Abstract
We investigated the anther culture (AC) efficiency of nine genotypes of winter bread wheat (Triticum aestivum L.). The genotype dependency was assessed during the induction of the androgenic callus, i.e., embryo-like structures (ELSs), green-, albino- and acclimatizated plantlets. The highest level of callus formation were shown for samples 120/20 (114.39 ELS per 100 anthers—ELS/100A) and 132/20 (16.26 ELS/100A). The number of green plantlets per 100 anthers (GP/100A) varied from 0 to 3.05 GP/100A with a mean of 0.71 GP/100A. The acclimatized plantlets (ADPs) per 100 regenerated green plantlets ranged in each combination, from 32.00 to 62.50 ADP/100GP with an average value of 35.92. Between 12.50 and 60.00 doubled haploid (DH) plants per 100 acclimatized plantlets (DH/100ADP), depending on the combination, with a mean of 29.41% were recovered. Fertile plants in the anthers culture 5 out of 9 studied genotypes were obtained. Seventeen dihaploid lines with complex rust resistance and common bunt (8–9 points) were obtained. Two clusters of complex resistance genes were identified in the ten studied DH lines: Lr26/Sr31/Pm8/Yr9 and Lr34/Yr18/Sr58/Pm38.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat is one of the leading food crops around the world. It provides roughly 20% of calories and protein for human nutrition globally. Just like other food crops, wheat can be affected by several diseases; and among the most destructive are the rust diseases. Wheat rust is air-borne and comes in three forms: stripe/yellow rust, stem rust and leaf rust. When any of these fungi enters the wheat plant through its leaves, the fungus takes the nutrients from the plant cells, leaving the plant unable to grow or develop grains. This puts the farmers’ entire wheat field at risk of infection during the whole growing season (https://www.isaaa.org/resources/publications/pocketk/60/default.asp, Bouvet et al. 2022; Martínez-Moreno et al. 2022).
Today, the level of development and technological techniques for obtaining linear wheat material (androgenesis in vitro or the haploproducer method) is at a high level, which allows using this method as an integral part of the selection process of this crop (Testillano 2019; Lantos and Pauk 2016). Biotechnological methods are of great importance for facilitating and accelerating the selection process. They make it possible to obtain new forms of wheat, resistant to various adverse factors, in the shortest possible time and without the use of large sown areas (Litvynenko 2010; Weigt et al. 2019; Dwivedi et al. 2015; Li et al. 2020). The production of haploids through anther culture has now become an integral part of the breeding programs of several agronomically important plants. However, approximately 170 species have been studied so far for haploid production through anther culture. (Upadhyay Richa 2022).
The strategies of modern selection consist in the need to create new varieties adapted to specific agroclimatic conditions and those characterized by complex resistance to diseases. In Ukraine, crop failure due to a complex of diseases is on average 12–18%, and in years of epiphytoties—25–50% or more. Resistance is the most sustainable method to control plant diseases. Remote hybridization is highly effective in the selection of wheat for resistance to rust and common bunt. Many of the resistance genes identified are derived from related species. At the Plant Breeding and Genetics Institute–National Center of Seed and Cultivar Investigations (PBGI–NCSCI), introgressive lines of bread wheat were obtained by crossing varieties of local selection with Aegilops cylindrica, Ae. variabilis, Triticum erebuni. According to the program of creation of initial breeding material of wheat with group resistance to pathogens of major diseases (types of rust and sooty, powdery mildew) in order to "connected" effective Lr, Yr, Sr, Pm-genes in one genotype, hybridization was carried out between lines and varieties of wheat, which are in the gene pool of the Department of Phytopathology and Entomology. Further, phytopathological assessment and selection of pathogen-resistant samples were carried out in field conditions on an artificially created infection environment. Hybrids, lines and varieties of wheat resistant to pathogens of brown (Puccinia triticina), yellow (Puccinia glumarum Erikss. etHenn.), stem rust (Puccinia graminis f. sp. tritici), powdery mildew (Blumeria graminis (DC) Speer f. sp. tritici) and hard soot (Tilletia caries) were selected (Babayants and Babayants 2014; Babayants et al. 2015a, b).
The aim of the research is to create homozygous dihaploid lines with group resistance, which have a complex of effective disease resistance genes, by in vitro androgenesis method.
Materials and methods
The study investigated nine selection lines of hybrids (F4–F5), provided by the Department of Phytopathology and Entomology of PBGI-NCSCI, obtained on the basis of donors, the resistance of which comes from wild relatives of wheat (Aegilops cilyndrica, Ae. variabilis, Triticum erebuni) (Babayants et al. 2015a, b, 2021). The presented lines had group resistance to brown rust, stem rust, yellow rust, powdery mildew, common bunt. The presented lines were obtained by the method of individual "pedigree" selection up to the fifth generation, however, most of them are heterozygotes.
As a method for obtaining doubled haploids (DH) of wheat, in vitro culture of isolated anthers was used.
Anther culture in vitro
Donor plants of winter bread wheat were grown in paddy fields at the experimental plots of the PBGI-NCSCI in Odesa, Ukraine. The ears were cut when vacuolated microspores of most of the anthers were in the early and middle–late uninucleate stage. The cytological control of the development stage of microspores in anthers was carried out by preparing temporary micropreparations of anthers stained with acetocarmine (4%) under a light microscope (Axiophot, Opton, West Germany). The cut ears in the covering leaf were placed in water solution of abscisic acid (ABA) at a concentration 0.5 mg L−1, wrapped with film, and placed in a climate chamber at the temperature of + 2–4 °C for 3–5 days.
Sterilization was carried out as follows: spikes were freed from the covering leaves, cut off the awns and placed in Petri dishes with a diameter of 150 mm; dishes were poured over with a solution of the commercial product Bilyzna (including sodium hypochlorite (NaOCl), active chlorine and alkalis) for 40 min and then drained. Further, the Petri dishes with spikes were filled with 0.05 N HCl (hydrogen chloride) solution for 10 min, after which they were washed five times with sterile distilled water.
Anthers were isolated aseptically in laminar box conditions and were cultured on the hard 190–2 nutrient medium (Table 1), which 10 ml mowed in test tube (ø = 15 mm, h = 200 mm) and exposed to the dark at + 30 °C first three days and further at + 24 °C for callus induction. For each combination, 600–700 anthers were explanted in three replicates. The 190–2 nutrient medium was supplemented with 90 g L−1 of sucrose, 400 mg L−1 l-glutamine, 400 mg L−1 l-proline, 100 mg L−1 myo-inositol, 3 g L−1 gelrite and different phytohormones: 2,4-D (2,4-dichlorophenoxyacetic acid) 1.5 mg L−1 and kinetin 0.5 mg L−1(Zambriborshch et al. 2020). Subsequently, after 3–6 weeks of cultivation, formed ELS on surface of the anthers (1–2 mm in size) were transferred to the proliferation and regeneration Murashige and Skoog (MS1) basal medium with addition of 0.2 mg L−1 2,4-D, 0.5 mg L−1 kinetin, 200 mg L−1 l-glutamine, 200 mg L−1 l-proline, 100 mg L−1 myo-inositol, 6 g L−1 agar (Agar Plant tissue culture grade, AppliChem) and 60 mg L−1 sucrose (Litvynenko et al. 2015) and cultivated in the dark for 21 days at 24 ± 2 °C before the emergence of regeneration centers. Subsequently, the calli (ELS) were transplanted to enhance the regeneration of green plants on MS2 medium with the 0.5 mg L−1 gibberellic acid and 25 mg L−1 malic acid, 1 g L−1 potato starch (Sigma), 30 mg L−1 sucrose, 6 g L−1 agar and were cultivated 2–3 weeks at 10–12 °C, of 16-h photoperiod, 2500–3000 lx.
Regenerants of green plantlets formed on ELS were transplanted into glass jars (200 ml) to the ½ MS hormone-free basal medium for rooting. The developed plantlets were acclimatized and transplanted into a greenhouse for further vernalization (45 days), growth to seed and evaluation. The ploidy level of the regenerated doubled haploid (DH) plants was also evaluated by fertilization analysis.
For each genotype, the following parameters of in vitro androgenesis were determined: the number of induction ELS (ELS/100A), green plantlets (GP/100A), albino plantlets (AP/100A) per 100 anthers. Also, we were determinated the acclimatized plantlets per 100 regenerated green plantlets—ADP/100GP, and the doubled haploid plants per 100 acclimatized plantlets (DH/100ADP) (Zambriborshch et al. 2020),
Phytopathological assessment
In 2022, field evaluation of dihaploid lines of genotypes 2/20, 3/20, 120/20, 132/20, 352/20 obtained in 2021 was carried out. This material was tested for resistance to brown, stem, yellow rust, powdery mildew, common bunt on an artificial complex infectious background of these diseases in a field nursery (Babayants and Babayants 2014).
The seeds were divided into half. One part was material for the study of resistance at the juvenile stage of plants (only brown rust and powdery mildew), and the second part—on adult plants (stem, brown rust, powdery mildew, common bund). One half of the seeds were germinated in a greenhouse, the sprouts at the 3-leaf stage (BBCH 12–13) were infected with (1) uredospores of brown rust and, separately, (2) conidia of powdery mildew. Previously, before infection, the sprouts were sprayed with water with the addition of Tween. The ones were used for better adhesion of the agents. Inoculation was carried out: (1) with a mixture of uredospores (10 mg/m2) and talc (in a ratio of 1:100); (2) powdery mildew conidia, previously propagated on the storage variety—Odeska napivkarlikova. After processing, plants were placed under PVC film to create a wet chamber. The type of lesions and its intensity were evaluated for the maximum development of the disease according to generally accepted scales (Table 2).
Phytopathological evaluation at the level of an adult plant was carried out in the field conditions of an artificial of leaf–stem diseases infectious nursery. For the organization of the latter, the composition of bread wheat varieties: Michigan, Odeska 26 and Odeska napivkarlikova was used as a reservoir of rust diseases (Babayants and Babayants 2014).
In the spring, inoculation with brown rust at the flag leaf phase (BBCH 60–63) at temperatures optimal for infection (18–20 °C) was carried out. Stem rust was infected in the earing phase (BBCH 63–65) at an air temperature of 20–22 °C. To create an artificial infectious background, a population of the pathogen collected in the fields of Southern Ukraine in the summer of last year and strengthened by the most virulent and aggressive races of the pathogen were used. Uredospores were stored at a temperature of 5 °C in sealed ampoules with nitrogen. Spores were activated by heating to 50 °C for 20 min. Inoculation with a mixture of uredospores and talc using a similar method as when inoculating juvenile plants was carried out.
The provocative background of yellow rust was created by sowing the absolutely susceptible line Erythrospermum 194/06, which acted as a reservoir of infection. In recent years, the disease has manifested itself every year with an intensity sufficient to conduct assessments. In 2022, the manifestations of the disease were at the level of mediocre epiphytotia.
Provocative infectious background for assessing resistance to powdery mildew was created by spreading last year's straw with cleistothecia (stored in PVC bags at 2–4 °C). In addition, vases with a fresh infection propagated on the accumulator variety were placed next to the experimental plants.
Phytopathological assessment of the resistance of the obtained DH lines of wheat to hard soot was carried out in a field infectious nursery under artificial infection of seeds. Inoculation by means of mechanical mixing of teliospores of the fungus and seeds before sowing was carried out (using 100 mg of spores per 10 g of seed).
The assessment was carried out according to generally accepted methods in points.
PCR analysis
Dihaploid lines of winter wheat selection of Department of Phytopathology and Entomology PBGI-NCSCI 2/20 (1 pc.), 3/20 (4 pc.), 120/20 (1 pc.), 132/20 (8 pc.), 352/20 (3 pcs.). PC).
DNA was isolated from green leaves using STAV buffer (Sivolap 1998). The Lr genes (Lr21, Lr24, Lr26, Lr34, LrAmigo), Sr genes (Sr24, Sr31, Sr58, SrAmigo), Pm genes (Pm8, Pm17, Pm38), Yr genes (Yr9, Yr18) in lines were detected by molecular-genetic markers (Table 3).
The conditions of PCR analysis with molecular markers of Lr genes was given in works (Spielmeyer et al. 2000; Mago et al. 2005; Lagudah et al. 2006; Gorash et al. 2014; Galaev and Sivolap 2015; Galaev 2016).
Statistical analysis
To evaluate effect of the genotype, the data of the androgenic parameters (number of ELS, regenerated-, green-, albino-, acclimatized plantlets) were analyzed by a one-way ANOVA (analysis of variance) using software Excel.
Results
In 2020 year, wheat genotypes resistant to brown rust, stem rust, yellow rust, powdery mildew and hard soot were homozygized. The given experimental material was first involved in the culture of anthers, that is, it had an unknown sensitivity to androgenesis in vitro. The results of studies of this material are shown in Table 4.
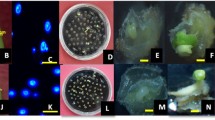
It was shown that at the first stage of in vitro androgenesis under the given experimental conditions, all studied genotypes formed calluses (ELS) (Fig. 1a). The percentage of induction of the latter from planted anthers ranged from 0.99 to 16.26 depending on the genotype, while the overall mean was 6.63 ELS/100A (Table 4).
At the next stage, plant regeneration (Fig. 1b) was obtained only in anther culture of seven out of nine genotypes. Genotypes No. 120/20 and 132/20 and at the stage of regeneration were characterized by the greatest regeneration potential (1.36 and 3.05%, respectively). Two other genotypes that showed a high level of ELS formation (No. 10/20 and No. 352/20) at the second stage of androgenesis according to the indicator "regeneration of green plantlets" did not stand out among this set of genotypes. The number of albino plantlets (Fig. 1c) was not high: from 0 to 0.57% depending on the genotype, while the overall mean was 0.18 AP/100A. Only in genotype No. 132/20 the number of albino regenerant plantless exceeded that of green plants. The albino plantlets were counted and discarded. After about 2–3 weeks, the plantlets were transplanted into glass jars on a hormone-free ½ MC medium for good rooting (Fig. 1c,d).
Androgenesis in in vitro anther culture of winter bread wheat: a formation of callus on the surface of anthers, medium 190–2; b regeneration of green plantlets after transplantation ELS on MS medium; c green and albino regenerated plantlets; d rooting of regenerants on a hormone-free MS nutrient medium with a filling-salt composition
At the later stages of adaptation to ex vitro conditions, vernalization and growing of regenerants (Fig. 2), many plants die due to various reasons: sharp physiological stress associated with a change in water balance after transfer from in vitro conditions, chromosomal instability of some regenerants, etc. (Tripathy 2018). According to the results of our research, at the stages of adaptation to soil conditions and vernalization, from 32 (No. 120/20) to 62 (No. 352/20) regenerants (ADP) per 100 received green plants were survived. On average, 35.92 ADP/100GP survived (Table 4). We obtained 51 plantlets from 6 of the 9 provided genotypes. The largest number of plants—25 ps. was regenerated in anther culture No. 132/20.
The rate of DH/100ADP varied from 12.50 to 60.00% across the combinations. The highest values of the DH plants were present in the genotypes №352/20 (Table 4).
The effect of the genotype on the ELS per 100 anthers (ELS/100A), the green plantlets per 100 anthers (GP/100A), the albino plantlets per 100 anthers (AP/100A), the acclimatized plantlets per 100 regenerated plantlets (GP/100RP) and DH plantlets per 100 acclimatized plantlets (DH/100ADP) was significant at P < 0.001 (Table 5).
Next, the obtained lines (propagated in the field in 2021) were evaluated in 2022 for resistance to various pathogens in the field conditions of an artificial infectious nursery (Table 6).
It was shown that all lines of the same genotype did not differ among themselves in qualitative and quantitative characteristics of resistance to the studied pathogens. Therefore, Table 6 shows general data on the stability of DH lines for individual hybrid combinations. It was established that only dihaploid lines of genotype 120/20 are susceptible to powdery mildew and yellow rust. However, all obtained dihaploid lines have been showed high (8–9 points) complex resistance to leaf, stem, yellow rust and common bunt.
In order to identify genes for resistance to brown leaf, stem and yellow rust, powdery mildew in the obtained dihaploid lines of bread wheat, PLR-analysis was performed using molecular markers of genes Lr, Sr, Pm, Yr (Table 7).
5 Lr genes, 4 Sr genes, 3 Pm genes and 2 Yr genes were tested in 18 dihaploid wheat lines. The LrAmigo/SrAmigo/Pm17 genes localized in the 1BL.1BS-3Ae#1L translocation of the Amigo type and the Lr21 resistance gene to brown leaf rust were not detected in the studied lines. In the DH 2/20 line, no loci associated with identification genes were found.
Two genotypes were detected in line 3/20: (1) DH1 − Lr26, Sr31, Pm3, Yr9 (frequency 17%) and (2) DH2, DH3, DH4, DH5, DH6 − Lr26 + Lr34, Sr31 + Sr58, Pm3 + Pm38, Yr9 + Yr18 (frequency 83%). In line 132/20, two genotypes were also found among the dihaploids: (1) DH1, DH2, DH3, DH4 − Lr26, Sr31, Pm3, Yr9 (frequency 50%); (2) DH5, DH6,DH7, DH8 − Lr26 + Lr34, Sr31 + Sr58, Pm3 + Pm38, Yr9 + Yr18 (frequency 50%).
The DH line of genotype 120/20 had genes Lr26, Sr31, Pm3, Yr9 in the genome, and the DH line 352/20—Lr26 + Lr34, Sr31 + Sr58, Pm3 + Pm38, Yr9 + Yr18.
Discussion
Availability of novel technologies has further enabled reduction in the breeding time, or an improved genetic gain using DHs in a breeding program. For example, through the development of DH wheat lines containing rust resistance genes, Wessels and Botes (2014) showed that integration of MAS and DH technology into conventional breeding processes could increase the speed of cultivar development. Large numbers of finished inbred lines are attained at once in a DH-based breeding pipeline compared to multiple years and stages in a conventional method (Hale et al. 2021).
The dependence of haploproduction efficiency in the culture of soft wheat anthers on the genotype does not make it possible to ensure predictability of results when working with any genotype and prompts researchers to search for possible activation of morphogenetic competence in vitro.
It was shown that under these experimental conditions, all studied genotypes were sensitive to the first stage of in vitro androgenesis. The percentage of ELS formation from planted anthers ranged from 0.99 ± 0.20 (No. 114/20) to 16.26 ± 0.74 (No. 132/20), with an average of 6.63%. It should be noted that this indicator in the culture of anthers of all studied samples had rather high values for the genetic material of the South of Ukraine. Hungarian researchers have achieved higher results in the formation of ESL. The number of embryo-like structures per 100 anthers (ELS/100A) ranged from 6.00 to 74.53 depending on the combination (genotype), while the overall mean was 35.84 ELS/100A (Kanbar et al. 2020). The maximum value of this index was relatively higher than that obtained by our research: 18% (El-Hennawy et al. 2011); and 14.7% (Grauda et al. 2014) The highest ELS/100A exceeding 100% was found in these studies: 119% (Kondic-Spika et al. 2008); and 169.40%, 190.40% in 2010, 2011 (Lantos et al. 2013).
Regarding our long-term in vitro studies of winter soft wheat androgenesis, the highest rate of ELS/100A was observed in the Kavkaz variety—38% (Zambriborshch et al. 2018). And on average, this indicator was at the level of 15%, which is high for our genotypes. Thus, the two genotypes in terms of the number of ELS per 100 anthers for No. 120/20 (14.4%) and No. 132/20 (16.3%) were at the level of winter wheat genotypes, which are haploproduction donors for the conditions of who are haploproduct donors for the conditions of Southern Ukraine.
Plant regeneration is a complex process that depends on many factors, the main of which is the genotype (Upadhyay Richa 2022). The ability of individual genotypes to regenerate green plantlets is different. Thus, in our studies, the GP/100A index ranged from 0 to 3.05%. A total of 142 green plantlets were obtained, of which more than half (75 pcs.) were from genotype 132/20.
The next stages of the biotechnology of obtaining linear wheat material by androgenesis—adaptation to ex vitro conditions, vernalization and growing of regenerants—are among the most critical. This is due to a high percentage of plantlets dying at these stages due to various reasons: severe water deficit in tissues after transfer from in vitro conditions, chromosomal imbalance of regenerants, etc. (Tripathy 2018).
According to the results of the current research, at the stage of adaptation to the soil, survived from 30 to 60% of green regenerants. A total of 51 plantlets were acclimatized, which is 35.92 of the green plantlets obtained. However, not all these plants had the genetic determinants that allowed the plant to undergo further stages of growth differentiation and seeds production. Normally, plants obtained in anther culture can be considered haploids because they have arisen from haploid microspores. However, the actual plants produced during regeneration might be a mixture of haploids, diploids or mixoploids, which can be explained by the defects in the development of microspores or callus tissue (Tripathy et al. 2019). The fusion or unequal division of nuclei, endomitosis inside the pollen grain, and disruption of meiosis could also lead to the development of plants other than haploids.
Cereals are characterized by the spontaneous doubling of chromosomes in haploid callus cells, which leads to the formation of numerous doubled haploids: depending on the type of crop and cultivation conditions, for example, for rice from 30 to 87% (Mishra and Rao 2016). During our study of androgenesis of winter wheat, we did not use diploidizers, that is, all 17 fertile plants obtained are the result of spontaneous diploidization. The frequency of the latter averaged 29.41%, which corresponds to similar results of 32.72% in Lantos and Pauk (2016). The values of DH/100ADP ranged from 12.50 to 44.44% depending on the genotype. The average value of spontaneous diploidization (DH/100ADP) was 28.40% (Lantos et al. 2019).
The aim of the work was to create homozygous lines of winter wheat with complex resistance to a number of diseases. It should be noted that conditions were created in the infectious nursery that contributed to the intensive development of various pathogens. All obtained DH lines were characterized by a high level of resistance to pathogens Puccinia triticina Eriks., Puccinia graminis Pers. f. Tritici Erikss. et Heen., Puccinia striiformis West., Blumeria graminis (DC) Speer f. sp. tritici., Tilletia caries (D.C.) Tul. Only DH lines of genotype 120/20 were susceptible to powdery mildew and yellow rust, while all other 16 lines showed high (8–9 points) complex resistance to brown, stem, yellow rust and hard soot.
In order to identify genes for resistance to brown leaf, stem and yellow rust, powdery mildew and hard soot in dihaploid lines of soft winter wheat, PLR analysis was performed using molecular markers for up to 5 Lr genes, 4 Sr genes, 3 Pm genes, 2 Yr genes. 10 years ago, plant material that had wheat–rye translocations 1BL.1RS and 1AL.1RS was actively involved in the wheat selection process at PBGI-NCSCI (Galaev and Sivolap 2015). Therefore, in the panel for the detection of resistance genes, among others, the primers for the markers of these DNA regions were selected. As a result, only two genes Lr26, Lr34 were found in the genome of DH lines, and the genes Lr21, Lr24, LrAmigo were not found. Among the Sr genes, only the Sr31 and Sr58 genes were detected, while the SrAmigo and Sr24 genes were not. Accordingly, the gene responsible for resistance to powdery mildew Pm17, which is also localized in the Amigo-type translocation 1BL.1BS-3Ae#1L, was not detected.
Two clusters of complex resistance genes were identified in the ten studied DH lines: Lr26/Sr31/Pm8/Yr9 and Lr34/Yr18/Sr58/Pm38. That is, these lines had at least two genes for resistance to brown (Lr26 + Lr34), leaf (Sr31 + Sr58), yellow (Yr9 + Yr18) rust and powdery mildew (Pm8 + Pm38). Six dihaploids were characterized by a genotype with a complex of different resistance genes Lr26, Sr31, Pm8, Yr9. So, the high level of resistance of DH to the disease complex shown in field tests was proved by molecular genetic analysis. Only in the genome of DH 2/20 no gene was detected. However, this line was characterized by resistance both in the juvenile and in the phase of an adult plant. This indicates the presence of oligogenes in its genome, which cause high resistance. Unfortunately, the limited set of markers did not allow them to be detected. It is obvious that this line carries other (undetected) resistance genes and needs further research.
As a result of the research, a linear homozygous material with complex resistance to various types of rust and common bunt have been obtained. These DH lines are an innovative starting material for wheat breeding and can be additionally involved in research on the genetic basis of this resistance.
Conclusion
By the method of in vitro anther culture, 17 homozygous dihaploid lines of different genotypes of winter bread wheat with complex resistance to various types of rust and common bunt were obtained. Genotypes No. 120/20 and 132/20 were characterized by the highest indicators of haploproduction: the percentage of callus formation (ELS/100A) was 14.39% and 16.26%, the percentage of formation of green plantlets (GP/100A) was 1.36 and 3.05%, respectively. The frequency of spontaneous diploidization (DH/100ADP) averaged 29.41%. All obtained dihaploid lines showed high (8–9 points) complex resistance to brown, stem, yellow rust and common bunt. PCR analysis was proved the presence of resistance genes Lr, Yr, Sr in DH lines, and they are a valuable starting material by wheat selection for immunity to group of pathogens.
References
Babayants O, Babayants L (2014) Basics of selection and methodology of assessments of wheat stability to pathogens of diseases. Odessa: 400 p. ISBN 978–966–413–479–5
Babayants O, Babayants L, Gorash A, Vasilev A, Traskovetskaya V, Galaev A (2015a) Physiologic specialization of Puccinia triticina Erikss and effectiveness of Lr-genes in the south of Ukraine during 2013–2014. Chil J Agric Res 75:443–450
Babaynts O, Babaynts L, Traskovetskaya V, Gorash A, Saulyak N and Galaev A (2015b) Race composition of Blumeria graminis (DC) Speer f. sp. tritici in South of Ukarine and effectiveness of Pm-genes in 2004–2013. Cereal Res Commun 43(3):449–458. https://doi.org/10.1556/0806.43.2015.011
Babayants O, Babayants L, Sauliak N, Ternovoy K, Vasiliev A, Bushulian M, Traskovetskaia V (2021) Unique source breeding material of wheat with group resistance to pathogens by pyramidation of effective Lr, Sr, Yr, Pm, Bt, Ut genes. Breeding of cereals and legumes in the context of climate change: directions and priorities. ABSTRACTS International Scientific Conference. Odesa, Ukraine (May 5, 2021), pp 122
Bouvet L, Holdgate S, James L et al (2022) The evolving battle between yellow rust and wheat: implications for global food security. Theor Appl Genet 135:741–753. https://doi.org/10.1007/s00122-021-03983-z)
Dwivedi S, Britt A, Tripathi L, Sharma S, Upadhyaya H, Ortiz R (2015) Haploids: constraints and opportunities in plant breeding. Biotech Adv 33:812–829. https://doi.org/10.1016/j.biotechadv.2015.07.001
El-Hennawy M, Abdalla A, Shafey S, Al-Ashkar I (2011) Production of doubled haploid wheat lines (Triticum aestivum L.) using anther culture technique. Ann Agric Sci 56:63–72. https://doi.org/10.1016/j.aoas.2011.05.008
Galaev A (2016) Effectiveness of different resistance genes to leaf rust and their combinations in interline hybrids of spring bread wheat (Triticum aestivum L.) in South Ukraine. Collect Sci Works SGI 28(68):109–121
Galaev A, Sivolap Yu (2015) Description of the bread wheat varieties of Ukrainian and Russian breeding by alleles of locus csLV34 closely connected with multipathogen resistance gene Lr34/Yr18/Pm38. Cytol Genet 49(1):13–19. https://doi.org/10.3103/S0095452715010041
Gorash A, Galaev A, Babayants O, Babayants L (2014) Leaf rust resistance of bread wheat (Triticum aestivum L.) lines derived from interspecific crosses. Zemdirbyste 101:295–302. https://doi.org/10.13080/za.2014.101.038
Grauda D, Miķelsone A, Ļisina N, Žagata K, Ornicāns R, Fokina O, Lapiòa L, Rashal I (2014) Anther culture effectiveness in producing doubled haploids of cereals. Proc Latvian Acad Sci Sec B Natural Exact Appl Sci 68:142–147. https://doi.org/10.2478/prolas-2014-0016
Hale B, Ferrie AMR, Chellamma S, Samuel JP, Phillips GC (2021) Androgenesis-based doubled haploidy: past, present, and future perspectives. Front Plant Sci 12:751230. https://doi.org/10.3389/fpls.2021.751230.PMID:35069615;PMCID:PMC8777211
Kanbar OZ, Lantos C, Chege PK, Kiss E, Pauk J (2020) Generation of doubled haploid lines from winter wheat (Triticum aestivum L.) breeding material using in vitro anther culture. Czech J Genet Plant Breed 56(4):150–158. https://doi.org/10.17221/113/2019-CJGPB
Kondic-Spika A, Kobiljski B, Hristov N (2008) Efficiency of anther culture technique in the production of wheat double haploids. Zbornik Matice Srpske za Prirodne Nauke, pp 35–40. https://doi.org/10.2298/zmspn0815035k
Lagudah E, McFadden H, Singh R, Huerta-Espino J, Bariana H, Spielmeyer W (2006) Molecular genetic characterization of the Lr34/Yr18 slow rusting resistance gene region in wheat. Theor Appl Genet 114(1):21–30. https://doi.org/10.1007/s00122-006-0406-z
Lantos C, Weyen J, Orsini JM, Gnad H, Schlieter B, Lein V, Kontoeski S, Jacobi A, Mihaly R, Broughton S, Pauk J (2013) Efficient application of in vitro anther culture for different European winter wheat (Triticum aestivum L.) breeding programmes. Plant Breed 132:149–154. https://doi.org/10.1111/pbr.12032
Lantos C, Purgel S, Ács K, Langó B, Bóna L, Boda K, Békés F, Pauk J (2019) Utilization of in vitro anther culture in spelt wheat breeding. Plants 8:436. https://doi.org/10.3390/plants8100436
Lantos C, Pauk J (2016) Anther culture as an effective tool in winter wheat (Triticum aestivum L.) breeding. Genetika Aug 52(8):910–8. https://doi.org/10.7868/s0016675816080075. PMID: 29368884
Li J, Cheng D, Guo S, Yang Z, Chen M, Chen C, et al (2020) Genomic selection to optimize doubled haploid-based hybrid breeding in maize. bioRxiv [Preprint]. https://doi.org/10.1101/2020.09.08.287672
Litvynenko M (2010) Biotechnological methods in selection of agricultural crops.Bull Agrarian Scie 6:11–14
Litvynenko M, Topal M, Shestopal O, Zambriborshch I, Galaev O (2015) Udoskonalena tekhnolohiya selekciynogo procesu pshenyci myakoi ozymoi z vykoryctannyam biotekhnolohichnykh i moleculyarno-genetychnykh metodiv. Naukovo-metodychnyi posibnyk. Odesa: Astroprint, 41p
Mago R, Bariana HS, Dundas IS, Spielmeyer W, Lawrence GJ, Pryor AJ, Ellis JG (2005) Development of PCR markers for the selection of wheat stem rust resistance genes Sr24 and Sr26 in diverse wheat germplasm. Theor Appl Genet 111(3):496–504. https://doi.org/10.1007/s00122-005-2039-z/
Martínez-Moreno F, Giraldo P, Nieto C, Ruiz M (2022) Resistance to leaf and yellow rust in a collection of Spanish bread wheat landraces and association with ecogeographical variables. Agronomy 12(1):187. https://doi.org/10.3390/agronomy12010187
Mishra R, Rao GJN (2016) In-vitro androgenesis in rice: adventages, constraints and future prospects. Rice Sci 23(2):57–68. https://doi.org/10.1016/j.rsci.2016.02.001
Resources Publications Pocket K Rust-resistant Wheat https://www.isaaa.org/resources/publications/pocketk/60/default.asp
Sivolap Y (1998) Application of analysis in genetic and breeding research: scientific method.guide/ed. YuM Sivolap. TO:Agrarian Sciences, 156p
Spielmeyer W, Huang L, Bariana H et al (2000) NBS-LRR sequence family is associated with leaf and stripe rust resistance on the end of homoeologous chromosome group 1S of wheat. Theor Appl Genet 101:1139–1144. https://doi.org/10.1007/s001220051590
Testillano PS (2019) Microspore embryogenesis: targeting the determinant factors of stress-induced cell reprogramming for crop improvement. J Exp Bot 70(11):2965–2978. https://doi.org/10.1093/jxb/ery464. (PMID: 30753698)
Tripathy S (2018) Anther culture for double haploid breeding in rice—a way forward. Rice Genomics Genet 9(1):1–6. https://doi.org/10.5376/rgg.2018.09.0001
Tripathy S, Swain D, Mohapatra P, Prusti A et al (2019) Exploring factors affecting anther culture in rice (Oryza sativa L.). J Appl Biol Biotechnol 7(02):87–92. https://doi.org/10.7324/JABB.2019.70216
Upadhyay Richa (2022) Chapter 7—Anther culture for haploid plant production. In: Avinash CR, Ajay K, Arpan M, Major S (eds) Advances in plant tissue culture. Academic Press, New York, pp 157–174. ISBN 9780323907958. https://doi.org/10.1016/B978-0-323-90795-8.00004-7
Weigt D, Kiel A, Siatkowski I, Zyprych-Walczak J, Tomkowiak A, Kwiatek M (2019) Comparison of the androgenic response of spring and winter wheat (Triticum aestivum L.). Plants (Basel) 9(1):49. https://doi.org/10.3390/plants9010049
Wessels E, Botes W (2014) Accelerating resistance breeding in wheat by integrating marker-assisted selection and doubled haploid technology. South African J Plant Soil 31:35–43. https://doi.org/10.1080/02571862.2014.903434
Zambriborshch I, Shestopal O, Chekalova M, Golub E (2020) The testing of haploproduction ability of soft winter wheat different hybrids in anther culture in vitro. Faktori Eksperimental’noi Evolucii Organizmiv 26:207–211. https://doi.org/10.7124/feeo.v26.1267
Zambriborshch I, Shestopal O, Boyko M (2018) Genotypic features of morphogenetic reactions of varieties and F1 hybrids of winter soft wheat at different stages of androgenesis in vitro. Factors of experimental evolution of organisms: Sb. nauk pr. K: Logos 22:252–256. https://doi.org/10.7124/FEEO.v22.957
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Communicated by Janusz Zimny.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zambriborshch, I., Shestopal, O., Traskovetskaya, V. et al. Obtaining dihaploid lines of winter bread wheat with complex resistance to rust and common bunt by anther culture in vitro. CEREAL RESEARCH COMMUNICATIONS (2024). https://doi.org/10.1007/s42976-023-00466-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42976-023-00466-3