Abstract
Introduction
Hematopoietic stem and progenitor cells (HSPC) are responsible for the reconstitution of blood cells and thought to contribute to peripheral tissue repair. Following acute exercise in young adults, HSPC are mobilized from their niche in bone marrow into circulation, however the kinetics of mobilization following exercise is not well understood in older adults. In the present study, we aimed to investigate how exercise intensity influences mobilization of specific subpopulations of HSPC, and how mobilization is affected by aging.
Methods
Healthy older men (OM; 69.9 ± 2.0 years) and young men (YM; 21.5 ± 0.8 years) performed three separate bouts of exercise on a cycle ergometer: 70% of their peak work rate (WRpeak) until volitional fatigue, 30% of their WRpeak work matched to the 70% WRpeak trial, and a high intensity interval training (HIIT) trial. Blood samples were collected before, immediately post, and 10, 30, and 60 min post-exercise. Total blood cells, hematocrit, and mononuclear cells isolated by density gradient centrifugation were quantified using flow cytometry.
Results
Mononuclear cells, CD34+, CD34+/CD38+, CD34+/CD110+, CD3+/CD(16 + 56)+, CD11c+/CD123−, and CD11c−/CD123+ cells per millilitre of blood increased immediately post-exercise, but predominately in the 70% WRpeak trial in both OM and YM.
Conclusion
Taken together, our evidence suggests that higher intensity exercise but also volume is necessary for the mobilization of HSPC. Furthermore, the pattern of mobilization was nearly identical in OM as compared to YM though to a lesser extent than their younger counterparts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mature hematopoietic cells are largely present in general circulation, while hematopoietic stem and progenitor cell populations (HSPC) are primarily found in their niche in the bone marrow. They are maintained in the niche through various adhesion proteins but can be released from the niche when induced to do so. Renewal of the approximate 10 billion mature blood cells in circulation requires proliferation and differentiation of HSPC and it is also widely accepted that HSPC may contribute to peripheral tissue repair [3]. On any given day between 1% and 5% of cells are exchanged between the bone marrow and general circulation and mobilization of HSPC from bone marrow can be influenced by various factors. Additionally, in the clinical setting, mobilization of bone marrow cells into circulation and subsequent harvest of cells is an important step in the treatment of hematopoietic malignancies, through stem cell transplants. A successful transplant can cure hematological malignancies, however several factors contribute to the success of a transplant, including the number of cells harvested and the general quality of a graft. Traditionally, the harvest of an adequate number of CD34+ cells, thought to represent the most primitive HSPC population, was the only factor considered during the harvest of HSPC for the purpose of transplantation. More recently, it has been established that the highest quality of a graft and therefore the greatest likelihood of success upon transplantation should include all HSPC populations.
A hallmark observation of aging is a loss in bone marrow cellularity associated with a reciprocal increase in bone marrow fat (Longo [20]. In fact, although the effect of aging on HSPC number is controversial the decline in their function is well documented [19] as is a difficulty in mobilizing enough functional HSPC in advanced age. For these reasons, clinically, there is a donor age restriction of 60 years after which one can no longer serve as a donor. HSPC are typically mobilized from bone marrow with granulocyte-colony stimulating factor (G-CSF) in order to provide sufficient cells for a graft [16]. G-CSF is a colony stimulating hormone that stimulates production of granulocytes but also has potent mobilization properties and is the most commonly used clinical mobilizing agent. Importantly, G-CSF fails to mobilize sufficient numbers of peripheral blood stem cells in up to 40% of patients [15]. This issue has been addressed with some degree of success by adding Plerixafor, a reversible CXCR-4 chemokine receptor antagonist that results in an increase in CD34+ cell mobilization [21]. Other strategies have used combinations of G-CSF with GM-CSF or chemotherapy agents and the addition of Plerixafor. While Plerixafor can significantly improve mobilization success, failure rates can remain as high as 30% in some patients [28]. There are clear limitations to pharmacological intervention, which begs the question of whether atypical methods for HSPC mobilization could be effective alternatives or be used in conjunction with pharmaceuticals to improve HSPC mobilization. One such alternative may be acute exercise.
The appearance of HSPC (CD34+ cells) in circulation have been shown to increase immediately following various exercise protocols, in both men and women and across various age ranges [1, 6, 7, 22, 23, 34,35,38]. Importantly, work by Baker et al. [4] recently reported that the mobilization of various HSPC populations could be mobilized into circulation in an intensity dependent manner. In this study, young men performed a 70% VO2peak test to volitional failure as well as a 30% VO2peak trial work matched to the 70% trial and blood draws were taken before and at various time-points following each trial. Various HSPC populations, known to be important for a successful graft, significantly mobilized following the 70% trial with the peak increase occurring immediately following exercise and returning to baseline levels within 30 min, while there was generally no increase in the 30% trial suggesting that the mobilization of HSPC into circulation occurs in an intensity dependent fashion. The importance of exercise intensity in mobilizing HSPC populations was also demonstrated in a cohort of wheelchair athletes who performed a 25 km time trial. Following this trial, the extent of circulating progenitor cells was positively correlated with lactate concentrations suggesting that higher exercise intensities better mobilized progenitor cells [23]. In addition, a recent study demonstrated that older men mobilized fewer circulating angiogenic cell populations, including CD34+ cells, after a 30 min trial at 70% of VO2max [29].
Collectively, there is little information on exercise and the importance of exercise intensity as a stimulus to mobilize HSPC. Moreover, there is even less information on mobilization of HSPC using exercise in older adults. The purpose of the present study was to further investigate the role of exercise intensity in mobilizing various HSPC populations in both young and older adults.
Methods
Participants
Eight young men (n = 8; 21.5 ± 0.8 year, mean ± SEM) and eight older men (n = 8; 69.9 ± 2.0 year, mean ± SEM) were recruited to participate in this study. Participants were asked to refrain from strenuous exercise but otherwise maintain their daily routine during participation in the study. Exclusion criteria included smoking, diabetes, the use of nonsteroidal anti-inflammatory drugs (NSAIDs) and/or statins, a history of respiratory disease and/or any major orthopedic disability or hip replacement. The study was approved by the Hamilton Health Sciences Integrated Research Ethics Board (14-734) and conformed to the guidelines outlined in the Declaration of Helsinki. Participants gave their informed written consent before inclusion into the study. For subject characteristics, see Table 1.
Exercise protocol
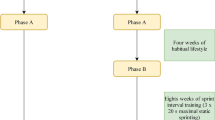
Participants reported to the laboratory to perform a ramp incremental exercise test (50 W baseline for 3 min followed by a 30 W/min ramp) on an electrically braked cycle ergometer (Excalibur Sport V2.0; Lode, Groningen, The Netherlands) for determination of peak VO2 (VO2peak) and peak work rate (WRpeak). Expired gas and ventilatory parameters were collected for the determination of VO2peak (Moxus Metabolic System; AEI Technologies, Pittsburgh, PA). Subjects were asked to maintain a cadence between 60 and 70 rpm during the test, and the test was terminated upon volitional fatigue. Following a 1-week washout period during which participants refrained from any structured exercise or physical activity, participants performed a cycling high intensity interval training (HIIT) session. Participants performed HIIT on a cycle ergometer (Kettler Racer GT Indoor Cycle Trainer, Kettler Alurad; Germany) while wearing a heart rate (HR) monitor (H7 Heart Rate Sensor; Polar Electro Canada, Lachine, QC). Following a 3 min warm-up at 25 W, subjects completed 10 × 60 s intervals at a workload which elicited ~ 90% maximal HR (HRmax), while maintaining a cadence of ≥ 90 rpm. Workload was adjusted as needed to maintain an average HR of ~ 90% HRmax over the 10 intervals. Intervals were interspersed with 60 s of rest where subjects cycled at a self-selected pace against 25 W. The workload of the high intensity bouts was based on HIIT recommendations [14]. Following another 1-week washout period, participants exercised at a cycler ergometer workload (Kettler Racer GT Indoor Cycle Trainer, Kettler Alurad; Germany) pertaining to 70% of their WRpeak until volitional fatigue following a 3 min warm-up at 25 W. Following a final 1-week washout period, participants performed a bout of cycling at 30% WRpeak (Kettler Racer GT Indoor Cycle Trainer, Kettler Alurad; Germany), with the work output matched to the 70% WRpeak bout following a 3 min warm-up at 25 W.
Blood collection and processing
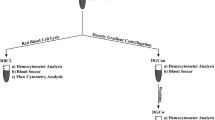
Fifteen milliliters of blood was collected via venous catheter before (pre), immediately after (post), and 10, 30, and 60 min post-exercise using heparinized vacutainers (0268795, Fisher Scientific Canada). Importantly, blood was drawn immediately following the final interval bout of the HIIT condition, before the active rest period. Total blood volume (mL) was recorded. Whole blood total cell counts were done using a countess automated cell counter (Invitrogen, Carlsbad, CA). Hematocrit was measured with microhematocrit tubes (22-274-913; Beckman Coulter). Mononuclear cells were then isolated from the remainder of the blood using Ficoll-Paque Plus [12] (17-1440-02; GE Healthcare Life Sciences), suspended in 1% bovine serum albumin in PBS, and were counted using a Countess automated cell counter.
Flow cytometry
Mononuclear cells were analyzed via flow cytometry (CyFlow Space, Partec) immediately after isolation from blood. The following antibodies were used in the analysis: CD34 (FAB7227G; R&D Systems), CD110 (FAB1016A; R&D Systems), CD11c (FAB1777N; R&D Systems), CD123 (FAB301P; R&D Systems), CD4 (FAB3791F; R&D Systems), CD38 (FAB2404A; R&D Systems), CD41 (FAB7616A; R&D Systems), and CD3/CD(16 + 56) cocktail (319101; BioLegend). Antibody concentrations used reflect manufacturer recommendations. At least 1 × 106 cells were stained for each antibody, with at least 5 × 105 events captured and analyzed. Unstained and single stain controls were used for compensation and gating. Final gates were based on FSC/SSC and two parameter plots. For analysis, doublets discrimination was applied [4, 9]. Data were analyzed and expressed as the number of stain-positive cells within viable mononuclear cells (MNC) per mL of total blood.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7 analysis software (San Diego, CA). Data and graphs are expressed as mean ± standard error (SE) with P < 0.05 considered to be statistically significant. Statistical differences between time-points and groups were determined using two-way repeated measures ANOVA, with Bonferroni corrections applied to adjust for multiple comparisons. To assess differences at baseline (pre) and the change in cells from pre to subsequent post-exercise timepoint between OM and YM, Student’s t test was performed and adjusted appropriately.
Results
Demographics
By design, the age of the OM was significantly greater than the YM (Table 1, P < 0.05). With the exception of height, there were no significant differences between OM and YM in weight or BMI (Table 1).
Total blood cell counts and hematocrit
No significant differences from pre were detected at any time point, in either age group, or between any of the exercise trials (data not shown).
Mononuclear cell numbers per mL of blood increased following exercise in an intensity but not age related manner. Total blood count and hematocrit were not significantly different from pre, mononuclear cells (MNC) impacted by exercise. In OM, following the 70% WRpeak trial, MNC number increased significantly (~ 211%) immediately post-exercise (P < 0.05). At post, MNC number was greater in the 70% WRpeak trial compared to the 30% and HIIT trials (Fig. 1a, P < 0.05). No significant differences in MNC number were observed in the 30% WRpeak or the HIIT trial, at any time point. In YM, following the 70% WRpeak trial, MNC number increased significantly by (~ 245%) immediately post exercise (P < 0.05). At post, MNC number was greater in the 70% WRpeak trial compared to the 30% and HIIT trials (Fig. 1b, P < 0.05). No significant differences in MNC number were observed in the 30% WRpeak or the HIIT trial, at any time point. There was no significant difference detected when comparing the change in MNC from pre to post between OM and YM (P < 0.05). In order to determine whether specific populations of HSPC and leukocytes were affected by exercise, cell populations were further analyzed via flow cytometry. The differences between these cell subpopulation at rest in OM compared to YM are expressed in Table 2.
Mononuclear cell number per mL of blood, as isolated through density gradient centrifugation, for the 70% WRpeak (○), 30% WRpeak (■) and HIIT (▽) exercise trials across the time course in OM (a) and YM (b). *P < 0.05 difference between the 70% WRpeak trial and the 30% WRpeak trial at time point. †P < 0.05 difference between 70% WRpeak trial and the HIIT trial at timepoint. ‡P < 0.05 significantly greater than pre in 70% WRpeak trial
CD34+ cells (general hematopoietic progenitors)
In OM, following the 70% WRpeak trial, CD34+ cell number per mL significantly increased from pre to immediately post exercise (~ 101%, P < 0.05). At the post timepoint, CD34+ cell number per mL was significantly greater in the 70% WRpeak trial as compared to the post timepoint in the 30% WRpeak and HIIT trials (Fig. 2a, P < 0.05). No significant differences in CD34+ cell number per mL were observed in the 30% WRpeak or the HIIT trial, at any time point. In YM, following the 70% WRpeak trial, CD34+ cell number per mL significantly increased from pre to immediately post-exercise (~ 352%, P < 0.05). CD34+ cell number per mL was significantly greater in the 70% WRpeak trial at the post timepoint, 10 min and 60 min post-exercise as compared to the HIIT trial (Fig. 2a, P < 0.05). CD34+ cell number per mL was greater into 70% WRpeak trial at the post and 60 min post-exercise timepoints as compared to the 30% WRpeak trial (Fig. 2a, P < 0.05). No significant differences in CD34+ cell number were observed in the 30% WRpeak or the HIIT trial, at any time point. There was a significantly greater increase in CD34+ cell number per mL in the YM as compared to the OM in response to the 70% WRpeak (P < 0.05).
CD34+ cells per mL blood for the 70% WRpeak (○), 30% WRpeak (■) and HIIT (▽) exercise trials across the time course in OM (a) and YM (b). *P < 0.05 difference between the 70% WRpeak trial and the 30% WRpeak trial at time point. †P < 0.05 difference between 70% WRpeak trial and the HIIT trial at timepoint. ‡P < 0.05 significantly greater than Pre in 70% WRpeak trial
CD34+/CD38− (most primitive and undifferentiated CD34+ population)
In OM, following the 70% WRpeak trial, CD34+/CD38− cell number per mL significantly increased from pre to immediately post-exercise (~ 56%, P < 0.05). At the post timepoint, CD34+/CD38− cell number per mL was significantly greater in the 70% WRpeak trial as compared to the post timepoint in the 30% WRpeak and HIIT trials (Fig. 3a, P < 0.05). No significant differences in CD34+ cell number per mL were observed in the 30% WRpeak or the HIIT trial, at any time point.
Specific hematopoietic stem cell (HSC) populations. Three different blood cell populations, analyzed by flow cytometry. CD34+/CD38− in OM (a) and YM (b); CD34+/CD110+ in OM (c) and YM (d); CD3−/CD(16 + 56)+ in OM (e) and YM (f). All values are expressed as positive cells per mL of blood for the 70% WRpeak (○), 30% WRpeak (■) and HIIT (▽) exercise trials across the time course. *P < 0.05 difference between the 70% WRpeak trial and the 30% WRpeak trial at time point. †P < 0.05 difference between 70% WRpeak trial and the HIIT trial at timepoint. ‡P < 0.05 significantly greater than pre in 70% WRpeak trial. §P < 0.05 significantly greater than pre in the HIIT trial
In YM, following the 70% WRpeak trial, CD34+/CD38− cell number per mL significantly increased from pre to immediately post-exercise (~ 251%, P < 0.05). There was no significant differences between the 70% WRpeak trial and the HIIT trial (P = 0.1, Fig. 3b) and the 30% WRpeak trial (P = 0.1, Fig. 3b). No significant differences in CD34+ cell number were observed in the 30% WRpeak or the HIIT trial, at any time point. There was no significant difference detected when comparing the change in CD34+/CD38− cell number per mL from pre to post in OM to YM (P < 0.05) in response to the 70% WRpeak (P < 0.05, Fig. 5).
CD34+/CD110+ (megakaryocyte progenitors)
In OM, following the 70% WRpeak trial, CD34+/CD110+ cell number per mL significantly increased from pre to immediately post-exercise (~ 150%, P < 0.05). At the post timepoint, CD34+/CD110+ cell number per mL was significantly greater in the 70% WRpeak trial as compared to the post timepoint in the 30% WRpeak and HIIT trials (Fig. 3c, P < 0.05). No significant differences in CD34+/CD110+ cell number per mL were observed in the 30% WRpeak or the HIIT trial, at any time point. In YM, following the 70% WRpeak trial, CD34+/CD110+ cell number per mL significantly increased from pre to immediately post-exercise (~ 373%, P < 0.05). CD34+/CD110+ cell number per mL was significantly greater in the 70% WRpeak trial at the immediately post and 60 min post-exercise timepoints as compared to the 30% WRpeak and HIIT trial (Fig. 3d, P < 0.05). No significant differences in CD34+/CD110+ cell number were observed in the 30% WRpeak or the HIIT trial, at any time point. There was a significantly greater increase in CD34+/CD110+ cell number per mL in the YM as compared to the OM in response to the 70% WRpeak (P < 0.05, Fig. 5).
CD3−/CD(16 + 56)+ (natural killer cells)
In OM, following the 70% WRpeak trial, CD3−/CD(16 + 56)+ cell number per mL significantly increased from pre to immediately post-exercise (~ 554% from pre, P < 0.05) and 10 min post-exercise (~ 171% from pre, P < 0.05). CD3−/CD(16 + 56)+ cell number per mL was significantly greater in the 70% WRpeak trial as compared to the 30% WRpeak trial immediately post and 10 min post-exercise (Fig. 3e, P < 0.05). When compared to the HIIT trial, CD3−/CD(16 + 56)+ cell number per mL was greater in the 70% WRpeak trial immediately post exercise (Fig. 3e, P < 0.05). No significant differences in CD3−/CD(16 + 56)+ cell number per mL were observed in the 30% WRpeak or the HIIT trial, at any time point. In YM, following the 70% WRpeak trial, CD3−/CD(16 + 56)+ cell number per mL significantly increased from pre to immediately post-exercise (~ 1125%, P < 0.05). In the HIIT trial, CD3−/CD(16 + 56)+ cell number per mL was increased from pre to immediately post-exercise (~ 11%, P < 0.05). CD3−/CD(16 + 56)+ cell number per mL was significantly greater in the 70% WRpeak trial as compared to the 30% WRpeak (Fig. 3f, P < 0.05) but not the HIIT trial immediately post-exercise. No significant differences in CD3−/CD(16 + 56)+ cell number were observed in the 30% WRpeak trial, at any time point. There was no significant difference detected when comparing the change in CD3−/CD(16 + 56)+ cell number per mL from pre to post between OM and YM in response to the 70% WRpeak (P < 0.05, Fig. 5). In comparing the response to the HIIT trial in OM and YM, CD3−/CD(16 + 56)+ cell number per mL did not reach significance (P = 0.07).
CD11c+/CD123− and CD11c−/CD123+ (dendritic type 1 and type 2 cells, respectively)
In OM, there was a main effect for time, with the CD11c+/CD123− cell number per mL being greater immediately post-exercise as compared to pre across all trials (Fig. 4a, P < 0.05). Following the 70% WRpeak trial, CD11c−/CD123+ cell number per mL significantly increased from pre to immediately post-exercise (~ 210%, P < 0.05). At the post timepoint, CD11c−/CD123+ cell number per mL was significantly greater in the 70% WRpeak trial as compared to the post timepoint in the 30% WRpeak and HIIT trials (Fig. 4c, P < 0.05). No significant differences in CD11c−/CD123+ cell number per mL were observed in the 30% WRpeak or the HIIT trial, at any time point.
Specific dendritic cell populations. CD11c+/CD123− in OM (a) and YM (b). CD11c−/CD123+ in OM (c) and YM (d). All values are expressed as positive cells per mL of blood for the 70% WRpeak (○), 30% WRpeak (■) and HIIT (▽) exercise trials across the time course. *P < 0.05 difference between the 70% WRpeak trial and the 30% WRpeak trial at time point. †P < 0.05 difference between 70% WRpeak trial and the HIIT trial at timepoint. ‡P < 0.05 significantly greater than pre in 70% WRpeak trial. #Main effect across all modalities, post significantly greater than pre, P < 0.05
In YM, following the 70% WRpeak trial, CD11c+/CD123− cell number per mL significantly increased from pre to immediately post, 30 min and 60 min post-exercise (~ 311%, ~ 128% and ~ 321%, from pre respectively P < 0.05). CD11c+/CD123− cell number per mL was significantly greater in the 70% WRpeak trial immediately post, 30 min and 60 min post-exercise timepoints as compared to the 30% WRpeak (Fig. 4b, P < 0.05), and immediately post timepoint as compared to the HIIT trial (Fig. 4b, P < 0.05). No significant differences in CD11c+/CD123− cell number were observed in the 30% WRpeak or the HIIT trial, at any time point. There was a significantly greater increase in CD11c+/CD123− cell number per mL in the YM as compared to the OM in response to the 70% WRpeak (P < 0.05).
Following the 70% WRpeak trial, CD11c−/CD123+ cell number per mL significantly increased from pre to immediately post (~ 505%, P < 0.05, Fig. 4b). No significant differences in CD11c−/CD123+ cell number were observed in the 30% WRpeak or the HIIT trial, at any time point. CD11c−/CD123+ cell number per mL were non-significantly increased immediately post (~ 67%, P = 0.07) following the 30% WRpeak trial. There was no significant difference detected when comparing the change in CD11c−/CD123+ cell number per mL from pre to post between OM and YM in response to the 70% WRpeak (P > 0.05, Fig. 5).
CD34+/CD41+ (myeloid and megakaryocyte progenitors)
In OM, there was a main effect for exercise modality, with the CD34+/CD41+ cell number per mL being greater in the 70% WRpeak trial as compared to the 30% WRpeak and the HIIT trial (P < 0.05, data not shown).
CD4+ (lymphocytes that coordinate the immune response)
In OM, following the 70% WRpeak trial, CD4+ cell number per mL significantly increased from pre to immediately post exercise (~ 68% from pre, P < 0.05). CD4+ cell number per mL was significantly greater in the 70% WRpeak trial as compared to the 30% WRpeak and HIIT trial immediately post exercise (P < 0.05, data not shown).
Discussion
In the present study, we observe that moderate-intensity, higher volume endurance exercise mobilizes mononuclear cells into circulation. More importantly, various hematopoietic stem and progenitor (HSPC) populations were mobilized into circulation with the greatest number of cells detected immediately after exercise cessation. Lower intensity endurance as well as high-intensity, low volume interval training failed to mobilize nearly all HSPC, suggesting that mobilization is both intensity and volume dependent. Additionally, although older men retain the capacity to mobilize HSPC through moderate intensity exercise, the extent of specific HSC and/or dendritic cell populations was altered in the time course of appearance as well as a presenting with a diminished response to exercise as compared to their younger counterparts.
A primary finding in this study was that exercise intensity is a critically important factor for successful mobilization of HSPC. Consistent with previous work [4], we found that 70% WRpeak to volitional fatigue was a sufficient stimulus for general mononuclear cell mobilization and various HSPC. It is important to note that the mobilization of specific HSPC was likely simply a reflection of general mononuclear cell mobilization and was not indicative of exercise mobilizing specific HSPC. In contrast to the moderate intensity, higher volume trial, we observed that 30% WRpeak work-matched to the 70% trial did not induce any appreciable mobilization of HSPC, reaffirming the notion that exercise intensity is a critical factor for HSPC mobilization in both OM and YM. It is widely accepted that various forms of exercise are associated with the release of cytokines, chemokines, metabolites, and growth factors including mobilizing agents such as IL-6, TNF-a, IL-1β, and IGF-1 [11, 25, 35]. The extent of the appearance of these various factors into circulation is often dependent on the intensity of exercise [2]. Accordingly, it is entirely possible that the intensity-dependent mobilization of bone marrow derived HSPC is associated with the intensity-dependent release of various cytokines and growth factors. It is also possible that the mobilization of HSPC associated with exercise is directly related to the redistribution of blood flow with exercise. The redistribution of blood at the onset of exercise not only affects the working muscles but there is also a significant redistribution of flow through the highly vascularized bone marrow niche [33, 34]. Increased flow through the niche could mobilize HSPC residing in the vascular niche of bone marrow. Cells residing in this niche are readily mobilized as compared to cells residing in the endosteal niche where cells are tightly adhered to the endosteum of bone [39]. Enhancing flow through the marrow cavity may mobilize cells into circulation from the vascular niche through a mass-action effect. Taken together, an increase in circulating factors and/or enhanced blood flow may lead to mobilization of HSPC from the marrow into circulation. Evidence from the current study supports this notion, with the highest level of HSPC mobilization detected immediately post-exercise. Interestingly, the HIIT trial, performed as 10 bouts of intermittent exercise at ~ 90% HRmax, did not lead to any appreciable mobilization of HSPC. This suggests that intensity of exercise alone is not sufficient to mobilize HSPC as the bouts of exercise performed in the HIIT trial were performed at a higher work rate than the 70% WRpeak trial. Perhaps a more provocative question is why are HSPC transiently mobilized in response to high intensity exercise. This is not a question that can be readily answered however one can speculate that it may simply be a blood flow issue. Perhaps enhanced blood flow through the marrow cavity results in HSPC in the vascular niche “leaking” into circulation without any biological function. Alternatively, the release of HSPC into circulation may be a generic stress response to increased sympathetic activity perhaps with the intent to aid in the repair of peripheral tissues. A recent clinical trial in human patients demonstrated that when Desipramine, a norepinephrine reuptake inhibitor, was used in conjunction with G-CSF there was an enhanced mobilization of HSPC [31]. It is possible that mobilization of HSPC is related to enhanced sympathetic activity during exercise, which would explain the rapid reuptake of HSPC with the cessation of exercise. Indeed, recent work suggests that CD34+ cell are mobilized through mechanisms involving β2—adrenergic receptors [1]. In this study, subjects completed 3, independent, 30 min cycling trials while on a placebo, a β1—adrenergic receptor antagonist or on a β1 and β2 adrenergic antagonist. CD34+ cell number only increased following exercise while on the placebo and the β1 adrenergic receptor antagonist suggesting that CD34+ cells are mobilized through mechanism involving β2—adrenergic receptors. Alternatively, the mobilization of HSPC may be related to the plethora of cytokines and growth factors associated with acute exercise. A recent study reported production of both medullary and extramedullary cytokines and mobilizing factors in response to exercise in mice, which may be a mechanism for exercise-induced HSPC mobilization [10].
It is well documented that there is a decrease in marrow cellularity with aging [4]. The decrease in marrow cellularity, with a complimentary increase in marrow fat in older adults could reduce the number of cells available for mobilization. Indeed, for several but not all HSPC, we detected fewer cells in OM as compared to YM at baseline (Table 2). Although a simple decrease in marrow cell number could contribute to a lower number of cells mobilized, there are likely other age-related factors that contribute to reduced HSPC in circulation. For example, Bonsignore et al. [7] found that there was an inverse correlation between circulating TNF-α and circulating HSPC quantity 24 h following exercise. TNF-α expression appears to be elevated in aging bone [27] and in circulation [17]. Therefore, it stands to reason that elevated levels of this cytokine, at rest in OM, may serve to reduce the capacity for complete mobilization. The reality, however, is that many different cytokines are known to play a role in mobilization of HSPC, many of which appear to be altered by aging such as IL-6, IL-1β [24], and IGF-1 binding protein [5]. Age-related changes in any one of many different cytokines could alter the mobilization kinetics of HSPC. Importantly, however the pattern of HSPC mobilization in OM was nearly identical to that in YM suggesting that even in advanced age older adults retain the capacity to mobilize HSPC in response to higher intensity exercise. This may or may not be relevant for clinical purposes where after the age of 60 years one can no longer act as a stem cell donor. This decision is primarily justified by the relative inability to harvest an adequate number of HSPC from older adults, as HSPC quality does not appear to be affected by aging [13].
Although it has been thought that mobilization of threshold doses of CD34+ cells is most important for a successful transplant [32], it is now apparent that mobilization of clinically relevant HSPC, beyond CD34+ alone, could mean the difference between a successful or an unsuccessful transplant. In fact, it has been shown that grafts with a greater content of CD34+/CD38− cells, a more primitive HSPC correlates with overall recipient survival [18]. Here we demonstrate that moderate intensity, higher volume exercise was capable of mobilizing these cells in both YM and OM (Fig. 3a, b, respectively). Similarly, the CD34+/CD110+ population, a platelet progenitor population increased in both OM and YM immediately following exercise before returning to baseline levels 10 min following exercise in the moderate intensity, higher volume trial (Fig. 4c, d, respectively). Increased graft content of this population correlates with increased platelet reconstitution following transplantation [30]. Immune reconstitution following transplant is absolutely critical in order to resist infection in myoablated patients. Thus, increased CD16+/CD56+ natural killer cell content in a graft is necessary for successful immune reconstitution [26]. Consistent with the previous HSPC responses to exercise, moderate intensity higher volume exercise increased CD16+/CD56+ cells immediately following exercise in both YM and OM (Fig. 3e, f, respectively). Finally, the presence of high type one dendritic cell content (CD11c+/CD123−), and high type two dendritic cell content (CD11c−/CD123+), have been shown to be predictive of overall survival following transplantation [8]. Again, consistent with the other tested populations we observed an increase in CD11c−/CD123+, and CD11c+/CD123− cells following moderate intensity high volume exercise in both older and younger populations (Fig. 4).
Collectively, we demonstrate that moderate intensity exercise at higher volume can effectively mobilize several HSPC. Interestingly, lower intensity exercise or high intensity lower volume exercise could not achieve the same level of mobilization. This suggests that the optimal form of exercise for HSPC mobilization requires a combination of intensity and volume. Having said this, even the higher volume group only exercised for ~ 17 min or ~ 16 min (YM and OM respectively) on average suggesting that it is not an extreme stimulus needed for mobilization of HSPC. We also show that although OM had lower cell count for some HSPC at baseline, OM were still quite responsive to the moderate intensity higher volume exercise stimulus suggesting that older men retain the ability to mobilize HSPC with exercise. Future studies should focus on the underlying mechanisms as to how exercise results in HSPC mobilization. Additionally, it is unclear whether the greatest concentration of exercise-mobilized cells is reflected at the end of exercise or whether cells are rapidly mobilized shortly after the onset of exercise and whether peak concentrations of cell populations are achieved during exercise rather than following the cessation of exercise. These questions can be addressed by taking blood draws throughout the exercise session rather than at the end of exercise and during the recovery period. Further, the combination of pharmaceutical approaches with exercise for optimal mobilization and HSPC collection should be examined as a potential optimal therapeutic approach.
References
Agha NH, Baker FL, Kunz HE, Graff R, Azadan R, Dolan C, Laughlin MS, Hosing C, Markofski MM, Bond RA, Bollard CM, Simpson RJ. Vigorous exercise mobilizes CD34 + hematopoietic stem cells to peripheral blood via the β2-adrenergic receptor. Brain Behav Immun. 2018;68:66–75. https://doi.org/10.1016/j.bbi.2017.10.001.
Antunes BM, Campos EZ, Thomatieli RV, dos Santos J, Rosa-Neto C, Franchini E, Bishop NC, Lira FS. Anti-inflammatory response to acute exercise is related with intensity and physical fitness. J Cell Biochem. 2019;120(4):5333–42. https://doi.org/10.1002/jcb.27810.
Attar EC, Scadden DT. Regulation of hematopoietic stem cell growth. Leukemia. 2004;18(11):1760–8. https://doi.org/10.1038/sj.leu.2403515.
Baker JM, Nederveen JP, Parise G. Aerobic exercise in humans mobilizes HSCs in an intensity-dependent manner. J Appl Physiol. 2017;122(1):182. https://doi.org/10.1152/japplphysiol.00696.2016.
Benbassat CA, Maki KC, Unterman TG. Circulating levels of insulin-like growth factor (IGF) binding protein-1 and -3 in aging men: relationships to insulin, glucose, IGF, and dehydroepiandrosterone sulfate levels and anthropometric measures. J Clin Endocrinol Metab. 1997;82(5):1484–91. https://doi.org/10.1210/jcem.82.5.3930.
Bonsignore MR, Morici G, Riccioni R, Huertas A, Petrucci E, Veca M, Mariani G, Bonanno A, Chimenti L, Gioia M, Palange P, Testa U. Hemopoietic and angiogenetic progenitors in healthy athletes: different responses to endurance and maximal exercise. J Appl Physiol. 2010;109(1):60–7. https://doi.org/10.1152/japplphysiol.01344.2009.
Bonsignore MR, Morici G, Santoro A, Pagano M, Cascio L, Bonanno A, Abate P, Mirabella F, Profita M, Insalaco G, Gioia M, Vignola AM, Majolino I, Testa U, Hogg JC. Circulating hematopoietic progenitor cells in runners. J Appl Physiol. 2002;93(5):1691–7. https://doi.org/10.1152/japplphysiol.00376.2002.
Dean R, Masci P, Pohlman B, Andresen S, Serafino S, Sobecks R, Kuczkowski E, Curtis J, Maciejewski J, Rybicki L, Kalaycio M, Hsi E, Theil K, Bolwell BJ. Dendritic cells in autologous hematopoietic stem cell transplantation for diffuse large B-cell lymphoma: graft content and post transplant recovery predict survival. Bone Marrow Transplant. 2005;36(12):1049–52.
De Lisio M, Parise G. Characterization of the effects of exercise training on hematopoietic stem cell quantity and function. J Appl Physiol (1985). 2012;113(10):1576–84.
Emmons R, Niemiro GM, Owolabi O, De Lisio M. Acute exercise mobilizes hematopoietic stem and progenitor cells and alters the mesenchymal stromal cell secretome. J Appl Physiol. 2016;120(6):624–32. https://doi.org/10.1152/japplphysiol.00925.2015.
Frystyk JAN. Exercise and the growth hormone-insulin-like growth factor axis. Med Sci Sports Exerc. 2010;42(1):58–66. https://doi.org/10.1249/MSS.0b013e3181b07d2d.
Fuss IJ, Kanof ME, Smith PD, Zola H. Isolation of whole mononuclear cells from peripheral blood and cord blood. Current protocols in immunology, Chapter 7: Unit 7.1. New York: Wiley; 2009. https://doi.org/10.1002/0471142735.im0701s85.
Garvin K, CF J, Sharp G, Berger A. Does the number or quality of pluripotent bone marrow stem cells decrease with age? Clin Orthop Relat Res. 2007;465:202–7. https://doi.org/10.1097/BLO.0b013e318159a9b8.
Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590(5):1077–84. https://doi.org/10.1113/jphysiol.2011.224725.
Giralt S, Costa L, Schriber J, DiPersio J, Maziarz R, McCarty J, Shaughnessy P, Snyder E, Bensinger W, Copelan E, Hosing C, Negrin R, Petersen FB, Rondelli D, Soiffer R, Leather H, Pazzalia A, Devine S. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20(3):295. https://doi.org/10.1016/j.bbmt.2013.10.013.
Guardiola P, Runde V, Bacigalupo A, Ruutu T, Locatelli F, Boogaerts MA, Pagliuca A, Cornelissen JJ, Schouten HC, Carreras E, Finke J, van Biezen A, Brand R, Niederwieser D, Gluckman E, de Witte TM, Subcommittee for Myelodysplastic Syndromes of the Chronic Leukaemia Working Group of the European Blood and Marrow Transplantation Group. Retrospective comparison of bone marrow and granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells for allogeneic stem cell transplantation using HLA identical sibling donors in myelodysplastic syndromes. Blood. 2002;99(12):4370–8.
Hasegawa Y, Sawada M, Ozaki N, Inagaki T, Suzumura A. Increased soluble tumor necrosis factor receptor levels in the serum of elderly people. Gerontology. 2000;46(4):185–8. https://doi.org/10.1159/000022157.
Hénon PH, Sovalat H, Bourderont D. Importance of CD34 + cell subsets in autologous PBSC transplantation: the mulhouse experience using CD34 + CD38-cells as predictive tool for hematopoietic engraftment. J Biol Regul Homeost Agents. 2001;15(1):62–7. http://www.ncbi.nlm.nih.gov/pubmed/11388746.
Lee J, Yoon S, Choi I, Jung H. Causes and mechanisms of hematopoietic stem cell aging. Int J Mol Sci. 2019;20(6):1272. https://doi.org/10.3390/ijms20061272.
Longo DL. Bone marrow in aging: changes? Yes; clinical malfunction? Not so clear. Blood. 2008;112(11):sci-1.
Milone G, Martino M, Spadaro A, Leotta S, Di Marco A, Scalzulli P, Cupri A, Di Martina V, Schinocca E, Spina E, Tripepi G. Plerixafor on-demand combined with chemotherapy and granulocyte colony-stimulating factor: significant improvement in peripheral blood stem cells mobilization and harvest with no increase in costs. Br J Haematol. 2014;164(1):113–23. https://doi.org/10.1111/bjh.12606.
Morici G, Zangla D, Santoro A, Pelosi E, Petrucci E, Gioia M, Bonanno A, Profita M, Bellia V, Testa U, Bonsignore MR. Supramaximal exercise mobilizes hematopoietic progenitors and reticulocytes in athletes. Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1496–503. https://doi.org/10.1152/ajpregu.00338.2005.
Niemiro GM, Parel J, Beals J, Van Vliet S, Paluska SA, Moore DR, Burd NA, De Lisio M. Kinetics of circulating progenitor cell mobilization during submaximal exercise. J Appl Physiol. 2017;122(3):675–82. https://doi.org/10.1152/japplphysiol.00936.2016.
Nilsson MI, Bourgeois JM, Nederveen JP, Leite MR, Hettinga BP, Bujak AL, May L, Lin E, Crozier M, Rusiecki DR, Moffatt C, Azzopardi P, Young J, Yang Y, Nguyen J, Adler E, Lan L, Tarnopolsky MA. Lifelong aerobic exercise protects against inflammaging and cancer. PLoS One. 2019;14(1):e0210863. https://doi.org/10.1371/journal.pone.0210863(Edited by Antonio Musaro).
Pedersen BK, Åkerström TCA, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103(3):1093–8. https://doi.org/10.1152/japplphysiol.00080.2007.
Porrata LF, Gastineau DA, Padley D, Bundy K, Markovic SN. Re-infused autologous graft natural killer cells correlates with absolute lymphocyte count recovery after autologous stem cell transplantation. Leuk Lymphoma. 2003;44(6):997–1000. https://doi.org/10.1080/1042819031000077089.
Puchta A, Naidoo A, Verschoor CP, Loukov D, Thevaranjan N, Mandur TS, Nguyen PS, Jordana M, Loeb M, Xing Z, Kobzik L, Larché MJ, Bowdish DM. TNF drives monocyte dysfunction with age and results in impaired anti-pneumococcal immunity. PLoS Pathog. 2016;12(1):e1005368. https://doi.org/10.1371/journal.ppat.1005368.
Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF, Westervelt P, Vij R, Abboud CN, Stockerl-Goldstein KE, Sempek DS, Smith AL, DiPersio JF. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14(9):1045–56. https://doi.org/10.1016/j.bbmt.2008.07.004.
Ross MD, Malone EM, Simpson R, Cranston I, Ingram L, Wright GP, Chambers G, Florida-James G. Lower resting and exercise-induced circulating angiogenic progenitors and angiogenic T cells in older men. Am J Physiol Heart Circ Physiol. 2018;314(3):H392–402. https://doi.org/10.1152/ajpheart.00592.2017.
Sartor MM, Garvin F, Antonenas V, Bradstock KF, Gottlieb DJ. Failure to achieve a threshold dose of CD34 + CD110 + progenitor cells in the graft predicts delayed platelet engraftment after autologous stem cell transplantation. Bone Marrow Transplant. 2007;40(9):851–7. https://doi.org/10.1038/sj.bmt.1705818.
Shastri A, Budhathoki A, Barta SK, Kornblum N, Derman O, Battini R, Raghupathy R, Verma AK, Frenette PS, Braunschweig I, Janakiram M. Stimulation of adrenergic activity by desipramine enhances hematopoietic stem and progenitor cell mobilization along with G-CSF in multiple myeloma: a pilot study. Am J Hematol. 2017;92(10):1047–51. https://doi.org/10.1002/ajh.24843.
Solá C, Maroto P, Salazar R, Mesía R, Mendoza L, Brunet J, López-Pousa A, Tabernero JM, Montesinos J, Pericay C, Martínez C, Cancelas JA, López-López JJ. Bone marrow transplantation: prognostic factors of peripheral blood stem cell mobilization with cyclophosphamide and filgrastim (r-MetHuG-CSF): the CD34+ cell dose positively affects the time to hematopoietic recovery and supportive requirements after high-dose chemotherapy. Hematology. 1999;4(3):195–209.
Spodaryk K, Dabrowski Z. Blood flow in different regions of bone marrow after short-term exercise. Acta Physiol Hung. 1991;77(1):13–7. http://www.ncbi.nlm.nih.gov/pubmed/1950588.
Stabley JN, Moningka NC, Behnke BJ, Delp MD. Exercise training augments regional bone and marrow blood flow during exercise. Med Sci Sports Exerc. 2014;46(11):2107–12. https://doi.org/10.1249/MSS.0000000000000342.
Suzuki K, Shigeyuki N, Mutsuo Y, Manabu T, Koki S, Kazuo S. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev. 2002;8:6–48. http://www.ncbi.nlm.nih.gov/pubmed/12690937.
Thijssen DHJ, Vos JB, Verseyden C, van Zonneveld AJ, Smits P, Sweep FC, Hopman MT, de Boer HC. Haematopoietic stem cells and endothelial progenitor cells in healthy men: effect of aging and training. Aging Cell. 2006;5(6):495–503. https://doi.org/10.1111/j.1474-9726.2006.00242.x.
Wardyn GG, Rennard SI, Brusnahan SK, McGuire TR, Carlson ML, Smith LM, McGranaghan S, Sharp JG. Effects of exercise on hematological parameters, circulating side population cells, and cytokines. Exp Hematol. 2008;36(2):216–23. https://doi.org/10.1016/j.exphem.2007.10.003.
Zaldivar F, Eliakim A, Radom-Aizik S, Leu S-Y, Cooper DM. The effect of brief exercise on circulating CD34 + stem cells in early and late pubertal boys. Pediatr Res. 2007;61(4):491–5. https://doi.org/10.1203/pdr.0b013e3180332d36.
Zhao M, Li L. Dissecting the bone marrow HSC niches. Cell Res. 2016;26(9):975–6. https://doi.org/10.1038/cr.2016.71.
Acknowledgements
G. P. was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Grant (1455843), J. P. N. by a NSERC Canadian Graduate Scholarship (CGS-D).
Author information
Authors and Affiliations
Contributions
JPN, JMB, GP designed the study; JPN, GI, VI, MEP performed data acquisition; JPN, JMB, GI, VI, performed data analysis and interpretation; JPN, JMB, GP provided statistical expertise; JPN and GP wrote the manuscript; JPN, GI, VI, MEP, GP reviewed the manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Nederveen, J.P., Baker, J., Ibrahim, G. et al. Hematopoietic Stem and Progenitor Cell (HSPC) Mobilization Responses to Different Exercise Intensities in Young and Older Adults. J. of SCI. IN SPORT AND EXERCISE 2, 47–58 (2020). https://doi.org/10.1007/s42978-019-00050-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42978-019-00050-4