Abstract
Movements, habitat use, and activity of herbivorous species are mainly influenced by trade-offs between food availability and daily/seasonal variation in predation risk. Nevertheless, studies conducted across several seasons and evaluating both spatial and temporal responses of meso-small herbivores are still scanty. Additionally, information on spatiotemporal behaviour is often lacking for cryptic, localised species, which may limit conservation actions. We evaluated how sex, time of day, seasonality, and habitat type—reflecting trade-offs in predation risk and access to food resources—influenced the spatial behaviour and locomotor activity of an herbivorous mammal, the Apennine hare, a threatened species endemic to central-southern Italy. In a 4-year study, we intensively radio-tracked 12 individuals, providing the first insights on the spatiotemporal ecology of this lagomorph. Sex affected neither home range size nor habitat selection. Home range size was larger during the night and in warm months, when hares moved to the most energetically rewarding habitat type for feeding activities, i.e., cultivations. At both study area and home range spatial scales, habitat selection did not vary between the cold and the warm months. At the study area scale, hares avoided deciduous woodland and human settlements, whereas selected the Mediterranean scrubwood and cultivations. Within home ranges, at night, Apennine hares selected risky patches, i.e., cultivations as feeding grounds, whereas in daylight, the safer Mediterranean scrubwood was used for cover and resting. Throughout the year, Apennine hares were mostly nocturnal and were more active in open than in concealed habitats, with no differences between sexes. Bright nights, i.e., with full moon and clear sky, inhibited activity in open areas but not in cover, likely to reduce predation risk. Our findings emphasised the role of feeding/antipredatory requirements in shaping spatiotemporal behaviour of meso-small mammals, with potential consequences for the conservation of threatened species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Individuals move to search for food, mates, and shelter, applying the best trade-off between their life requirements and potential threats (e.g., insects: Kareiva 1983; fish: Abecasis and Erzini 2008; birds: Davies and Lundberg 1984; mammals: Castillo et al. 2012). This trade-off can be mediated by habitat use, i.e., through the use of high-quality but risky feeding patches, or safer patches with a lower availability of food resources, as predicted by the “landscape of fear” framework (hereafter, LOF: Bleicher 2017, for a review). Consequently, amongst herbivorous species, ranging movements and temporal activity patterns are generally built upon physiological requirements while limiting predation risk (e.g., Linkie and Ridout 2011; Prugh and Golden 2014) and/or encounters with potential competitors (Stewart et al. 2002; Goldwater et al. 2012). Access to food resources and their availability have been shown to predict ranging movements and activity amongst mammalian herbivores (Fryxell and Sinclair 1988; Smith and Litvaitis 2000; Korslund and Steen 2006). At the same time, constrains related to variation in predation risk over time of day, seasons, and habitat types also influence their spatial movements and temporal activity (Prugh and Golden 2014 for a review). However, studies conducted across several seasons and evaluating both spatial and temporal data of wild herbivores are still scanty. In this work, we evaluated how intrinsic (sex) and environmental factors reflecting trade-offs in predation risk and access to food resources (time of day, seasonality, and habitat type) influenced the spatial behaviour and the activity of an herbivorous mammal, the Apennine hare Lepus corsicanus.
The Apennine hare has been for long reported as a subspecies of the widely distributed European brown hare L. europaeus (Miller 1912; Toschi 1965). Then, morphometric (Palacios 1996; Riga et al. 2001), morphologic (Rugge et al. 2009; Fattorini et al. 2018), and molecular (both mitochondrial and nuclear genes: Pierpaoli et al. 1999; Alves and Melo-Ferreira 2007; Alves et al. 2008) analyses have confirmed L. corsicanus as a well-divergent species, endemic to central and southern Italy (Amori and Castiglia, 2018), with a fragmented population (Dori et al. 2018). In Sicily, where it is the only hare present (Lo Valvo et al. 1997), the extent of occurrence is continuous (Angelici and Luiselli 2001; Mori et al. 2014a). The Apennine hare has also been introduced to Corsica, where it occurs mainly in the northern and eastern part of the island (Scalera and Angelici 2003; Pietri 2015). Loss of suitable habitats, population isolation, low population densities, and illegal hunting represent the main threats to the Apennine hare (Angelici and Luiselli 2007; Fulgione et al. 2009; Mori et al. 2014a). Competition with European hares released for hunting purposes has been suggested (Fulgione et al. 2009; Barbar and Lambertucci 2018), but not confirmed yet, and only a few cases of interspecific aggression have been observed (Angelici et al. 2010). Hybridisation between European brown hare and Apennine hare has been detected only in Corsica (Pietri et al. 2011), whereas genetic analyses for mainland Italy and Sicily did not detect introgression (Mengoni et al. 2015). Where these species coexist, according to hunting-bag data, the Apennine and the European hare seem to show an altitude partitioning, with the former detected at higher elevations than the latter (Angelici and Luiselli 2007).
The knowledge of behavioural ecology of a species can be particularly helpful for its conservation (Caro 1999; Caro and Sherman 2012, for reviews). Despite the high conservation value of this taxon endemic to central-southern Italy (IUCN risk category: “Vulnerable”; Amori and Castiglia 2018), no study on behaviour and ecology of the Apennine hare has been conducted. If so, information on its ranging movements, habitat use and selection, as well as activity are needed to understand the main ecological requirements of this species. According to a preliminary habitat suitability model, the Mediterranean shrubwood alternated with open areas represent the most suitable habitat for this species (Angelici et al. 2010), although field data are necessary to test its validity. To fill this gap, we have conducted the first radio-tracking study on spatiotemporal behaviour of the Apennine hare. We evaluated whether movements of hares, their habitat selection, and diel activity would change with sex (i.e., larger home ranges in males: Averianov et al. 2003, for L. europaeus), time of day (e.g., Gilbert and Boutin 1991 for L. americanus; Santilli et al. 2014 for L. europaeus), season, and habitat type. We expect that (1) home range size would be larger in males than females, especially during the warm months (Averianov et al. 2003). In addition, according to the LOF conceptual framework, we expect that: (2) open areas, i.e., the riskiest but most energetically rewarding amongst the habitat types, would be used more at night and in warm months than during daylight hours and cold months, respectively, and (3) Apennine hares would avoid bright moonlight nights in open areas.
Materials and methods
Study area
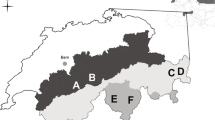
Our study area included a hunting preserve, “La Marsiliana” (about 3.000 ha; Manciano, Grosseto, 43°29′ N, 11°22′ E; 120–245 m a.s.l.), in a deciduous woodland area surrounded by fallows and cultivations. This site was characterized by a sub-Mediterranean climate, with dry summers and relatively mild temperatures throughout the year. Weather data of our study area (mean hourly temperature; hourly rainfall) were provided by Servizio Idrologico Regionale—Regione Toscana. Average monthly temperatures were always below 30 °C in summer and above 0 °C in winter (Torniai 2019). We divided the year arbitrarily in two 6-month periods based on temperatures recorded in our study area through the study years (Carbone 2019; Torniai 2019): a warm one (April–September, mean ± SD = 19.1 ± 6.1 °C) and a cold one (October–March, mean ± SD = 9.4 ± 1.3 °C). Monthly average rainfall has been about 72 mm, with a peak in autumn. A habitat mapping of the study area has been carried out through the analysis of satellite aerial photographs, and confirmed by field investigations (Torniai 2019). About 83.1% of the area was covered by deciduous woodland (Quercus petraea, Q. cerris, Carpinus betulus, Ostrya carpinifolia, and Fraxinus ornus). Cultivations (lucerne and cereals) and Mediterranean shrubwood (i.e. “macchia”: Quercus ilex, Arbutus unedo, Juniperus spp., Smilax aspera, Pistacia lentiscus, Phyllirea latifolia, and Rubus ulmifolius) covered, respectively, 9.5% and 4.2% of the study area. The remaining 3.2% was covered by human settlements (Fig. 1). Potential predators of hares were free-ranging cats and dogs, the grey wolf Canis lupus, red fox Vulpes vulpes, stone marten Martes foina, common buzzard Buteo buteo, and black kite Milvus migrans (Carbone 2019; Torniai 2019; Fattorini et al. 2018).
Radio-tracking and home range estimation
Hares were drive-netted in three areas of about 100 ha each (Pielowski 1972; Rühe and Hohmann 2004) and adults (i.e., no distal epiphyseal knob in ulna, diagnostic of juveniles: Stroh 1931; weight > 2.5 kg: Amori et al. 2008) were fitted with VHF radio-collars (Holohil, Canada) and released. The presence of the distal epiphyseal knob in ulna was always determined by the same observer (G.R.), to avoid diagnosis variability due to different observers. Sex of captured hares was assessed by genital examination (Toschi 1965). Between 2015 and 2018, seven males and five females of Apennine hare were monitored. All hares were radio-tracked for 24–48 h/week/individual (1 fix/4.5 h), for 12–13 months (n. fixes/individual, median ± interquartile range: 246 ± 129; total n. of fixes = 3333). Fixes collected in the first week from radio-tagging were discarded from our analyses. At least three bearings collected within 15 min were used to estimate each location, which was given by the coordinates of the centre of the polygon error, assessed through triangulation (Kenward 1987). If the capture occurred in the middle of a cold (or a warm) period, we pooled together the fixes from the “first” and the “second” cold (or warm) periods. For each individual, each 6-month period (cold and warm periods) was treated independently.
Before data collection, the mean location error was determined by positioning several radio-tags in 150 known locations/operator, at ground level, and by calculating the difference between actual and estimated locations (mean location error ± SD = 22.2 ± 4.0 m: see Bartolommei et al. 2012). Boundaries of each study area were defined by a total 100% minimum convex polygon (MCP) encompassing all the radio-locations, with a 150 m wide buffer area (Castillo et al. 2012). 6-month home range sizes were estimated through the MCP 95% and the 95% fixed kernel (Ker 95%), calculated through the statistical software R version 3.3.1, packages ade4 (Dray and Dufour 2007), adehabitat (Calenge 2006), and HRTools (Preatoni and Bisi 2013). The last package was also used to assess the minimum number of fixes to estimate home range sizes reliably, following the procedure by Seaman et al. (1999). As to the 95% fixed kernel, we used an ad hoc smoothing parameter (hadhoc) to prevent over- or undersmoothing (Berger and Gese 2007; Kie et al. 2010).
Given the moderate-sample size of individual home ranges, we could not use any regression-like analysis to assess differences in home range according to sex, time of day, and season, which would require a greater number of data-points (Zuur et al. 2009). We also avoided to use parametric tests, because our sample size did not allow to detect properly if any assumption was met. Rather, we performed non-parametric two-sample tests and used more robust calculations of P values based on Monte Carlo algorithms. We used the Wilcoxon paired test to compare home range and core area sizes between the warm and cold months, as well as between daytime and nighttime, at the individual hare level. The Monte Carlo significance value was based on 99,999 random reassignments of values to factors (warm vs cold period and daytime vs night), within each individual. We used the Mann–Whitney test to compare home range and core area sizes between males and females. The Monte Carlo significance value was based on 99,999 random permutations. Two-sample tests were performed through the software Past (Hammer et al. 2001).
Habitat selection analysis
Individual-based habitat selection was assessed by comparing the proportion of habitat used by each individual hare against the available proportion of habitat, at two levels (second- and third-order habitat selection, i.e., at the study site and home range scales, respectively; Johnson 1980). Testing parametrically the habitat selection (Aebischer et al. 1993) would require meeting multivariate normality of log ratio of proportions. Conversely, we used the non-parametric methodology proposed by Fattorini et al. (2014), based on a permutation of sign tests and used also on the closely related brown hare (Fattorini et al. 2017). We obtained an overall statistic value for the simultaneous assessment of habitat selection in all the habitat types by combining P values from each test through a permutation procedure (Pesarin 2001; Fattorini et al. 2014). We considered four habitat types: deciduous woodland, cultivations, Mediterranean shrubwood, and human settlements. Sign tests on the original data were applied to assess if a habitat was proportionally used according to its availability, for each individual hare, or if it was over- or underutilised (Fattorini et al. 2014). Non-parametric testing of habitat selection was performed through the package phuassess for R 3.3.1 (Fattorini et al. 2017). Differences in habitat selection (within home ranges) between cold and warm periods, between the cold/warm period and the total year, and between sexes, were tested through Z tests (Hald 1967).
Analysis of locomotor activity
The activity of radio-tagged Apennine hares was assessed through an activity switch, detecting the variation of signal intensity within 60 s (Garshelis and Pelton 1980). For c. 5% fixes, we could not obtain the signal of the activity switch because of malfunctions or reception problems; therefore, the analysis of activity was based on 2989 fixes from all the individuals monitored. However, this slightly lower sample size should have not affected the biological meaning of our results. We analysed activity using generalised additive mixed models (GAMMs; Zuur et al. 2009), to evaluate non-linear effects of predictors on activity of hares. GAMMs can model the shape of non-linear relationships through non-parametric smoothing functions to generate predictions, while including parametric fixed and random predictor terms (Wood 2006, 2013). Our response variable was whether the monitored individual was active (presence of activity) or not (absence of activity). We modelled the probability of an individual to be active through binomial errors (link: logit), in relation to potentially influencing factors, based on previous information on other hare species (e.g., Tapper and Barnes 1986; Holley 2001; Schai-Braun et al. 2012). GAMMs were conducted at two levels, (I) by considering the whole data set (n = 2989 fixes) and (II) the subset of night data (n = 1458 fixes).
As a first step (I), we evaluated the effects of sex, habitat type, and temporal/environmental predictors on hare activity. We pooled habitats by comparing open (cultivations) and concealed areas (Mediterranean shrubwood and woodland), as the latter are expected to provide higher antipredator cover for this herbivorous species. Hence, we built different full GAMMs, to run alternative model selections. Each full GAMM tested the effects of sex (parametric) and the interaction between habitat type (parametric) and one continuous predictor (non-parametric smoothing function), while accounting for other confounding factors as random intercepts. We used such a conservative approach to assess separately the non-linear effect of each single continuous predictor while still accounting for the others as random effects, thus avoiding multicollinearity due to potentially inter-related continuous predictors and/or interactions.
Temporal/environmental continuous predictors which we tested in interaction with habitat type, in separate model sets, were: time of day (as decimal hours from midnight); Julian day (as days elapsed from 1 January); mean hourly temperature (°C); hourly rainfall (mm). When included as random factors, we categorised temporal/environmental variables as following: time of day as hourly period; Julian day as month; mean hourly temperature as temperature classes of 5 °C, from − 5 to 35 °C; hourly rainfall as rainfall classes of 0 mm, 0–2 mm, > 2 mm. In each model, the individual hare was treated as a random intercept to account for data pseudoreplication. When included as fixed effects, both time of day and month were modelled as cyclic cubic regression splines to take into account the circularity of these variables, to reach reliable population-level predictions across days and years of our study. Thus, the value of the smoother at the far left point (i.e., 12:00 PM and 1 January) was the same as the one at the far right point (i.e., 11:59 PM and 31 December). The other continuous predictors were modelled as thin-plate regression splines.
For each full GAMM, each one having a specific combination of random intercepts to account for confounding factors (i.e., a specific random structure), we performed a model selection to rank all the possible (six) combinations of fixed effects, as each of them could represent a different a priori hypothesis (Burnham and Anderson 2000). The null model, i.e., the one retaining the random part only, was also included in model selection, allowing an assessment of model performance relative to a fixed baseline (Mac Nally et al. 2018). Model selection was based on the Akaike’s Information Criterion corrected for small sample sizes (AICc) and accounted for nesting (sensu Richards et al. 2011): models were selected if they had ∆AICc ≤ 2 (Burnham and Anderson 2000), provided that they were not more complex versions of a better model (i.e., a model having a lower AICc value). For each model set, predictions (± 95% confidence intervals) of hare activity were obtained from the best model, i.e., the model with the greatest weight. Best models were validated through visual inspection of residuals (Zuur et al. 2009). We also performed explorative analyses by manually refitting each full GAMM with a different number of knots. This precaution allowed us to check whether we used an optimal basis dimension of the smoothers modelling non-linear relationships, to achieve a balance between model fit and number of parameters (sensu Wood 2019) i.e., without overfitting models (Wood 2017). We found no important change in model fit with increasing number of knots, suggesting that it was optimal (Wood 2017). In addition, the relatively high effective degrees of freedom (cf. Table S2) should not be a problem, because our sample size included much more than ten observations per predictor (Bolker et al. 2009). We also provided the adjusted R2 for each best model, as a measure of goodness of fit. Model selection and GAMMs were performed, respectively, through the functions dredge (R package MuMIn; Barton 2013) and gam (R pacakge mgcv; Wood 2019), whilst predictions were plotted through the function plot_smooth (R package itsadug; van Rij et al. 2017).
As a further step (II), we performed a model selection on night data by considering only those fixes recorded before the dawn and after the dusk, to evaluate the joint effects of moonlight and sky cover on hare activity (Stokes et al. 2001; Mori et al. 2014b; Prugh and Golden 2014). We tested the interaction between the non-parametric smoothing function of sky brightness index (ranging from 0 to 4, see Fig. S1 in Appendix 1) with habitat type, by controlling the other factors as parametric predictors and random intercepts, following the approach of (I). Model selection and analysis were conducted as for (I).
We assessed also the overlap between locomotor activity patterns of male and female individuals through the R package overlap (Meredith and Ridout 2014), removing inactive fixes (i.e., 22.1% of total radio-locations) from the data set. We estimated the overlap coefficient Δ, which ranges between 0 (no overlap) and 1 (maximum overlap: Linkie and Ridout 2011; Meredith and Ridout 2014). We computed the Δ4 overlap estimator, i.e., the coefficient to be used when also the lowest sample of the pair comparison exceeded 75 locations (Linkie and Ridout 2011; Meredith and Ridout 2014). The Watson’s test for homogeneity was used to compare the distribution of active fixes of male and female hares between cold and warm months, through the R package circular (Lund et al. 2017).
Results
Home range size
An average number of 24 ± 6 fixes/individual in the cold period and 28 ± 3 in the warm one were the minimum to reliably assess home range size. Home ranges of Apennine hares were significantly larger during the warm period with respect to the cold one (Wilcoxon paired test; MCP 95%: W = 73, P = 0.005; Ker 95%: W = 71, P = 0.009; Fig. 2). The same result was obtained for core areas estimated by Ker, but not for those estimated by MCP (MCP 50%: W = 41, P = 0.909; Ker 50%: W = 75, P = 0.002). Both nocturnal home ranges and core areas were significantly larger than diurnal ones (MCP 95%: W = 78, P < 0.001; Ker 95%: W = 78, P < 0.001; MCP 50%: W = 78, P < 0.001; Ker 50%: W = 78, P < 0.001).
No significant difference was found between males and females in home range and core area sizes throughout the year (MCP 95%: U = 16, P = 0.876; Ker 95%: U = 10, P = 0.266; MCP 50%: U = 13, P = 0.530; Ker 50%: U = 14, P = 0.636), in the warm period (MCP 95%: U = 13, P = 0.531; Ker 95%: U = 12, P = 0.432; MCP 50%: U = 8, P = 0.150; Ker 50%: U = 9, P = 0.201) as well as in the cold one, except for core areas estimated by Ker (MCP 95%: U = 14, P = 0.637; Ker 95%: U = 7, P = 0.111; MCP 50%: U = 14, P = 0.637; Ker 50%: U = 1.5, P = 0.007). We found no difference between sexes in nocturnal home ranges/core areas (MCP 95%: U = 17, P = 1; Ker 95%: U = 11, P = 0.341; MCP 50%: U = 11, P = 0.342; Ker 50%: U = 17, P = 1), but diurnal home ranges and core areas were significantly larger in female hares, except for core areas estimated by MCP (MCP 95%: U = 3, P = 0.017; Ker 95%: U = 5.5, P = 0.050; MCP 50%: U = 8, P = 0.148; Ker 50%: U = 0, P = 0.002).
Habitat selection within the study area
Apennine hares used habitats in a nonrandom manner (cold period, P = 0.002; warm period, P = 0.001). Within the study area, individuals selected the Mediterranean shrubwood and cultivations, and avoided human settlements and deciduous woodland (Fig. 3), both in the cold and the warm periods.
Habitat selection within home ranges
Within home ranges, overall, Apennine hares showed a nonrandom habitat use (cold period, P = 0.003; warm period, P = 0.001). The Mediterranean shrubwood was selected positively both in the cold and in the warm period. Cultivations were only overutilised during the warm period, whereas deciduous woodland was underutilised throughout the year (Fig. 4a). At night (overall P value: cold period = 0.002; warm period = 0.001), cultivations were selected throughout the year, whereas deciduous woodland was underutilised (Fig. 4b). During daylight (overall P value: cold period = 0.003; warm period = 0.013), cultivations were avoided throughout the year; deciduous woodland was selected over the cold period, whereas the Mediterranean shrubwood was preferred in the warm one (Fig. 4c). Habitat selection did not differ between cold and warm periods on the 24 h cycle, nor between the cold/warm period and the total year period, nor between sexes (Z tests, P = 0.226–0.425).
Locomotor activity patterns
Rankings of candidate models predicting hare activity are summarised in Table S1 (Appendix 1). Because alternative best models were more complex versions of the best ones, despite having ΔAICc < 2, our rankings showed only a selected model per model set. The effect of sex on the probability of being active was never supported in selected models, although it was present in all the second best models (Table S2). Overall, all models showed that hares were more active in open than in concealed habitats (Fig. 5).
Diel locomotor activity of the Apennine hare in relation to a time of day, b Julian day, c temperature and nocturnal locomotor activity in relation to d sky brightness, in both open (light grey items) and concealed areas (dark grey items). Lines and shaded areas show predicted values ± 95% confidence intervals estimated at the population level by best GAMMs, which account for other influencing factors and hare identity as random effects
In cover, hare activity increased from 05:00 PM, with a peak between 00:00 and 05:00 AM, and decreased from 06:00 AM, with the lowest activity between 10:00 am and 05:00 PM (Fig. 5a). In open areas, the probability of being active approached 1 around the clock, with a slight decrease between 09:00 AM and 04:00 PM (Fig. 5a). Hare activity varied throughout the year, in cover: the highest probability of being active was between January and March and in June–August (Fig. 5b), whereas hares showed the lowest activity in April–May and throughout September–December. In open areas, the probability of being active was high throughout the year, with a slight decrease in February (Fig. 5b). Activity of hares increased with increasing ambient temperature both in open areas, where hares were almost ever active when temperature was greater than 10 °C (Fig. 5c), and in cover, throughout the range of measured temperature (Fig. 5c). Rainfall was not supported as an influencing variable of hare activity in selected models, although it was present in the third and fourth best models (Table S1 in Appendix 1). At night, the probability of being active decreased with increasing sky brightness, but this relationship was not significant in cover, where the joint effect of moonlight and sky cover had no effect (Fig. 5d).
The temporal activity overlap between sexes was almost complete (Δ4: 0.91, cold months; 0.93, warm months; 0.98, annual), but their temporal activity patterns were different between the cold and the warm periods (Watson’s test for homogeneity for males: U2 = 1.71, P < 0.001; Watson’s test for homogeneity for females: U2 = 2.04, P < 0.001).
Discussion
Seasonality, time of day, habitat type, and sex are important factors influencing spatiotemporal behaviour of herbivores (e.g., Bisi et al. 2011; Owen-Smith and Goodall 2014; Fattorini et al. 2019). Here, we tested whether the above factors affected phenology of movements, habitat use, and activity of a meso-small herbivore, as clues for potential trade-offs between feeding and antipredatory necessities. The Apennine hare lives at low densities in southern Tuscany (Macchia et al. 2005), at the limit of its distribution range, which prevented us to carry out a higher number of captures. Thus, our study was based on a limited sample size, although it provided the first insights on the spatiotemporal behaviour of this threatened lagomorph.
In mammals, home range size can increase with increasing body size and energy constraints (Jenkins 1981; Lindstedt et al. 1986; Kelt and Van Vuren 1999). This relationship has been widely confirmed for herbivorous mammals, including lagomorphs (McNab 1986; Swihart 1986). The annual home range of the Apennine hare (mean ± SD = 46 ± 16 ha) was smaller than that of the European brown hare (Tapper and Barnes 1986; Kovacs and Buza 1987; Giovannini et al. 1988; Zilio et al. 1997; Meriggi et al. 2015: mean ± SD = 67 ± 12 ha; Carbone 2019, N = 3 ind., in our study area: mean ± SD = 295 ± 104 ha), which is larger in body size (European brown hare: 2.5–6.5 kg; Apennine hare: 1.8–3.5 kg; Toschi 1965; Riga et al. 2001). The home range size of the Apennine hare was similar to that of the Iberian hare L. granatensis (36–40 ha.), of similar body mass (mean weight: 2.5 kg: Schai-Braun and Hackländer 2016), in southern Spain (Carro et al. 2011), inhabiting comparable scrubland environments. Conversely, the North-American showshoe hare L. americanus (mean weight: 2.4 kg: Schai-Braun and Hackländer 2016) and the European mountain hare L. timidus (mean weight: 3.5 kg: Schai-Braun and Hackländer 2016) move over large annual areas, often > 50 ha, but they are typical of resource-poor habitats (i.e., mountain prairies and boreal forests: Hewson 1989; Bisi et al. 2011). In the European brown hare, annual home ranges are c. 15–17% larger in males than in females (Averianov et al. 2003). Conversely, similarly to the Iberian hare (Carro et al. 2011), the size of annual home ranges of the Apennine hare did not differ significantly between sexes, thus not supporting our prediction (i) that males ranged on a wider area than females.
In the Apennine hare, a significant variation of home range size occurred between the cold and the warm periods. In the latter, when the Mediterranean shrubwood has been found the least productive also for other mammalian species (e.g. red fox: Lucherini and Lovari 1996; wild boar Sus scrofa: Massei et al. 1997; crested porcupine Hystrix cristata: Lovari et al. 2013), Apennine hare significantly increased home range size to move to cultivated areas to feed. Within the study area, Apennine hare apparently avoided woodland throughout the year while positively selecting the Mediterranean shrubwood and cultivations. At the home range scale, Apennine hares selected the Mediterranean shrubwood both in the cold and in the warm months, mostly in daylight hours, confirming this lagomorph as a species typical of concealed habitats (Angelici et al. 2010). In sub-Mediterranean climate countries, shrublands are covered with dense vegetation and a rich understorey throughout the year, therefore providing cover, as well as the best thermic, wind-sheltered conditions (Robbins 1983; Lucherini et al. 1995; Lombardi et al. 2007). Apennine hares may locate their diurnal resting sites in this concealed habitat, often in hollows of the ground protected by bushes or shrubs, as observed in other lagomorph species (Moreno et al. 1996 for the wild rabbit, Oryctolagus cuniculus; Carro et al. 2011, for L. granatensis; Neumann et al. 2011, for L. europaeus). In the cold period, diurnal fixes of the Apennine hares were located more in deciduous woodland than in Mediterranean shrubwood, possibly because of location of their main food resources (e.g., Fagaceae, Aceraceae, and Araliaceae: Buglione et al. 2018). Hares use to vary often the location of their resting sites in concealed habitats, possibly to increase predator avoidance (Angelici et al. 1999; Neumann et al. 2011). From the cold to the warm period, Apennine hares increased their use of cultivations, which became the most selected habitat type to search for food (Buglione et al. 2018), presumably because cultivations provided them with an abundant and clumped food resource (Altieri 1999; Hockings et al. 2009), thus shortening foraging time (Altmann and Muruthi 1988; Cavallini and Lovari 1991; Weterings et al. 2018). Therefore, our prediction (ii), i.e., which open areas would be used mostly at night and in warm months, was fulfilled.
A different parental investment between sexes and, in turn, potential differences in their spatiotemporal behaviour have been found in polygynous species (Lagomorphs: Cowan and Bell 1986). Conversely, male and female Apennine hares shared both habitat use and temporal activity, with an extensive intersexual overlap (i.e., over 90%). Apennine hares were mainly active in open areas (i.e., cultivations) at night, with a peak between midnight and the 05.00 AM, while showing the lowest activity between the 10.00 AM and the 05.00 PM, in line with the behaviour of the similar European brown hare (Santilli et al. 2014). In the warm period, when nights get shorter, some peaks of diurnal activity were also recorded, as in other nocturnal species (Corsini et al. 1995; Schai-Braun et al. 2012). Activity in closed habitats was the lowest in spring (April–May, i.e., at birth peaks: Amori et al. 2008), when hares start to range mostly in open areas (i.e., cultivations), as well as in autumn, when trophic resources in that areas are suggested to be the lowest (Buglione et al. 2018).
Nocturnal foraging is common amongst herbivorous mammals; nevertheless, moon presence could make them easily detectable by potential predators (rodents: e.g., Daly et al. 1992; Fattorini and Pokheral 2012; ungulates: e.g., Carnevali et al. 2016; Palmer et al. 2017). Light intensity, i.e., the sky brightness resulting from the joint effects of moon phase and cloud covering, appeared to reduce the Apennine hare nocturnal activity in open areas, but not in concealed ones. This is in line with the behavioural ecology of other hare species, e.g., the snowshoe hare L. americanus (Gilbert and Boutin 1991) and the black-tailed jackrabbit L. californicus (Smith 1990), and fulfilled our prediction (iii) that Apennine hares avoid moonlight nights, particularly in open areas.
Among mammals, most prey species use bright light as an indirect cue of predation susceptibility, shifting their habitat use from open to closed habitats, e.g., for concealment (Clarke 1983; Upham and Hafner 2013; Prugh and Golden 2014; Weterings et al. 2019); accordingly, hunting success of the red fox, i.e., the main natural predator of the Apennine hare (Amori et al. 2008; Fattorini et al. 2018), is the highest on bright moonlight nights with clear sky (Molsher et al. 2000).
Although movements and activity of predators should be studied to support the landscape of fear hypothesis, our results strongly suggest that predator avoidance is a major factor influencing the spatiotemporal behaviour of the Apennine hare, as in other hare species (Daly et al. 1992; Holley 1993; Beaudoin et al. 2004; Weterings et al. 2019). In addition, competition with the larger, coexisting European brown hare, restocked for hunting purposes, has been suggested (Angelici and Luiselli 2007). Although our data cannot confirm this hypothesis, as brown hare select open areas (e.g., Tapper and Barnes 1986; Santilli et al. 2014) and show cathemeral locomotor activity patterns (Schai-Braun et al. 2012), the suggestion that Apennine hares is forced to select scrubland areas when in sympatry with the European hare may find support (Schai-Braun and Hackländer 2016). In turn, predation risk and, possibly, potential competitors seem to shape activity and habitat use of Apennine hare. To this end, patches with dense vegetation cover close to fields should be preserved in areas earmarked for the conservation of this Italian endemic species. Our findings emphasised the role of seasonality, time of day, and habitat type as antipredatory/feeding requirements in shaping spatiotemporal behaviour of meso-small mammals, with potential consequences for the conservation of threatened species.
Change history
25 January 2021
The original version of this article unfortunately contained two mistakes.
References
Abecasis D, Erzini K (2008) Site fidelity and movements of gilthead sea bream (Sparus aurata) in a coastal lagoon (Ria Formosa, Portugal). Estuar Coast Shelf Sci 79:758–763
Aebischer NJ, Robertson PA, Kenward RE (1993) Compositional analysis of habitat use from animal radio-tracking data. Ecology 74:1313–1325
Altieri M (1999) The ecological role of biodiversity in agroecosystems. Agr Eco Env 74:19–31
Altmann J, Muruthi P (1988) Differences in daily life between semiprovisioned and wild-feeding baboons. Am J Prim 15:213–221
Alves PC, Melo-Ferreira J (2007) Are Lepus corsicanus and L. castroviejoi conspecific? Evidence from the analysis of nuclear markers. Conservazione di Lepus corsicanus, Stato delle conoscenze. IGF Pub Napoli 1:45–52
Alves PC, Melo-Ferreira J, Branco M, Suchentrunk F, Ferrand N, Harris DJ (2008) Evidence for genetic similarity of two allopatric European hares (Lepus corsicanus and L. castroviejoi) inferred from nuclear DNA sequences. Mol Phyl Evol 46:1191–1197
Amori G, Castiglia R (2018) Mammal endemism in Italy: a review. Biogeographia 33:19–31
Amori G, Contoli L, Nappi A (2008) Fauna d’Italia. Mammalia II: Erinaceomorpha, Soricomorpha, Lagomorpha, Rodentia. Edizioni Calderini, Bologna, Italy
Angelici FM, Luiselli L (2001) Distribution and status of the Apennine hare Lepus corsicanus in continental Italy and Sicily. Oryx 35:245–249
Angelici FM, Luiselli L (2007) Body size and altitude partitioning of the hares L. europaeus and L. corsicanus living in sympatry and allopatry in Italy. Wildl Biol 13:251–257
Angelici FM, Riga F, Boitani L, Luiselli L (1999) Use of dens by radiotracked brown hares Lepus europaeus. Behav Proc 47:205–209
Angelici FM, Petrozzi F, Galli A (2010) The Apennine hare Lepus corsicanus in Latium, Central Italy: a habitat suitability model and comparison with its current range. Hystrix 21:177–182
Averianov A, Niethammer J, Pegel M (2003) Lepus europeus Pallas, 1778–Feldhase. In: Krapp F (eds) Handbuch der Säugietere Europas. Band 3/II: Hasentiere. Lagomorpha. Aula-Verlag, Kempten, pp 35–104
Barbar F, Lambertucci SA (2018) The roles of leporid species that have been translocated: a review of their ecosystem effects as native and exotic species. Mamm. Rev. 48:245–260
Bartolommei P, Francucci S, Pezzo F (2012) Accuracy of conventional radio telemetry estimates: a practical procedure of measurement. Hystrix 23:12–18
Bartoń K (2013) Package “MuMIn.” http://cran.rproject.org/web/packages/MuMIn/index.html. Accessed 9 Jan 2019
Beaudoin C, Crete M, Huot J, Etcheverry P, Cotè SD (2004) Does predation risk affect habitat use in snowshoe hares? Ecoscience 11:370–378
Berger KM, Gese EM (2007) Does interference competition with wolves limit the distribution and abundance of coyotes? J Anim Ecol 76:1075–1085
Bisi F, Nodari M, Dos Santos Oliveira NM, Masseroni E, Preatoni DG, Wauters LA, Tosi G, Martinoli A (2011) Space use patterns of mountain hare (Lepus timidus) on the Alps. Eur J Wildl Res 57:305–312
Bleicher SS (2017) The landscape of fear conceptual framework: definition and review of current applications and misuses. PeerJ 5:e3772
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Buglione M, Maselli V, Rippa D, de Filippo G, Trapanese M, Fulgione D (2018) A pilot study on the application of DNA metabarcoding for non-invasive diet analysis in the Italian hare. Mammal Biol 88:31–42
Burnham KP, Anderson DR (2000) Model section and multimodel inferences: a practical-theoretic approach, 2nd edn. Springer, London
Calenge C (2006) The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519
Carbone R (2019). Metanalisi del comportamento spazio-temporale della lepre europea Lepus europaeus. In: Bachelor Dissertation in Scienze Faunistiche, Università degli Studi di Firenze
Carnevali L, Lovari S, Monaco A, Mori E (2016) Nocturnal activity of a “diurnal” species, the northern chamois, in a predator-free Alpine area. Behav Proc 126:101–107
Caro T (1999) The behaviour-conservation interface. Trends Ecol Evol 14:366–369
Caro T, Sherman PW (2012) Vanishing behaviors. Cons Lett 5:159–166
Carro F, Soriguer RC, Beltràn F, Andreu AC (2011) Heavy flooding effects on home range and habitat selection of free-ranging Iberian hares (Lepus granatensis) in Doñana National Park (SW Spain). Acta Theriol 56:375–382
Castillo DF, Luengos Vidal EM, Casaneve EB, Lucherini M (2012) Habitat selection of Molina’s hog nosed skunks in relation to prey abundance in the Pampas grassland of Argentina. J Mammal 93:716–721
Cavallini P, Lovari S (1991) Environmental factors influencing the use of habitat in the red fox, Vulpes vulpes. J Zool (Lond) 223:323–339
Clarke JA (1983) Moonlight’s influence on predator/prey interactions between short-eared owls (Asio flammeus) and deer-mice (Peromyscus maniculatus). Behav Ecol Socibiol 13:205–209
Corsini MT, Lovari S, Sonnino S (1995) Temporal activity patterns of crested porcupines Hystrix cristata. J Zool (Lond) 236:43–54
Cowan DP, Bell DJ (1986) Leporid social behaviour and social organization. Mammal Rev 16:169–179
Daly M, Behrends PR, Wilson MI, Jacobs LF (1992) Behavioural modulation of predation risk: moonlight avoidance and crepuscular compensation in a nocturnal desert rodent, Dipodomys merriami. Anim Behav 44:1–9
Davies NB, Lundberg A (1984) Food distribution and a variable mating system in the dunnock, Prunella modularis. J Anim Ecol 1984:895–912
Dori P, Scalisi M, Mori E (2018) “An American near Rome”… and not only! In: Presence of the eastern cottontail in Central Italy and potential impacts on the endemic and vulnerable Apennine hare. Mammalia, https://doi.org/10.1515/mammalia-2018-0069
Dray S, Dufour AB (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22:1–20
Fattorini N, Pokheral CP (2012) Activity and habitat selection of the Indian crested porcupine. Ethol Ecol Evol 24:377–387
Fattorini L, Pisani C, Riga F, Zaccaroni M (2014) A permutation-based combination of sign tests for assessing habitat selection. Env Ecol Stat 21:161–187
Fattorini L, Pisani C, Riga F, Zaccaroni M (2017) The R package “phuassess” for assessing habitat selection using permutation-based combination of sign tests. Mammal Biol 83:64–70
Fattorini N, Burrini L, Morao G, Ferretti F, Romeo G, Mori E (2018) Splitting hairs: how to tell hair of hares apart for predator diet studies. Mammal Biol 85:84–89
Fattorini N, Brunetti C, Baruzzi C, Chiatante G, Lovari S, Ferretti F (2019) Temporal variation in foraging activity and grouping patterns in a mountain-dwelling herbivore: environmental and endogenous drivers. Behav Process 167:103909
Fryxell JM, Sinclair ARE (1988) Causes and consequences of migration by large herbivores. Trends Ecol Evol 3:237–241
Fulgione D, Maselli V, Pavarese G, Rippa D, Rastogi RK (2009) Landscape fragmentation and habitat suitability in endangered Italian hare (Lepus corsicanus) and European hare (Lepus europaeus) populations. Eur J Wildl Res 55:385–396
Garshelis DL, Pelton MR (1980) Activity of black bears in the Great Smoky Mountains National Park. J Mammal 61:8–19
Gilbert BS, Boutin S (1991) Effect of moonlight on winter activity of snowshoe hare. Arctic Alpine Res 23:61–65
Giovannini A, Trocchi V, Savigni G, Spagnesi M (1988) Immissione in un’area controllata di lepri d’allevamento: analisi della capacità di adattamento all’ambiente mediante radio-tracking. Atti del I Convegno Nazionale dei Biologi della Selvaggina 14:271–299
Goldwater N, Perry GL, Clout MN (2012) Responses of house mice to the removal of mammalian predators and competitors. Austral Ecol 37:971–979
Hald A (1967) Statistical theory with engineering applications. Wiley, New York
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hewson R (1989) Grazing preferences of mountain hares on heather moorland and hill pastures. J Appl Ecol 26:1–11
Hockings KJ, Anderson JR, Matsuzawa T (2009) Use of wild and cultivated foods by chimpanzees at Bussou, Republic of Guinea: feeding dynamics in a human-influenced environment. Am J Prim 71:636–646
Holley AJF (1993) Do brown hares signal to foxes? Ethology 94:21–30
Holley AJF (2001) The daily activity period of the brown hare (Lepus europaeus). Mammal Biol 66:357–364
Jenkins SH (1981) Common patterns in home range-body size relationships of birds and mammals. Am Nat 118:126–128
Johnson DH (1980) The comparison of usage and availability measurements for evaluating resource preference. Ecology 61:65–71
Kareiva PM (1983) Local movement in herbivorous insects: applying a passive diffusion model to mark-recapture field experiments. Oecologia 57:322–327
Kelt DA, Van Vuren D (1999) Energetic constraints and the relationship between body size and home range area in mammals. Ecology 80:337–340
Kenward R (1987) Wildlife radiotagging. Academic Press, London
Kie JG, Matthiopoulos J, Fieberg J, Powell RA, Cagnacci F, Mitchell MS, Gaillard JM, Moorcroft PR (2010) The home-range concept: are traditional estimators still relevant with modern telemetry technology? Philos Trans R Soc B 365:2221–2231
Korslund L, Steen H (2006) Small rodent winter survival: snow conditions limit access to food resources. J Anim Ecol 75:156–166
Kovacs C, Buza C (1987) Home range size of the brown hare in Hungary. Trans 18th IUGB Congress Krakow Poland 18:267–270
Lindstedt SL, Miller BJ, Buskirk SW (1986) Home range, time, and body size in mammals. Ecology 67:413–418
Linkie M, Ridout MS (2011) Assessing tiger-prey interactions in Sumatran rainforests. J Zool (Lond) 284:224–229
Lo Valvo M, Barera A, Seminara S (1997) Biometrics and status of the Italian hare (Lepus corsicanus, de Winton 1898) in Sicily. Natural Sicil 21:67–74
Lombardi L, Fernàndez N, Moreno S (2007) Habitat use and spatial behaviour in the European rabbit in three Mediterranean environments. Basic Appl Ecol 8:453–463
Lovari S, Sforzi A, Mori E (2013) Habitat richness affects home range size in a monogamous large rodent. Behav Proc 99:42–46
Lucherini M, Lovari S (1996) Habitat richness affects home range size in the red fox. Behav Proc 36:103–106
Lucherini M, Lovari S, Crema G (1995) Habitat use and ranging behaviour of the red fox (Vulpes vulpes) in a Mediterranean rural area: is shelter availability a key factor. J Zool (Lond) 237:577–591
Lund U, Agostinelli C, Arai H, Gagliardi A, Portugues EG, Giunchi D, Irisson JO, Pocernich M, Rotolo F (2017) Package circular. http://mirrors.ucr.ac.cr/CRAN/web/packages/circular/circular.pdf. Accessed 23 Dec 2018
Mac Nally R, Duncan RP, Thomson JR, Yen JD (2018) Model selection using information criteria, but is the “best” model any good? J Appl Ecol 55:1441–1444
Macchia M, Riga F, Trocchi V (2005) Preliminary data on distribution and comparative ecology of Italian hare (Lepus corsicanus De Winton, 1898) and European brown hare (Lepus europaeus Pallas, 1778) in the Grosseto province (Tuscany, Italy). In: Pohlmeyer K (ed) Extended abstract of the XXVII congress of the international union of game biologists. Hannover, Germany, pp 402–404
Massei G, Genov PV, Staines BW, Gorman ML (1997) Factors influencing home range and activity of wild boar (Sus scrofa) in a Mediterranean coastal area. J Zool (Lond) 242:411–423
McNab BK (1986) The influence of food habits on the energetics of eutherian mammals. Ecol Monogr 56:1–19
Mengoni C, Mucci N, Randi E (2015) Genetic diversity and no evidences of recent hybridization in the endemic Italian hare (Lepus corsicanus). Conserv Genetics 16:477–489
Meredith M, Ridout M (2014) Overview of the overlap package. http://cran.cs.wwu.edu/web/packages/overlap/vignettes/overlap.pdf. Accessed 12 Dec 2018
Meriggi F, Meriggi A, Pezzotti A (2015) Selezione dell’habitat da parte della lepre comune (Lepus europaeus P.) in un’area della Pianura Padana Nord Occidentale. In: Technical Report, Dipartimento di Scienze della Terra e dell’Ambiente, Università degli Studi di Pavia, Pavia, Italy
Miller GS (1912) Catalogue of the mammals of Western Europe. British Museum Editions, London
Molsher RL, Gifford EJ, McIlroy JC (2000) Temporal, spatial and individual variation in the diet of red foxes (Vulpes vulpes) in central New South Wales. Wildl Res 27:593–601
Moreno S, Delibes M, Villafuerte R (1996) Cover is safe during the day but dangerous at night: the use of vegetation by European wild rabbits. Can J Zool 74:1656–1660
Mori E, Menchetti M, Mazza G, Scalisi M (2014a) A new area of occurrence of an endemic Italian hare inferred by camera trapping. Boll Mus Civ Sci Nat Torino 30:123–130
Mori E, Nourisson DH, Lovari S, Romeo G, Sforzi A (2014b) Self-defence may not be enough: moonlight avoidance in a large, spiny rodent. J Zool (Lond) 294:31–40
Neumann F, Schai-Braun S, Weber D, Amrhein V (2011) European hares select resting places for providing cover. Hystrix 22:291–299
Owen-Smith N, Goodall V (2014) Coping with savanna seasonality: comparative daily activity patterns of African ungulates as revealed by GPS telemetry. J Zool (Lond) 293:181–191
Palacios F (1996) Systematics of the indigenous hares of Italy traditionally identified as Lepus europaeus Pallas, 1778 (Mammalia: Leporidae). Bonner Zool Beiträge 5:59–91
Palmer MS, Fieberg J, Swanson A, Kosmala M, Packer C (2017) A ‘dynamic’ landscape of fear: prey responses to spatiotemporal variations in predation risk across the lunar cycle. Ecol Lett 20:1364–1373
Pesarin F (2001) Multivariate permutation tests: with applications in biostatistics. Wiley Editions, New York
Pielowski Z (1972) Home range and degree of residence of the European hare. Acta Theriol 17:93–103
Pierpaoli M, Riga F, Trocchi V, Randi E (1999) Species distinction and evolutionary relationships of the Italian hare (Lepus corsicanus De Winton, 1898) as described by mitochondrial DNA sequencing. Mol Ecol. 8:1805–1817
Pietri C (2015) Range and status of the Italian hare Lepus corsicanus in Corsica. Hystrix 26:166–168
Pietri C, Alves PC, Melo-Ferreira J (2011) Hares in Corsica: high prevalence of Lepus corsicanus and hybridization with introduced L. europaeus and L. granatensis. Eur J Wildl Res 57:313–321
Preatoni DG, Bisi F (2013) HRTools: commodity functions for home range calculation. https://r-forge.r-project.org/R/?group_id=1531. Accessed 12 Dec 2018
Prugh LR, Golden CD (2014) Does moonlight increase predation risk? Meta-analysis reveals divergent responses of nocturnal mammals to lunar cycles. J Anim Ecol 83:504–514
Richards SA, Whittingham MJ, Stephens PA (2011) Model selection and model averaging in behavioural ecology: the utility of the IT-AIC framework. Behav Ecol Sociobiol 65:77–89
Riga F, Trocchi V, Toso S (2001) Morphometric differentiation between the Italian hare (Lepus corsicanus De Winton, 1898) and European brown hare (Lepus europaeus Pallas, 1778). J Zool (Lond) 253:241–252
Robbins CT (1983) Wildlife feeding and nutrition. Academic Press, New York, p 123
Rugge C, Mallia E, Perna A, Trocchi V, Freschi P (2009) First contribute to the characterization of coat in Lepus corsicanus and Lepus europaeus by colorimetric determinations. Ital J Anim Sci 8:802–804
Rühe F, Hohmann U (2004) Seasonal locomotion and home range characteristics of European hares (Lepus europaeus) in an arable region in central Germany. Eur J Wildl Res 50:101–111
Santilli F, Paci G, Bagliacca M (2014) Winter habitat selection by the European hare (Lepus europaeus) during feeding activity in a farmland area of southern Tuscany (Italy). Hystrix 25:51–53
Scalera R, Angelici FM (2003) Rediscovery of the Apennine Hare Lepus corsicanus in Corsica. Boll Mus Civ Sci Nat Torino 20:161–166
Schai-Braun SC, Hackländer K (2016) Family Leporidae. Hares and rabbits. In: Wilson DE, Lacher TE, Mittermeier RA (eds) Handbook of the mammals of the world. Volume 6. Lagomorphs and Rodents I. Lynx Editions, Barcelona, pp 62–148
Schai-Braun SC, Rödel HG, Hackländer K (2012) The influence of daylight regime on diurnal locomotor activity patterns of the European hare (Lepus europaeus) during summer. Mammal Biol 77:434–440
Seaman DE, Millspaugh JJ, Kernohan BJ, Brundige GC, Raedeke KJ, Gitzen RA (1999) Effects of sample size on kernel home range estimates. J Wildl Manag 63:739–747
Smith GW (1990) Home range and activity patterns of black-tailed jackrabbits. Great Basin Naturalist 50:249–256
Smith DF, Litvaitis JA (2000) Foraging strategies of sympatric lagomorphs: implications for differential success in fragmented landscapes. Can J Zool 78:2134–2141
Stewart KM, Bowyer RT, Kie JG, Cimon NJ, Johnson BK (2002) Temporospatial distributions of elk, mule deer, and cattle: resource partitioning and competitive displacement. J Mammal 83:229–244
Stokes MK, Slade NA, Blair SM (2001) Influences of weather and moonlight on activity patterns of small mammals: a biogeographical perspective. Can J Zool 79:966–972
Stroh G (1931) Zwei sichere Altersmerkmale beim Hasen. Berliner Tierärztl Wschr 47:180–181
Swihart RK (1986) Home range-body mass allometry in rabbits and hares (Leporidae). Acta Theriol 31:139–148
Tapper SC, Barnes RFW (1986) Influence of farming practice on the ecology of the brown hare (Lepus europaeus). J Appl Ecol 23:39–52
Torniai L (2019). Comportamento spaziale della lepre italica Lepus corsicanus. MSc thesis dissertation in Scienze e Gestione della Risorse Faunistico-Ambientali, Dipartimento di Scienze e Tecnologie Agrarie, Alimentari, Ambientali e Forestali—DAGRI, Università degli Studi di Firenze
Toschi A (1965) Fauna d’Italia VII, Mammalia (Lagomorpha-Rodentia-Carnivora-Artiodactyla-Cetacea). Edizioni Calderini, Bologna
Upham NS, Hafner JC (2013) Do nocturnal rodents in the Great Basin Desert avoid moonlight? J Mammal 94:59–72
van Rij J, Wieling M, Baayen R, van Rijn H (2017) “itsadug”: interpreting time series and autocorrelated data using GAMMs.” R package version 2.3. Accessed 12 Aug 2019
Weterings MJA, Moonen S, Prins HHT, van Wieren SE, van Langewelde FW (2018) Food quality and quantity are more important in explaining foraging of an intermediate-sized mammalian herbivore than predation risk or competition. Ecol Evol 8:8419–8432
Weterings MJA, Ewert SP, Peereboom JN, Kuipers HJ, Kuijper DPJ, Prins HHT, Jansen PA, van Langewelde FW, van Wieren SE (2019) Implications of shared predation for space use in two sympatric leporids. Ecol Evol. https://doi.org/10.1002/ece3.4980
Wood SN (2006) Generalized additive models: an introduction with R. Chapman and Hall/CRC, Boca Raton
Wood SN (2013) A simple test for random effects in regression models. Biometrika 100:1005–1010
Wood SN (2017) Generalized additive models: an introduction with R, 2nd edn. CRC/Taylor & Francis, Boca Raton
Wood SN (2019). Package ‘mgcv’. Mixed GAM computation vehicle with automatic smoothness estimation. https://cran.r-project.org/web/packages/mgcv/mgcv.pdf. Accessed 28 Dec 2019
Zilio A, Martinoli A, Chiarenzi B, Preatoni D (1997) Analisi della dispersione e sopravvivenza durante le prime fasi successive al rilascio in lepri (Lepus europaeus) di cattura utilizzate per il ripopolamento. Atti del III Convegno Nazionale dei Biologi della Selvaggina 14:271–299
Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer Science & Business Media Editions, New York
Acknowledgements
We are indebted to Don Filippo Corsini and to Dr. Cesare Moncini for their logistic and practical support, without which this study could not have been conducted. We are also grateful to Pietro Micca and Luciano Lombrichi for helping us in many ways during our field work. Netting sessions were organised by the personnel of the Tuscan Regional Council (former Grosseto Province): Massimo Machetti, Claudia Biliotti, Guido Donnini, Sonia Longhi, Fiora Meschi, Maurizio Zaccherotti, and, in particular, Gianluca Carfì. Enzo Mori and local hunters participated as volunteers during netting sessions. We thank all students who helped us in the collection of field data, in particular Martina Calosi and Marta Zanchi. Riccardo Fornaroli (Università di Milano Bicocca) provided us with precious technical help in GIS analysis. We thank the Servizio Idrologico Regionale—Regione Toscana, who provided us the meteorological data.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
EM participated in study planning, collected most data, estimated home ranges and conducted habitat selection analysis, wrote the first draft, and participated in writing up all drafts. SL participated in study planning and in writing up all drafts. FC, CG, CG, LT collected data. GR participated in study planning, led hare-netting sessions, and participated in writing up the last draft. FF helped in study planning and in data collection, as well as participated in writing up the last draft. NF collected data, performed non-parametric tests and statistical modelling, and participated in writing up all drafts. All the authors participated to hare-netting sessions, as well as read and approved the manuscript. Two anonymous reviewers and the Associate Editor greatly improved our first draft with their comments.
Corresponding author
Ethics declarations
Conflict of interest
Authors certify that they have no affiliation with or involvement in any organization or entity with any financial or non-financial interest in the subject matter or materials discussed in this manuscript. Thus, they have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Handling editor: Heiko Rödel.
The original online version of this article was revised: The caption of Fig. 4 was incorrect and the electronic supplementary material has been exchanged because Table S1 was incorrectly named Table S2.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mori, E., Lovari, S., Cozzi, F. et al. Safety or satiety? Spatiotemporal behaviour of a threatened herbivore. Mamm Biol 100, 49–61 (2020). https://doi.org/10.1007/s42991-020-00013-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-020-00013-1