing so, promote heterozygosity in offspring. Indeed, whether a given vocalisation’s specific information content is selected for per se, or arises due to differences in vocal production anatomy, we would expect receivers to attend to any available information when it is adaptive for them to do so. Playback experiments are now required to investigate whether female badgers can discriminate between churrs from males resident in their own versus unfamiliar social groups, and whether they use this ability to select more distantly related individuals as mating partners.
References
Annavi G, Newman C, Buesching CD, Macdonald DW, Burke T, Dugdale HL (2014) Heterozygosity–fitness correlations in a wild mammal population: accounting for parental and environmental effects. Ecol Evol 4:2594–2609
Bachorowski JA, Owren MJ (1999) Acoustic correlates of talker sex and individual talker identity are present in a short vowel segment produced in running speech. J Acoust Soc Am 106:1054–1063
Blumstein DT, Munos O (2005) Individual, age and sex-specific information is contained in yellow-bellied marmot alarm calls. Anim Behav 69:353–361
Briefer EF, Maigrot A-L, Mandel R, Freymond SB, Bachmann I, Hillmann E (2015) Segregation of information about emotional arousal and valence in horse whinnies. Sci Rep 4:9989–9911
Buesching CD, Jordan N (2019) The social function of latrines: a hypothesis-driven research approach. In: Buesching CD (ed) Chemical signals in vertebrates. Springer, Cham, pp 94–103
Buesching CD, Macdonald DW (2004) Variations in scent-marking behaviour of European badgers Meles meles in the vicinity of their setts. Acta Theriol 49:235–246
Buesching CD, Stankowich T (2017) Communication amongst the musteloids: signs, signals, and cues. In: Macdonald D, Newman C, Harrington LA (eds) Biology and conservation of the musteloids (badgers, otters, skunks, raccoons and their kin). Oxford University Press, Oxford, pp 149–166
Buesching CD, Heistermann M, Hodges JK, Zimmerman E (1998) Multimodal oestrus advertisement in a small nocturnal prosimian Microcebus murinus. Folia Primatol 69:295–308
Buesching CD, Newman C, Macdonald DW (2002a) Variations in colour and volume of the subcaudal gland secretion of badgers (Meles meles) in relation to sex, season and individual-specific parameters. Mamm Biol 67:147–156
Buesching CD, Waterhouse JS, Macdonald DW (2002b) Gas-chromatographic analyses of the subcaudal gland secretion of the European badger (Meles meles) part I: chemical differences related to individual parameters. J Chem Ecol 28:41–56
Buesching CD, Stopka P, Macdonald DW (2003) The social function of allo-marking in the European badger (Meles meles). Behaviour 140:965–980
Charlton BD (2014) Vocal distinctiveness in the harsh coughs of southern hairy-nosed wombats (Lasiorhinus latifrons). Acta Acust united Ac 100:719–723
Charlton B, Zhang Z, Snyder R (2009) Vocal cues to identity and relatedness in giant pandas (Ailuropoda melanoleuca). J Acoust Soc Am 126:2721–2732
Charlton BD, Ellis WAH, McKinnon AJ, Brumm J, Nilsson K, Fitch WT (2011) Perception of male caller identity in koalas (Phascolarctos cinereus): acoustic analysis and playback experiments. PLoS ONE 6:e20329
Charlton B, Taylor A, Reby D (2013) Are men better than women at acoustic size judgements?. Biol, Lett, p 9
Charrier I, Mathevon N, Jouventin P (2001) Mother's voice recognition by seal pups - Newborns need to learn their mother's call before she can take off on a fishing trip. Nature 412:873–873
Christian S (1993) Behavioural ecology of the Eurasian badger: space use, territoriality and social behaviour. PhD thesis, University of Sussex.
Clutton-Brock TH, Albon SD (1979) The roaring of red deer and the evolution of honest advertising. Behaviour 69:145–170
Davies JM, Lachno DR, Roper TJ (1988) The anal gland secretion of the European badger (Meles meles) and its role in social communication. J Zool 216:455–463
Dugdale HL, Griffiths A, Macdonald DW (2011) Polygynandrous and repeated mounting behaviour in European badgers, Meles meles. Anim Behav 82:1287–1297
East ML, Hofer H (1991) Loud calling in a female-dominated mammalian society. 2. Behavioral contexts and functions of whooping of spotted Hyaenas Crocuta-Crocuta. Anim Behav 42:651–669
Ellis WAH, Bercovitch FB, FitzGibbon S, Roe P, Wimmer J, Melzer A, Wilson R (2011) Koala bellows and their association with the spatial dynamics of free-ranging koalas. Behav Ecol 22:372–377
Evans PGH, Macdonald DW, Cheeseman CL (1989) Social structure of the Eurasian badger (Meles meles): genetic evidence. J Zool 218:587–595
Fant G (1960) Acoustic theory of speech production. Mouton, The Hague
Fell RJ, Buesching CD, Macdonald DW (2006) The social integration of European badger (Meles meles) cubs into their natal group. Behaviour 143:683–700
Fitch WT (1997) Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J Acoust Soc Am 102:1213–1222
Fitch WT (2002) Primate vocal production and its implications for auditory research. In: Ghazanfar AA (ed) Primate audition: ethology and neurobiology. CRC Press, Boca Raton, FL, pp 87–108
Fitch WT, Hauser MD (1995) Vocal production in nonhuman-primates—acoustics, physiology, and functional constraints on honest advertisement. Am J Primatol 37:191–219
Frommolt KH, Goltsman ME, MacDonald DW (2003) Barking foxes, Alopex lagopus: field experiments in individual recognition in a territorial mammal. Anim Behav 65:509–518
Furuyama T, Kobayasi KI, Riquimaroux H (2016) Role of vocal tract characteristics in individual discrimination by Japanese macaques (Macaca fuscata). Sci Rep 6:1–8
Grady RM, Hoogland JL (1986) Why do male black-tailed prairie dogs (Cynomys ludovicianus) give a mating call? Anim Behav 34:108–112
Hardouin L, Tabel P, Bretagnolle V (2006) Neighbour-stranger discrimination in the little owl, Athene noctua. Anim Behav 72:105–112
Harrington FH, Mech LD (1979) Wolf howling and its role in territory maintenance. Behaviour 68:207–249
Insley SJ (2000) Long-term vocal recognition in the northern fur seal. Nature 406:404–405
Kruuk H, Gorman ML, Leitch A (1984) Scent-marking with the subcaudal gland by the European badger. Anim Behav 32:899–907
Lemasson A, Mikus M-A, Blois-Heulin C, Lodé T (2014) Vocal repertoire, individual acoustic distinctiveness, and social networks in a group of captive Asian small-clawed otters (Aonyx cinerea). J Mammal 95:128–139
Macdonald DW, Newman C, Buesching CD (2015) Badgers in the rural landscape—conservation paragon or farmland pariah? Lessons from the Wytham Badger Project. In: Macdonald DW, Feber RE (eds) Wildlife conservation on farmland. Conflict in the Countryside. Oxford University Press, Oxford, pp 65–95
Manno T, Nesterova A, DeBarbieri L, Kennedy S (2007) Why do male Columbian ground squirrels give a mating call? Anim Behav 74:1319–1327
McComb K, Moss C, Sayialel S, Baker L (2000) Unusually extensive networks of vocal recognition in African elephants. Anim Behav 59:1103–1109
McComb K, Reby D, Baker L, Moss C, Sayialel S (2003) Long-distance communication of acoustic cues to social identity in African elephants. Anim Behav 65:317–329
McShane LJ, Estes JA, Riedman ML, Staedler MM (1995) Repertoire, structure, and individual variation of vocalizations in the sea otter. J Mammal 2:414–427
Minter LJ, DeLiberto TJ (2008) Seasonal variation in serum testosterone, testicular volume, and semen characteristics in the coyote (Canis latrans). Theriogenology 69:946–952
Mumm CA, Knornschild M (2014) The vocal repertoire of adult and neonate giant otters (Pteronura brasiliensis). PLoS ONE 9:e112562
Neal E, Cheeseman CL (1996) Badgers. T & AD Poyser, London
Noonan MJ, Tinnesand HV, Müller CT, Rosell F, MacDonald DW, Buesching CD (2020) Knowing me, knowing you: anal gland secretion of European badgers codes for individuality, sex and social group membership. J Chem Ecol
Owren MJ, Rendall D (2001) Sound on the rebound: bringing form and function back to the forefront in understanding nonhuman primate vocal signaling. Evol Anthropol 10:58–71
Owren MJ, Seyfarth RM, Cheney DL (1997) The acoustic features of vowel-like grunt calls in chacma baboons (Papio cyncephalus ursinus): implications for production processes and functions. J Acoust Soc Am 101:2951–2963
Radwan J, Tkacz A, Kloch A (2008) MHC and preferences for male odour in the bank vole. Ethology 114:827–833
Reby D, McComb K (2003) Anatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Anim Behav 65:519–530
Reby D, Joachim J, Lauga J, Lek S, Aulagnier S (1998) Individuality in the groans of fallow deer (Dama dama) bucks. J Zool 245:79–84
Reby D, Cargnelutti B, Hewison AJ (1999) Contexts and possible functions of barking in roe deer. Anim Behav 57:1121–1128
Reby D, Hewison M, Izquierdo M, Pepin D (2001) Red deer (Cervus elaphus) hinds discriminate between the roars of their current harem-holder stag and those of neighbouring stags. Ethology 107:951–959
Reby D, Andre-Obrecht R, Galinier A, Farinas J, Cargnelutti B (2006) Cepstral coefficients and hidden Markov models reveal idiosyncratic voice characteristics in red deer (Cervus elaphus) stags. J Acoust Soc Am 120:4080–4089
Rendall D (2003) Acoustic correlates of caller identity and affect intensity in the vowel-like grunt vocalizations of baboons. J Acoust Soc Am 113:3390–3402
Roper TJ, Shepherdson DJ, Davies JM (1986) Scent marking with faeces and anal secretion in the European badger (Meles meles): seasonal and spatial characteristics of latrine use in relation to territoriality. Behaviour 97:94–117
Savill P, Perrins C, Kirby K, Fisher N (2010) Wytham Woods: Oxford's ecological laboratory. OUP, Oxford
Schwensow N, Eberle M, Sommer S (2008) Compatibility counts: MHC-associated mate choice in a wild promiscuous primate. Proc Roy Soc B 275:555–564
Semple S (2001) Individuality and male discrimination of female copulation calls in the yellow baboon. Anim Behav 61:1023–1028
Soltis J, Leong KM, Savage A (2005) African elephant vocal communication II: rumble variation reflects the individual identity and emotional state of callers. Anim Behav 70:589–599
Stewart PD, Macdonald DW (1997) Age, sex, and condition as predictors of moult and the efficacy of a novel fur-clip technique for individual marking of the European badger (Meles meles). J Zool 241:543–550
Stoeger AS, Charlton BD, Kratochvil H, Fitch WT (2011) Vocal cues indicate level of arousal in infant African elephant roars. J Acoust Soc Am 130:1700–1710
Stoeger AS, Baotic A, Li D, Charlton BD (2012) Acoustic features indicate arousal in infant giant panda vocalisations. Ethology 118:896–905
Taylor A, Charlton BD, Reby D (2016) Vocal production by terrestrial mammals: source, filter and function. In: Suthers RA, Fitch WT, Fay RR, Popper A (eds) Vertebrate sound production and acoustic communication. Springer International Publishing, Berlin, pp 229–259
Tinnesand HV, Buesching CD, Noonan MJ, Newman C, Zedrosser A, Rosell F, Macdonald DW (2015) Will trespassers be prosecuted or assessed according to their merits? A consilient interpretation of territoriality in a group-living carnivore, the European Badger (Meles meles). PLoS ONE 10:e0132432–e132420
Titze IR (1994) Principles of voice production. Prentice Hall, Englewood Cliffs
Townsend S, Charlton B, Manser M (2014) Acoustic cues to identity and predator context in meerkat barks. Anim Behav 94:143–149
Vannoni E, McElligott AG (2007) Individual acoustic variation in fallow deer (Dama dama) common and harsh groans: a source-filter theory perspective. Ethology 113:223–234
Wong J, Stewart PD, Macdonald DW (1999) Vocal repertoire in the European badger (Meles meles): Structure, context, and function. J Mammal 80:570–588
Yamaguchi N, Dugdale HL, Macdonald DW (2006) Female receptiveity, embryonic diapause, and superfetation in the European badger (Meles Meles): implications for the reproductive tactics of males and females. Q Rev Biol 81:1287–1297
Acknowledgements
A UCD Career Development Award provided financial support for this study. The fieldwork and data collection for this study were covered by the University of Oxford’s Natural England license 2014-5710-SCI-SCI and Home Office license PPL 30/2385. University College Dublin’s Animal Research Ethics Committee (AREC-E-16-15-Charlton) approved the capture of the acoustic data. We would like to thank Joni Avenell for her help processing the video data and Nigel Fisher for logistical support on-site.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Heiko Rödel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Charlton, B.D., Newman, C., Macdonald, D.W. et al. Male European badger churrs: insights into call function and motivational basis. Mamm Biol 100, 429–438 (2020). https://doi.org/10.1007/s42991-020-00033-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-020-00033-x