Abstract
Effective and easy-to-apply monitoring techniques are necessary to detect alien species at their first stage of invasion, allowing rapid removal or delimitation of the invaded range for eradication or control actions. Monitoring tools should be effective in detecting the target species, reduce false absences and allow an early detection. The coypu (Myocastor coypus) is a large semi-aquatic rodent native to subtropical and temperate South America, introduced all over the world for its valuable fur. We tested tracking plates in the framework of a coypu occupancy study to take into account false absences and define a standardized monitoring protocol for the species with a limited engagement of staff. We set 60 linear transects, each with 3 tracking plates, along artificial water bodies within the rice district in northwestern Italy and checked them for six consecutive days. For the analyses, we fitted single-season occupancy models to our detection history data. We detected coypu presence at least once in 29 out of the 60 investigated transects (48%). When modeling occupancy and detection probability constant in time and space, the estimate Ψ was 0.48 and detection probability p was 0.60. A minimum of four consecutive visits to the transects provided reliable detection. Coypu’s probability of presence was significantly driven by the amount of surface covered by rice plantations around the investigated water courses. The proposed method may function as a tool for the rapid detection of coypu on large-scale monitoring projects and in case of new colonization, and as a basis for subsequent prompt control actions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasion is one of the greatest threats to biodiversity conservation and a leading driver of extinction for many taxa (Bellard et al. 2016), as well as a major cause of economic losses (Pimentel et al. 2005). It is, therefore, urgent to develop effective management strategies aimed at containing these impacts. To implement early warning and rapid response systems or management actions, monitoring is pivotal. Effective monitoring techniques are necessary to detect species at their first stage of invasion, allowing a rapid removal, or to delimit the invaded range for eradication or control (Byers et al. 2002; Bogich et al. 2008; Simberloff et al. 2013).
Monitoring tools should be effective in detecting the target species, even at low densities, and preferably be cheap and easy to apply. A common sampling problem, especially for elusive species, is the failure to detect a species’ presence despite it being present, generating false absences. It usually happens when the population size is small, the individuals are difficult to sample, or sampling effort is limited (Gu and Swihart 2004). Imperfect detection might neglect sites where the species is inaccurately deemed to be absent, limiting the effectiveness of management actions (Ancillotto et al. 2018). Given the importance of an accurate detection, especially for introduced species, there is a need to design and test new monitoring techniques that are inexpensive, require low effort, reduce false absences and provide an early detection.
The coypu (Myocastor coypus), is a large semi-aquatic rodent native to subtropical and temperate South America, introduced all over the world for its valuable fur (Carter and Leonard 2002; Bertolino et al. 2012). While perceived in some regions as a valuable resource, in most of the countries this species is considered a pest because of its impact on ecosystems, crops and irrigation systems (Carter and Leonard 2002; Panzacchi et al. 2007; Bertolino et al. 2012). In the invaded range, coypus can alter natural habitats because their feeding activities destroy marsh vegetation (Boorman and Fuller 1981; Carter et al. 1999) or directly impact animals such as waterbirds (Bertolino et al. 2011). Moreover, this rodent can damage cereal crops, especially in agricultural areas connected by canals to aquatic environments (Abbas 1988; Panzacchi et al. 2007), and undermine water control structures through their burrows (Carter and Leonard 2002; Panzacchi et al. 2007). Consequently, the species is managed in many countries to reduce its negative impacts, including Italy where it is widespread (Bertolino et al. 2015; Loy et al. 2019).
The methods commonly used to detect the presence of coypu include the direct observations of animals (Marini et al. 2011) or the detection of active burrows (D’Adamo et al. 2000), feeding signs (Corriale et al. 2006) or paths used by the animals (Balestrieri et al. 2016). All these methods require a search in the field that could be time expensive. Furthermore, they have not been tested against the presence and absence of the species, but see Balestrieri et al. (2016) for a quantitative approach. A technique not yet tested with coypu, but widely used to detect many other mammal species (Zielinski and Truex 1995; Gillies et al. 2003; Connors et al. 2005; DeSa et al. 2012), is the use of tracking plates: plates covered by a plastic medium used to detect the footprints of animals.
In this study, we tested the effectiveness of tracking plates for coypu detection. The method was tested in the framework of an occupancy model to take into account false absence and evaluate covariates that influence coypu presence. The purpose of this research was the definition of a standardized monitoring protocol for the species with a limited engagement of staff.
Materials and methods
Study area
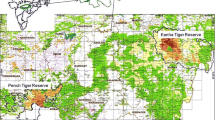
Our study was conducted within the River Po Valley in northwestern Italy (Piedmont region, from 45° 11′ N 8° 06′ E to 45° 08′ N 8° 20′ E; Fig. 1). The climate in this area is subcontinental with hot summers and cold winters. Abundant rainfall occurs mainly in spring and fall, with occasional snowfalls in winter. Part of the territory falls within the Park of the Rivers Po and Orba, a system of natural reserves established to protect residual wetlands and freshwater habitats.
Our study area along the Po River, Northwestern Italy (a). The zoomed area b shows the sampling areas (n = 60, colored circles) and the land cover classification according to Land Cover Piemonte (1:25,000). In c, we see a tracking plate set on the back of a canal with feet and tail tracks imprinted in the sand (d)
The landscape is dominated by the Po riverbed and its riparian ecosystem, intensive rice growing and dense artificial drainage network on the left of the river and hilly broadleaved forests, grassland and crop fields on its right (Fig. 1). The paddy field water network consists of narrow, long canals subjected to seasonal regulated drying out (autumn/winter) and flooding (spring/summer), functional to rice growth. Permanent water bodies not undergoing flow rate regulation also occur. These are artificial ponds, quarry lakes and major channels. Residual vegetation on the banks is mainly represented by spontaneous grass and herbs, with sparse woody vegetation, common reed Phragmites australis and broadleaf cattail Typha latifolia. Riparian vegetation on both sides of the Po riverbed hosts assemblages with different species of willows (Salix spp.), poplars (Populus spp.), European alder (Alnus glutinosa) and hygrophilous herbs, shrubs and bushes. On the right of the Po river, the majority of water bodies include artificial ponds and lakes, and minor rivers.
Field survey
We selected 60 areas to survey along artificial canals within the paddy fields, the banks of the Po and other small rivers, ponds and other water bodies. In each area, we set a linear transect with three tracking plates placed at 100-m intervals, in close proximity and in parallel to water bodies (Fig. 1). Tracking plates were made of squared (60 × 60 cm) plywood covered with a thin layer of vaseline to better hold the overlying thicker layer of sand, necessary to track impression, and baited with a few pieces of carrot. Between June and September 2018, 15 transects were set up at a time and inspected once a day for 6 consecutive days, and then moved to other areas. During each inspection, detected tracks (Fig. 1) were identified, measured, photographed and then deleted, the plates were reactivated by adding sand and renewing bait when necessary.
One of the assumptions of occupancy models requires that the target species is not misidentified. Coypu is much larger than other rodent species present in the area, like rats (Rattus rattus and R. norvegicus) and Italian water voles (Arvicola italicus, Castiglia et al. 2016); it has also webbed hind feet that make the footprint easily recognizable.
Transects were set up at a distance of at least 1.5 km from each other, with the aim of avoiding transects being visited by the same individual (Doncaster and Micol 1989; Reggiani et al. 1993). Transects were placed regardless of the boundaries of Natural Reserves, to homogeneously survey the study area.
During the survey, we also recorded the presence of footprints (outside the tracking plates), paths, and burrows along each transect, as well as any observed animals.
Statistical analyses
Analyses on the effectiveness of tracking plates and of factors influencing coypu’s distribution were performed using occupancy models developed by MacKenzie et al. (2002). These models allow the estimation of the species probability of presence (Ψ) and detection (p) at a given sampling site, taking into account imperfect detection, i.e., failure in detecting a species when present, resulting in false absences (MacKenzie et al. 2006).
For each transect, we compiled the detection history: non-detection, 0; detection, 1. We then fitted single-season occupancy models (MacKenzie et al. 2002) using the program PRESENCE 12.25 (Hines 2006). We selected six covariates that we hypothesized could influence the probability of coypu presence (Table 1). We expected the probability of presence in a given transect to be associated with the water body type (lentic/lotic) and width at water level, water speed (zero, low, high) and the cover of vegetation (tall grasses, shrubs and trees) taller than 80 cm on water bodies banks. Tall vegetation was included in the analysis because it represents potential shelter from local predators, e.g., canids, mustelids and herons (Bertolino et al. 2012). We also calculated the surface covered by paddy fields and other crops in a buffer area using Land Cover Piemonte 1:25,000 (Giannetti et al. 2003). The mean canal lengths (linear extension) in adult coypu individual home ranges is estimated to be about 1190 m (Doncaster and Micol 1989), while, according to Reggiani et al. (1993), in the Mediterranean area, coypus avoid crops beyond a 100 m distance from water bodies. We then built in QGIS software rectangular buffers of 20 ha (1000 m long, 200 m wide) centered on each transect to measure the cover of the two landscape covariates. Quantitative covariates were standardized to z scores. Covariates were tested for correlations before their inclusion in the models.
Considering the limited sample size (n = 60), we used the corrected AICc (Burnham and Anderson 2002). This value is not provided in the PRESENCE output, so this and related statistics were computed using the formulas reported in Symonds and Moussalli (2011) and Richards et al. (2011). The goodness of fit was assessed using the measure for overdispersion (\(\hat{c}\)) with 2000 bootstraps, correcting with quasi-AICc (QAICc) if necessary (Hines 2006).
We evaluated the minimum number of visits necessary to be confident of the coypu absence from an area, using the formula N = ln(αlevel)/ln(1 − p) (Reed 1996), where α represents the probability of type I error (fixed at 0.05) and p the detection probability.
We then evaluated the influence of the six variables hypothesized to be related to site occupancy by building all possible models. We held detection probability (p) constant, because landscape covariates could not change within the 6-day survey. Models were ranked according to AICc (or QAICc) values and selected with a two-step process. First, we considered all models with ΔAICc < 6 from the model with the lowest AICc (Richards et al. 2011). Then we considered if in nested models adding 1 or more parameters increased the fit, as measured by the log-likelihood value (Burnham and Anderson 2002).
We also used Akaike weights (wgt) to assess the strength of evidence in support of each model (Srivathsa et al. 2014). Finally, model-averaging was used to obtain parameter estimates and associated standard errors for covariates present in the selected models (Burnham and Anderson 2002). Model averaging was computed using the spreadsheet developed by Brian R. Mitchell (www.uvm.edu/~bmitchel/software.html) and using the second formula proposed by Burnham and Anderson (2002; formula 6.12, p. 345). Averages were computed over all models, with regression coefficients considered to be zero in models that do not contain the corresponding covariate and normalizing Akaike weights by dividing by their sum. Effect sizes of covariates were computed as regression coefficients after model averaging divided by their standard errors (Burnham and Anderson 2002).
Results
We detected coypu presence at least once in 29 out of the 60 investigated transects (naive estimate 0.48). When modeling occupancy and detection probability constant in time and space, the estimate Ψ was 0.48 and detection probability p was 0.60. With p(t) AICc was higher, therefore p was kept constant in the subsequent modeling stages. The minimum number of visits per site necessary to establish coypu’s absence from an area was 3.2.
We also recorded animals’ paths along 16 transects, footprints along 8 transects and burrows in 3, while animals were observed only in 1 transect. These data could not be analyzed with occupancy models because they were too limited (footprints and observed animals) or the framework was not applicable for the short duration of the survey (no change in paths and burrows expected in 6 days).
Models including covariates outperformed the reference constant model. Since overdispersion was found in the most general model, the value was corrected (\(\hat{c}\) = 2.35). The results for the first ten and the constant models are reported in Table 2; all other models have higher ΔQAIC.
Eight models were included within ΔQAICc < 6 and nearly all included rice as a covariate (Table 2). However, models from 4 to 8 add more covariates without improving the fit of the models. Therefore, we retained only the first three models, which included also other crops and vegetation as covariates. Models 1 and 3 are nested with the simplest model 2 which has only one parameter; however, the increase in the parameters (1 and 2, respectively) is compensated by a decrease in the likelihood and, therefore, there is support also for these models.
Model averaged values (Table 3) indicated that the effect size of rice is 3 times greater than other crops and 10 times greater than vegetation, supporting the overwhelming importance of this covariate. The relation between rice cover and Ψ is explained by a cubic curve (Fig. 2).
Discussion
The results of our research demonstrate that tracking plates constitute an efficient and rapid indirect method for the effective detection of coypu’s presence. This allows the definition of a standardized protocol for large-scale and long-term monitoring of the species, feasible in the face of limited resources. In the landscape investigated, with the estimated probability of detection, a minimum of four consecutive visits was necessary to ensure that in the presence of coypu non-detection will only occur with a probability of 5% or less.
On the other hand, during the surveys, we recorded only few signs of the species presence, and animals were observed only along a transect. Though it was not possible to apply occupancy models to these data for a proper statistical comparison, the most common signs of presence, the paths used by animals, were recorded only in 55% of the transects where coypu were recorded with tracking plates.
The positive association between coypu’s occurrence and rice fields cover confirms what was previously known on the species habitat preferences. It inhabits a wide range of freshwater habitats, e.g., rivers, streams, channels, including those more artificial like rice fields (Reggiani et al. 1993; D’Adamo et al. 2000; Bertolino and Ingegno 2009). Rice fields could be considered as a partial surrogate of natural aquatic habitats, which, however, are flooded during plants growth and dried out after harvest. The paddy drainage network seems to promote the population’s spatial segregation in favor of rice plantations, despite seasonal flooding and drying out of fields and canals. As hypothesized by Bertolino et al. (2005), the drying out of canals from September to April is probably compensated by permanent wetlands, e.g., scattered quarry lakes, large channels, and artificial ponds, which may act as shelter areas and guarantee the long-term persistence of vital populations and subsequent recolonization in spring (Bertolino and Ingegno 2009). Our results seem to confirm the observed descending gradient in species distribution at increasing distance from the rice plantations. Along the Po river banks and in the landscape on the right of the river where the water network is less extended and land use heterogeneity at the landscape scale increases, coypu is sporadic. Further research in this area may focus on the study of potential seasonal shifting of the species ranges as a consequence of drainage network management (canals flooding and drying out), also investigating potential source–sink dynamics.
The coypu has huge impacts on ecosystems through clearing of aquatic vegetation, with indirect negative effects on animal species requiring plants for shelter and their reproduction (Willner et al. 1979; Foote and Johnson 1993; Bertolino et al. 2005). Furthermore, it has important economic costs associated with crop and drainage system damage (Abbas 1988; Panzacchi et al. 2007). For this reason, the species is included in the Union list of invasive species under the EU Regulation on IAS (1143/2014). The regulation requires member states to monitor listed invasive alien species and report their distribution every 6 years. Furthermore, species on this list should be managed to avoid spreading and to mitigate impacts.
The tracking plate method developed here could be used to investigate coypu distribution where the species is spreading or where it is managed for spatial containment or population control. The proposed protocol allows to model the probability of detection and together with Reed’s formula the minimum effort necessary to reduce false absences. The method is easy to apply and cheap, could easily inform and direct control activities, allowing a better evaluation of their effects, and could be used as an effective tool for implementing an early warning system. In this respect, further calibration in other habitats may be useful to demonstrate the general validity of tracking plates for coypu monitoring.
References
Abbas A (1988) Impact du ragondin (Myocastor coypus Molina) sur une culture de mais (Zea mays) dans le Marais Poitevin? Acta Oecol 9:173–189
Ancillotto L, Notomista T, Mori E, Bertolino S, Russo D (2018) Assessment of detection methods and vegetation associations for introduced Finlayson’s squirrels (Callosciurus finlaysonii) in Italy. Environ Manag 61:875–883
Balestrieri A, Zenato M, Fontana E, Vezza P, Remonti L, Caronni FE, Saino N, Prigioni C (2016) An indirect method for assessing the relative abundance of introduced pest Myocastor coypus (Rodentia) in agricultural landscapes. J Zool 298:37–45
Bellard C, Genovesi P, Jeschke JM (2016) Global patterns in threats to vertebrates by biological invasions. Proc R Soc B. https://doi.org/10.1098/rspb.2015.2454
Bertolino S, Ingegno B (2009) Modelling the distribution of an introduced species: the coypu Myocastor coypus (Mammalia, Rodentia) in Piedmont region, NW Italy. Ital J Zool 76:340–346
Bertolino S, Perrone A, Gola L (2005) Effectiveness of coypu control in small Italian wetland areas. Wildl Soc Bull 33:714–720
Bertolino S, Angelici C, Monaco E, Monaco A, Capizzi D (2011) Is the coypu (Myocastor coypus) a nest predator or a nest destroyer? Hystrix Ital J Mamm 22:333–339
Bertolino S, Guichón ML, Carter J (2012) Myocastor Coypus Molina (coypu). In: Francis R (ed) Handbook of global freshwater invasive species. Earthscan, London, pp 149–156
Bertolino S, Colangelo P, Mori E, Capizzi D (2015) Good for management, not for conservation: an overview of research, conservation and management of Italian small mammals. Hystrix Ital J Mamm 26:25–35
Bogich TL, Liebhold AM, Shea K (2008) To sample or eradicate? A cost minimization model for monitoring and managing an invasive species. J Appl Ecol 45:1134–1142
Boorman LA, Fuller RM (1981) The changing status of reed swamp in the Norfolk broads. J Appl Ecol 18:241–269
Burnham KP, Anderson DR (2002) Model selection and inference—a practical information-theoretic approach, 2nd edn. Springer, New York
Byers JE, Reichard S, Randall JM, Parker IM, Smith CS, Lonsdale WM, Atkinson IAE, Seastedt TR, Williamson M, Chornesky E, Hayes D (2002) Directing research to reduce the impacts of nonindigenous species. Conserv Biol 16:630–640
Carter J, Leonard BP (2002) A review of the literature on the worldwide distribution, spread of, and efforts to eradicate the coypu (Myocastor coypus). Wildl Soc Bull 30(1):162–175
Carter J, Foote AL, Johnson-Randall LA (1999) Modelling the effects of coypu (Myocastor coypus) on wetland loss. Wetlands 19:209–219
Castiglia R, Aloise G, Amori G, Annesi F, Bertolino S, Capizzi D, Mori E, Colangelo P (2016) The Italian peninsula hosts a divergent mtDNA lineage of the water vole, Arvicola amphibius sl, including fossorial and aquatic ecotypes. Hystrix It J Mamm 27:99–103
Connors MJ, Schauber EM, Forbes A, Jones CJ, Goodwin BJ, Ostfeld RS (2005) Use of track plates to quantify predation risk at small spatial scales. J Mamm 86:991–996
Corriale MJ, Arias SM, Bó RF, Porini G (2006) Habitat-use patterns of the coypu Myocastor coypus in an urban wetland of its original distribution. Acta Theriol 51:295–302
D’Adamo P, Guichón ML, Bó RF, Cassini MH (2000) Habitat use of the coypu (Myocastor coypus) in agro-systems of the Argentinean Pampas. Acta Theriol 45:25–33
DeSa MA, Zweig CL, Percival HF, Kitchens WM, Kasbohm JW (2012) Comparison of small-mammal sampling techniques in tidal salt marshes of the central gulf coast of Florida. Southeast Nat 11:89–100
Doncaster CP, Micol T (1989) Annual cycle of a coypu (Myocastor coypus) population: male and female strategies. J Zool Lond 217:227–240
Foote AL, Johnson LA (1993) Plant stand development in Louisiana coastal wetlands: coypu grazing effects on plant biomass. In: Proceedings of the 13th annual conference of the society of wetland scientists, New Orleans, Louisiana, Society of wetland scientists. Utica, Mississippi, pp 265–271
Giannetti F, Gottero F, Terzuolo PG (2003) Use of high resolution satellite images in the forest inventory and mapping of Piemonte region (Italy). In: Corona P, Köhl M, Marchetti M (eds) Advances in forest inventory for sustainable forest management and biodiversity monitoring. Forestry sciences. Springer, Dordrecht, pp 87–95
Gillies CA, Leach MR, Coad NB, Theobald SW, Campbell J, Herbert T, Graham PJ, Pierce RJ (2003) Six years of intensive pest mammal control at Trounson Kauri Park, a Department of Conservation “mainland island”, June 1996–July 2002. N Z J Zool 30(4):399–420
Gu W, Swihart RK (2004) Absent or undetected? Effects of non-detection of species occurrence on wildlife–habitat models. Biol Conserv 116:195–203
Hines JE (2006) PRESENCE: software to estimate patch occupancy and related parameters—USGS-PWRC. http://www.mbr-pwrc.usgs.gov/software/presence.html. Accessed 18 May 2019
Loy A, Aloise G, Ancillotto L et al (2019) Mammals of Italy: an annotated checklist. Hystrix It J Mamm 30(2):87–106
MacKenzie DI, Nichols JD, Lachman GB, Droege S, Royle JA, Langtimm JA (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255
MacKenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey LL, Hines JE (2006) Occupancy estimation and modeling. Inferring patterns and dynamics of species occurrence. Elsevier Academic Press, Burlington, MA
Marini F, Ceccobelli S, Battisti C (2011) Coypu (Myocastor coypus) in a Mediterranean remnant wetland: a pilot study of a yearly cycle with management implications. Wetl Ecol Manag 19:159–164
Panzacchi M, Bertolino S, Cocchi R, Genovesi P (2007) Cost/benefit analysis of two opposite approaches to pest species: permanent control of Myocastor coypus in Italy versus eradication in East Anglia (UK). Wildl Biol 13:159–171
Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ 52:273–288
Reed JM (1996) Using statistical probability to increase confidence of inferring species extinction. Conserv Biol 10:1283–1285
Reggiani G, Boitani L, D’Antoni S, De Stefano R (1993) Biology and control of the coypu in the Mediterranean area. Suppl Ric Biol Selvag 21:67–100
Richards SA, Whittingham MJ, Stephens PA (2011) Model selection and model averaging in behavioural ecology: the utility of the IT-AIC framework. Behav Ecol Sociobiol 65:77–89
Simberloff D, Martin J, Genovesi P, Maris V, Wardle DA, Aronson J, Courchamp F, Galil B, García Berthou E, Pascal M, Pyšek P, Sousa R, Tabacchi E, Vilà M (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66
Srivathsa A, Karanth KK, Jathanna D, Kumar NS, Karanth KU (2014) On a dhole trail: examining ecological and anthropogenic correlates of dhole habitat occupancy in the Western Ghats of India. PLoS ONE 9:e98803
Symonds MR, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 65:13–21
Willner GR, Chapman JA, Pursley D (1979) Reproduction, physiological responses, food habitats, and abundance of nutria on Maryland marshes. Wildl Monogr 65:1–43
Zielinski WJ, Truex RL (1995) Distinguishing tracks of marten and fisher at track-plate stations. J Wildl Manag 59:571–579
Acknowledgements
We thank Marco Granata for significant help during field work and the personnel of the Park of the Rivers Po and Orba who provided logistical and technical support during field work. Two referees provided useful comments on an early draft of the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Emmanuel Serrano.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roviani, D., Artioli, P. & Bertolino, S. Evaluating the effectiveness of footprint platforms to detect invasive mammals: coypu (Myocastor coypus) as a case study. Mamm Biol 100, 213–218 (2020). https://doi.org/10.1007/s42991-020-00021-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-020-00021-1