Abstract
The use of new and efficient methods for fertilizing plants is of economic and environmental significance. A 2-year field experiment was conducted to investigate the effect of nano (micro and macro) and chemical fertilizers (N, P, K) on grain sorghum yield and biochemical properties including phosphorus (P), calcium (Ca), protein, crude fat and tannin contents under drought stress. The experiment was setup in a split plot randomized complete block design with four replications. The drought stress treatment (main plots) consisted of stoppage of irrigation before (D1) and after flowering (D2), and control (normal) irrigation (D3). The single and the combined use of nano- and chemical fertilizers including control (without fertilization) were used as the sub plots. In control (non-stress) conditions, there were not any differences between nano-fertilizer with chemical fertilizer on nutrient uptake (P and Ca), crop yield, and seed protein content. However, nano-fertilizer enhanced sorghum yield and biochemical properties in drought stress conditions. In D1, grain yield (6240 kg ha−1), and crude fat (5.29%), and in D2, P uptake (3498.87 mg kg−1), protein (21.5%) and tannin (0.373%) were most affected by nano- and chemical fertilizers. Control irrigation and chemical fertilization resulted in the highest grain yield, which was not significantly different from control irrigation with macro- and micro-fertilizer (6213.3 kg ha−1). The tested nano-fertilizers can be used as a suitable source of nutrients for planting sorghum plants in the arid- and semi-arid areas of the world, with economic and environmental benefits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Grain sorghum (Sorghum bicolor), as a resistant plant to drought and salinity and with high nutritional value, is one of the important components of poultry and livestock nutrition, especially in arid and semi-arid regions of the world. This plant with a 95% nutritional similarity with corn is highly considered by poultry breeders for nutritional purposes (Obizoba 1988). Sorghum is used all over the world in various forms such as baked bread, grilled, alcoholic and nonalcoholic beverages, etc. Grain sorghum, like other grains, is a good source of starch and protein, and as a gluten-free grain, can be used to treat celiac disease (Ratnavathi and Komala 2016). Phenolic compounds, flavonoids and various antioxidants of grain sorghum make it more useful for nutritional applications. Accordingly, grain sorghum can reduce the risks of various diseases including cancer, rheumatoid, etc., by reducing the number of free radicals (Vanamala et al. 2018; Rashawn et al. 2021).
Sorghum contains vitamin B, and its mineral content is highly variable (Kulamarva et al. 2009). In crude sorghum, the total oil composition ranges from 3.91 to 3.58%, and about 84% of which contains oleic and linoleic acid. The tannin content in sorghum pericarp is one of the factors affecting sorghum nutritional values including digestibility and metabolism energy. The presence of tannins in grain sorghum reduces its digestibility and nutritional quality. Dense tannin is found in some species of sorghum, and on the basis of tannin presence into the inner and outer shell, sorghum is classified into different types (Queiroz et al. 2018; Shirmohammadli et al. 2018).
The nutritional application of grain sorghum is determined by the amount of grain protein and phenolic compounds (especially tannins), which are affected by different agronomic factors such as application of fertilizers. Although phenolic compounds have some benefits such as enhancing plant tolerance in intense sunlight, resistance to drought stress and prevention of pre-harvest germination, they also negatively affect protein digestion (Zhao et al. 2020) and nutrient uptake (Lin et al. 2016) in different plant tissues. Despite being resistant to drought stress, the physiological properties of grain sorghum are negatively affected by biotic and abiotic stresses through the production of reactive oxygen species. These species reduce the concentration of photosynthetic pigments and disrupt the metabolic balance of plant (Proietti et al. 2015; Getachew et al. 2016).
Different types of fertilizers help the plant in stress conditions by improving the nutritional status of plant. Nutrition, water and their interactions as well as their allocation to the plant affect plant resistance under stress (Sabet and Mortazaeinezhad 2018; Asadi et al. 2019). Chemical fertilizers have been extensively and globally used in the past six decades to produce sufficient food resources; however, it is not economically and environmentally recommendable at it is subjected to leaching (Miransari and Mackenzie 2015; Miransari and Smith 2019). Accordingly, the use of modern and efficient fertilization techniques such as nanotechnology for efficient fertilizer production to reduce environmental pollution has been proposed, tested and used (Aghajani and Soleymani 2017).
Nanotechnology has provided solutions to increase the value of agricultural crops and to solve environmental issues (Seleiman et al. 2021). The interactions of plant cells with nanoparticles lead to altered plant gene expression and related biological pathways, which ultimately affect plant growth. However, the absorption efficiency and effect of different nanoparticles on the growth and metabolic functions of different plants are different (Zhao et al. 2017). Nanoparticles are more reactive due to their higher surface area increasing their absorption by plant (Sadeghi et al. 2021). The main goal of using nano-fertilizer has been to further reducing fertilizer use and improving plant nutrient efficiency (Raliya et al. 2017).
Some researchers believe that nano-fertilizers under environmental fluctuations such as temperature, soil acidity and humidity can help the plant to grow better and because of their controlled uptake are preferable to chemical fertilizer (Raliya et al. 2017; Guo et al. 2018).
Due to specific climatic conditions (warm and dry and little rainfall) in the province of Sistan-va-Baluchestan, Iran, most plants are subjected to drought stress at different stages of their growth and depending on the type and severity of the stress, their yield is affected. Sorghum as a resistant plant to drought stress (Sanjari et al. 2021), with optimum amount of yield and short growing season and easy production is a remarkable crop for the farmers of the region. Large quantities of conventional chemical fertilizers are used for sorghum planting, affecting plant biochemical properties including the nutritional values of cereal grains (Dimkpa et al. 2017).
With respect to the above-mentioned details, and because more has yet to be investigated on the use of nano-fertilizer affecting sorghum growth and quality under drought stress, the present research (a 2-year field experiment) was proposed. The objective was to test the effects of nano (macro + micro)-fertilizers on the yield and the biochemical properties of grain sorghum under drought stress conditions of Sistan-va-Baluchestan province, Iran.
Materials and methods
This experiment was carried out in the summer of 2017 and 2018 at the Agricultural and Natural Resources Research Station of Sistan (Zahak city, Iran) with the eastern latitude of 30.54°, and northern longitude of 61.41° and an altitude of 483 m. The climate of the experimental site is hot and dry, with an average annual temperature of 23 °C and a humidity of 38%. Soil physical and chemical properties were determined using the standard methods (Miransari et al. 2008).
The experiment was set up in a split plot randomized complete block design with four replications with plots measuring 15 m2. Main plots included three levels of water stress including 1) before flowering (the field was irrigated at planting and was re-irrigated at the appearance of the first flower) (D1)), after flowering (the field was not irrigated after emergence of 50% of flowers until harvest) (D2) and control (regular irrigation) (D3). The single and combined use of micro-and-macro nano- and chemical fertilizers were devoted to the subplots. The nano-fertilizer was sprayed in two different stages including the beginning of stemming and two weeks later (before flowering) at 2 g.L−1 by a rear sprayer after calibrating at 1 atm.
Different growth stages were considered based on the Zadox system. The nano-micro-fertilizer with a balanced combination of iron (Fe), zinc (Zn), manganese (Mn), copper (Cu), boron (B), molybdenum (Mo), calcium (Ca), magnesium (Mg) and sulfur (S), and nano-macro-fertilizer with nitrogen (N), phosphorus (P) and potassium (K) were used for the experiment. Chemical fertilizer (incorporated into the soil) was used as urea (200 kg.ha−1, half before planting and half before flowering), triple superphosphate (250 kg.ha−1) and potassium sulfate (75 kg.ha−1). The plants were harvested after the dough stage, when upper leaves turned yellow. The four middle rows (8 m2) (ignoring 50-cm margins from the two sides of each row) were harvested, and the grain yield was calculated on the basis of a 12% moisture.
The grains were analyzed for the contents of Ca and P, crude fat, protein and tannin in the laboratory. The percentage of seed fat was calculated according to National Standard No. 10700 with the following details of the sample was weighed. The Soxhlet balloon was brought to a constant weight and weighed. Soxhlet set was then closed, and five grams milled sample was inserted into the cartouche and was treated with N-hexane solvent, and the extraction operation was performed for 6 to 8 h. The solvent was then removed, and the Soxhlet balloon was oven dried and weighed. Using the following equation, the percentage of fat was calculated.
Seed P was determined according to National Standard No. 513 using a spectrophotometer at 430 nm and the following equation (the details of measurement are available at request).
W = reading from device.
Seed Ca was obtained according to National Standard Method No. 1–10,701 using the following equation.
v = consumed potassium permanganate 0.1 N.
Seed tannin (%) was measured according to National Standard No. 9341 IS IRI 9341 as follows. (1) Tannin was extracted by centrifugation by dimethylformamide and by adding ferric ammonium citrate and ammonia solution to the upper part of the fluid (2) the absorption at 525 nm was determined by spectrometer, and (3) the amount of sample tannin was determined using standard curves and tannic acid.
The percentage of seed crude protein was measured by Kjeldahl method and according to the National Iranian Standards Organization 19,052 in the laboratory. Nitrogen content was measured using a fully automated Kjeldahl apparatus including three stages of digestion, distillation and titration. After titration, N (%) was calculated using the following equation (Mossé and Baudet 1983). The conversion coefficient of protein was used for the calculation of crude protein content as a percentage of dry matter mass.
X = titration reading.
W = sample weight.
Data were analyzed using EXCEL and SAS version 9.2 software, and means were compared by the Duncan's multiple range test at the 5% level of probability.
Results
Analysis of variance
According to the analysis of variance, both drought stress and fertilization significantly (p ≤ 0.05) affected sorghum grain yield, P, Ca, protein, fat, and tannin contents. The interactions of the experimental treatments also significantly affected all the measured traits except grain fat percentage (p ≤ 0.1). The effect of year and its interaction with fertilization was just significant on seed tannin (Table 1).
Grain yield
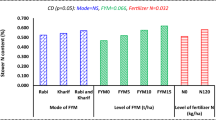
The highest grain yield resulted from the control irrigation and chemical fertilizer application (6240 kg/ha) and was not significantly different from control irrigation with macro- and micro-fertilizer (6213.3 kg/ha). Accordingly, both treatments increased seed yield almost equally (Fig. 1a). The least seed yield was resulted by D2 without fertilization (2738.8 kg/ha) (Table 2).
Grain sorghum: a yield, b P, and c Ca affected by nano- and chemical fertilizer under drought stress. D1 (irrigation before flowering), D2 (irrigation after flowering), D3 (control, normal irrigation). F1 = macro, F2 = micro, F3: chemical fertilizer, F4: macro + micro F5 = control (without fertilizer). Mean values followed by the same letters are not significantly different at P ≤ 0.05 using Duncan’s multiple range test
P and Ca uptake
Treatment D2 resulted in the highest grain P at different fertilization levels ranging from 3486.5 to 3498.87 mg kg−1 significantly higher than D1 (3308.62–3358 mg kg−1) and D3 (3411.12–3470.87 mg kg−1) (Fig. 1b). Treatment D3 (control) and macro- and micro- (5903.37 mg kg−1) or chemical fertilizer (5894.8 mg kg−1) resulted in the highest grain Ca. However, treatment D2 resulted in the least grain Ca at different levels of fertilization (5520.75–5575.25 mg kg−1) (Fig. 1c).
Grain protein
The highest grain protein was related to D2 and micro (21.5%), not significantly different from D2 and chemical fertilizer (21.08%), however, significantly higher than D1 (13.15–13.81%) and D3 (13.14–13.57%) (Fig. 2a).
Grain sorghum: a protein, b fat, and c tannin affected by nano- and chemical fertilizer under drought stress. D1 (irrigation before flowering), D2 (irrigation after flowering), D3 (control, normal irrigation). F1 = macro, F2 = micro, F3: chemical fertilizer, F4: macro + micro F5 = control (without fertilizer). Mean values followed by the same letters are not significantly different at P ≤ 0.05 using Duncan’s multiple range test
Crude fat
The highest grain crude fat was related to D1 and macro-fertilizer (5.29%) followed by D3 and macro + micro and D3 without fertilizer application. The least grain crude fat was resulted by D2 at different levels of fertilization ranging from 3.3–3.63%. (Fig. 2b).
Tannin
D2 and macro resulted in the highest grain tannin (0.373%), and D2 and micro resulted in the least grain tannin (0.126%). The grain tannin of D1 at different fertilization levels was in the range of 0.165–0.26%, and of D3 was in the range of 0.163–0.26% (Fig. 2c).
Discussion
The tested nano-fertilizer application enhanced sorghum yield and biochemical properties in drought stress conditions. Grain yield, Ca and crude fat were most affected by nano- and chemical fertilizer in D1, and P, protein and tannin were most affected by nano- and chemical fertilizer in D2.
The highest grain yield was related to D3 and chemical fertilizer (containing N, P and K), which is due to the positive role of N in promoting vegetative growth, soil fertility, chlorophyll concentration and the favorable role of P in stimulating the formation of trioses and carbon dioxide stabilization, as well as the positive effect of K on maintaining photosynthetic activities in an optimal mode. Accordingly, the above-mentioned factors increase grain yield by affecting plant metabolic and physiological processes (de Oliveira et al. 2020). The positive effects of N and P chemical fertilizer on sorghum grain yield has also been shown by different researchers (Fontes et al. 2017; Jankowski et al. 2020). There was not a significant difference between chemical fertilizer with D3 + macro + micro- fertilizer on sorghum grain yield, which is similar to the results of the Rehab et al. (2020) examining the effect of N, P, K fertilizer and micronutrients on sorghum.
The lowest grain yield was observed by D2 and non-fertilization, indicating sorghum yield vulnerability, at seed filling stage, under drought stress conditions. Dehydration by drought stress, before harvest, can significantly reduce crop yield by affecting stomatal opening and decreasing the activity of enzymes in the Kelvin’s cycle, and subsequent seed filling in sorghum (Awasthi et al. 2014; Farooq et al. 2018).
The D1 and D2 stress levels with nano- and chemical fertilizer affected seed protein percentage as the highest was related to D2 + micro (Fig. 2a) and the least to D3 + without fertilizer. Noori et al. (2021) found the least and the highest seed protein contents were realized by optimum irrigation and severe drought stress, respectively. During the seed filling period, under drought stress, plant uses the protein-making mechanism to deal with the stress.
The presence of Fe, Zn and molybdenum (Mo) in the micro-treatment can significantly affect crop yield, due to the following: (1) the significant role of Zn in protein synthesis and improvement of nutritional conditions, (2) the effect of Mo on the activity of nitrogenase and nitrate reductase, and (3) the effects of Fe on plant photosynthesis and protein synthesis, which increases seed protein content. Micronutrients can also enhance seed protein percentage by improving the yield of optical photosystems, especially under drought stress (Dimkpa et al. 2020; Li et al. 2020a; Wu et al. 2020). In the case of sorghum, high concentration of proline under drought stress can be a good source for seed transfer and can increase seed protein concentration (Nxele et al. 2017).
Grain P was the highest in D2 by nano- and chemical fertilizer, not statistically different from each other (Dimkpa et al. 2019; Aqaei et al. 2020). It is likely that under drought stress before harvest, plant increased sugar and proline contents for the osmotic regulation of the roots and increased P uptake resulted in the more allocation of P to the grains. The least grain P also was observed with D1 + without fertilization (Zehetner et al. 2018; Ghaffari et al. 2019). Sorghum plants were exposed to water deficit in their vegetative phase, without any fertilizer application, so the absorbed P was not enough for allocation to the grain in later stages.
During the vegetative growth stage, plant consumes all available P to meet plant current need for growth and development; however, during the reproductive phase because the grains are a stronger destination for P uptake, more P is transferred to the grains (Wang et al. 2018). There were not any significant differences between the fertilizer treatments, in terms of P uptake, indicating that fertilizer application was not advantageous for increasing sorghum grain P under drought stress before harvest.
Grain Ca was also affected by drought stress and fertilization. The highest grain Ca was observed with D3 + macro + micro- and D3 + chemical fertilizer. The combined use of micro- and macro-nutrients increased grain nutrient concentration including Ca in sorghum. The least grain Ca was related to D3 + control (without) fertilizer, significantly different from the other treatments. The presence of potassium in chemical fertilizer and macro + micro can reduce calcium uptake under drought stress (Zamani et al. 2020). Calcium is mainly transmitted to the root surface by mass flow, and the moisture deficiency limits calcium uptake and transport to the roots. Plant reduces evapotranspiration under drought stress, resulting in the decreased uptake and distribution of calcium in different plant tissues (Liang and Zhang 2018). It is also possible that under drought stress conditions, long-term wetting and drying of the soil will release K ions from the clay layers and increase K concentration in the soil, and subsequent K uptake, which decreases Ca uptake (Park et al. 2019).
In the present research, grain Ca content in D2 was the least, indicating that the plant uses other mechanisms for stress tolerance rather than increase in Ca content, which can also affect P concentration. According to the results, the treatments with the least Ca content had the highest P content. There is a high probability of antagonistic and synergistic among nutrients in sorghum (Aqaei et al. 2020).
The highest grain fat was related to D1 and D3, and there were not any significant differences among the different treatments of fertilization. During the grain filling period, first protein and then oil is formed; the longer the grain filling period, the more oil is produced. But if the grain filling period is short (for example, the grain is subject to drought stress during the filling period), due to the earlier formation of proteins, the grains will have a higher protein percentage. Moreover, due to the higher environmental temperature and water stress during the shorter grain filling stage, the seeds contain less fat. It is because under such conditions the ability to convert carbohydrates to fat is reduced and the oxidation of some unsaturated fatty acids reduces seed oil content (Farooq et al. 2017; Asghari et al. 2018).
The decrease in lipids, under drought stress, can also be the result of reduction in: (1) seed size, (2) activity of lipid-synthesizing enzymes, (3) the amount of carbohydrates available for the storage tissues, and (4) lipase activity (Wang et al. 2019; Li et al. 2020b). Mertz-Henning et al. (2018) found a negative relationship between seed oil and protein under drought stress conditions. Table 2 also shows that the treatments with the highest protein content have the least fat content and vice versa.
Treatment D2 + macro resulted in the highest percentage of grain tannin, probably due to the presence of P and N affecting the production of plant phenolic compounds (Jiang et al. 2020). These results were in line with the results of Alhaithloul (2019) indicating the increased concentration of tannins in Artemisia sieberi in dry conditions (dehydration). According to Jyothi and Hebsur (2017), nano-fertilizer increases the total amount of phenolic and antioxidant compounds by affecting the chemical properties of cereals.
The least amount of seed tannin was related to D2 + micro, which also resulted in the highest percentage of seed protein. This could be because when seed protein is present in large quantities, a relatively hydrophobic layer between the protein-tannin bond prevents protein from sedimentation. There were not significant differences among different treatments, indicating that the percentage of sorghum seed tannin, when seed is exposed to drought stress during filling stage, is mostly affected by dehydration (Kaur and Asthir 2017; Alhaithloul et al. 2020).
Due to water stress at different stages of plant growth, nutrient uptake process is disrupted in different plant tissues. According to the results, under normal and non-stress irrigation conditions, the fertilizer application treatments similarly affected sorghum yield and biochemical properties. The treatments differ only in seed tannin and seed fat percentage. Accordingly, a combination of micro- and macro-nano-fertilizer can be applied in non-stress conditions to increase seed yields. In addition, the combination of micro + macro-fertilizer contained higher nutrients than chemical fertilizer (N, P, K). However, the tested nano-fertilizer enhanced sorghum yield and biochemical properties in drought stress conditions. Grain yield, Ca, and crude fat were most affected by nano- and chemical fertilizer in D1, and P, protein and tannin were most affected by nano- and chemical fertilizer in D2. The results indicate that the tested micro- and macro-fertilizer can favorably improve sorghum growth and biochemical properties in drought stress conditions. However, drought stress before and after flowering, and other environmental conditions determine the recommendation of superior fertilizer treatment between nano- and chemical fertilizer.
Data availability statement
All data generated or analyzed during this study are included in this published article.
References
Aghajani A, Soleymani A (2017) Effects of nano-fertilization on growth and yield of bean (Phaseolus vulgaris L.) under water deficit conditions. Curr Nanosci 13:194–201
Alhaithloul HAS (2019) Impact of combined heat and drought stress on the potential growth responses of the desert grass Artemisia sieberi alba: relation to biochemical and molecular adaptation. Plants 8:416
Alhaithloul HA, Soliman MH, Ameta KL, El-Esawi MA, Elkelish A (2020) Changes in ecophysiology, osmolytes, and secondary metabolites of the medicinal plants of Mentha piperita and Catharanthus roseus subjected to drought and heat stress. Biomolecules 10:43
Aqaei P, Weisany W, Diyanat M, Razmi J, Struik PC (2020) Response of maize (Zea mays L.) to potassium nano-silica application under drought stress. J Plant Nutr 43:1205–1216
Asadi E, Mohammadi Ghehsareh A, Moghadam EG, Hoodaji M, Zabihi HR (2019) Improvement of pomegranate colorless arils using iron and zinc fertilization. J Clean Prod 234:392–399
Asghari BAH, Heravan EM, Alizadeh BAHRAM, Abad HHS, Madani HAMID (2018) Oil content, seed yield and morphological changes of canola cultivars in response to different sowing dates. Crop Res 53:38–44
Awasthi R, Kaushal N, Vadez V, Turner NC, Berger J, Siddique KH, Nayyar H (2014) Individual and combined effects of transient drought and heat stress on carbon assimilation and seed filling in chickpea. Funct Plant Biol 41:1148–1167
de Oliveira DF, de Sousa Lopes L, Gomes-Filho E (2020) Metabolic changes associated with differential salt tolerance in sorghum genotypes. Planta 252:1–18
Dimkpa CO, White JC, Elmer WH, Gardea-Torresdey J (2017) Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J Agric Food Chem 65:8552–8559
Dimkpa CO, Singh U, Bindraban PS, Elmer WH, Gardea-Torresdey JL, White JC (2019) Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci Total Environ 688:926–934
Dimkpa CO, Andrews J, Sanabria J, Bindraban PS, Singh U, Elmer WH, Gardea-Torresdey JL, White JC (2020) Interactive effects of drought, organic fertilizer, and zinc oxide nanoscale and bulk particles on wheat performance and grain nutrient accumulation. Sci Total Environ 722:137808
Farooq M, Nadeem F, Gogoi N, Ullah A, Alghamdi SS, Nayyar H, Siddique KH (2017) Heat stress in grain legumes during reproductive and grain-filling phases. Crop Pasture Sci 68:985–1005
Farooq M, Ullah A, Lee DJ, Alghamdi SS, Siddique KH (2018) Desi chickpea genotypes tolerate drought stress better than kabuli types by modulating germination metabolism, trehalose accumulation, and carbon assimilation. Plant Physiol Biochem 126:47–54
Fontes GP, Tomlinson PJ, Roozeboom KL, Ruiz Diaz DA (2017) Grain sorghum response to nitrogen fertilizer following cover crops. Agron J 109:2723–2737
Getachew G, Putnam DH, De Ben CM, De Peters EJ (2016) Potential of sorghum as an alternative to corn forage. Am J Plant Sci 7:1106–1121
Ghaffari H, Tadayon MR, Nadeem M, Cheema M, Razmjoo J (2019) Proline-mediated changes in antioxidant enzymatic activities and the physiology of sugar beet under drought stress. Acta Physiol Plant 41:23
Guo H, White JC, Wang Z, Xing B (2018) Nano-enabled fertilizers to control the release and use efficiency of nutrients. Curr Opin Environ Sci Health 6:77–83
Jankowski KJ, Sokólski MM, Dubis B, Załuski D, Szempliński W (2020) Sweet sorghum-Biomass production and energy balance at different levels of agricultural inputs. A six-year field experiment in north-eastern Poland. Eur J Agron 119:126119
Jiang Y, Zhang H, Qi X, Wu G (2020) Structural characterization and antioxidant activity of condensed tannins fractionated from sorghum grain. J Cereal Sci 92:102
Jyothi TV, Hebsur NS (2017) Effect of nanofertilizers on growth and yield of selected cereals-a review. Agric Rev 38(2):112–120
Kaur G, Asthir B (2017) Molecular responses to drought stress in plants. Biol Plant 61:201–209
Kulamarva AG, Sosle VR, Raghavan GSV (2009) Nutritional and rheological properties of sorghum. Int J Food Prop 12:55–69
Li P, Wang A, Du W, Mao L, Wei Z, Wang S, Yuan H, Ji R, Zhao L (2020) Insight into the interaction between Fe-based nanomaterials and maize (Zea mays) plants at metabolic level. Sci Total Environ 738:139795
Li G, Liang Z, Li Y, Liao Y, Liu Y (2020b) Exogenous spermidine regulates starch synthesis and the antioxidant system to promote wheat grain filling under drought stress. Acta Physiol Plant 42:1–14
Liang C, Zhang B (2018) Effect of exogenous calcium on growth, nutrients uptake and plasma membrane H+-ATPase and Ca2+-ATPase activities in soybean (Glycine max) seedlings under simulated acid rain stress. Ecotoxicol Environ Saf 165:261–269
Lin D, Xiao M, Zhao J, Li Z, Xing B, Li X, Kong M, Li L, Zhang Q, Liu Y, Chen H (2016) An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 21:1374
Mertz-Henning LM, Ferreira LC, Henning FA, Mandarino JM, Santos ED, Oliveira MC, Nepomuceno AL, Farias JR, Neumaier N (2018) Effect of water deficit-induced at vegetative and reproductive stages on protein and oil content in soybean grains. Agronomy 8:3
Miransari M, Mackenzie AF (2015) Development of soil N testing for wheat production using soil residual mineral N. J Plant Nutr 38:1995–2005
Miransari M, Smith D (2019) Sustainable wheat (Triticum aestivum L.) production in saline fields: a review. Crit Rev Biotechnol 39:999–1014
Miransari M, Bahrami HA, Rejali F, Malakouti MJ (2008) Using arbuscular mycorrhiza to alleviate the stress of soil compaction on wheat (Triticum aestivum L.) growth. Soil Biol Biochem 40:1197–1206
Mossé J, Baudet J (1983) Crude protein content and aminoacid composition of seeds: variability and correlations. Plant Foods Hum Nutr 32:225–245
Noori MS (2021) Alleviation of drought stress in wheat (Triticum aestivum L.) by mineral fertilization. J Stress Physiol Biochem 17:82–93
Nxele X, Klein A, Ndimba BK (2017) Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S Afr J Bot 108:261–266
Obizoba IC (1988) Nutritive-value of malted, dry-milled or wet-milled sorghum and corn. Cereal Chem 65:447–449
Park SM, Lee J, Jeon EK, Kang S, Alam MS, Tsang DC, Alessi DS, Baek K (2019) Adsorption characteristics of cesium on the clay minerals: structural change under wetting and drying condition. Geoderma 340:49–54
Proietti I, Frazzoli C, Mantovani A (2015) Exploiting nutritional value of staple foods in the world’s semi-arid areas: risks, benefits, challenges and opportunities of sorghum. Healthcare 3:172–193
Queiroz VAV, da Silva Aguiar A, de Menezes CB, de Carvalho CWP, Paiva CL, Fonseca PC, da Conceição RRP (2018) A low calorie and nutritive sorghum powdered drink mix: Influence of tannin on the sensorial and functional properties. J Cereal Sci 79:43–49
Raliya R, Saharan V, Dimkpa C, Biswas P (2017) Nanofertilizer for precision and sustainable agriculture: current state and future perspectives. J Agric Food Chem 66:6487–6503
Rashawn AK, Yones HA, Karim N, Taha EM, Chen W (2021) Potential processing technologies for developing sorghum-based food products: an update and comprehensive review. Trends Food Sci Technol in press
Ratnavathi CV, Komala VV (2016) Sorghum grain quality. In: Sorghum biochemistry. ISBN: 9780128031575 Academic Press, pp. 1–61.
Rehab IF, Kordy AM, Salim B (2020) Assessment of sorghum (Sorghum bicolor L.) productivity under different weed control methods, mineral and nano fertilization . Egypt Acad J Biol Sciences H Botany 11:1–11
Sabet H, Mortazaeinezhad F (2018) Yield, growth and Fe uptake of cumin (Cuminum cyminum L.) affected by Fe-nano, Fe-chelated and Fe-siderophore fertilization in the calcareous soils. J Trace Elem Med Biol 50:154–160
Sadeghi, A., Ataabadi, M., Abolhasani, M.H., 2021. Chromium removal from a contaminated soil using nano zero-valent iron and magnetite affected by temperature and moisture. Soil Sediment Contam (in press).
Sanjari S, Shobbar ZS, Ghanati F, Afshari-Behbahanizadeh S, Farajpour M, Jokar M, Khazaei A, Shahbazi M (2021) Molecular, chemical, and physiological analyses of sorghum leaf wax under post-flowering drought stress. Plant Physiol Biochem 159:383–391
Seleiman MF, Almutairi KF, Alotaibi M, Shami A, Alhammad BA, Battaglia ML (2021) Nano-fertilization as an emerging fertilization technique: Why can modern agriculture benefit from its use? Plants 10:2
Shirmohammadli Y, Efhamisisi D, Pizzi A (2018) Tannins as a sustainable raw material for green chemistry: A review. Ind Crops Prod 126:316–332
Vanamala JK, Massey AR, Pinnamaneni SR, Reddivari L, Reardon KF (2018) Grain and sweet sorghum (Sorghum bicolor L. Moench) serves as a novel source of bioactive compounds for human health. Crit Rev Food Sci Nutr 58:2867–2881
Wang J, Chen Y, Wang P, Li YS, Wang G, Liu P, Khan A (2018) Leaf gas exchange, phosphorus uptake, growth and yield responses of cotton cultivars to different phosphorus rates. Photosynthetica 56:1414–1421
Wang X, Mao Z, Zhang J, Hemat M, Huang M, Cai J, Zhou Q, Dai T, Jiang D (2019) Osmolyte accumulation plays important roles in the drought priming induced tolerance to post-anthesis drought stress in winter wheat (Triticum aestivum L). Environ Exp Bot 166:103804
Wu S, Hu C, Yang X, Tan Q, Yao S, Zhou Y, Wang X, Sun X (2020) Molybdenum induces alterations in the glycerolipidome that confer drought tolerance in wheat. J Exp Bot 71:5074–5086
Zamani S, Naderi MR, Soleymani A, Nasiri BM (2020) Sunflower (Helianthus annuus L.) biochemical properties and seed components affected by potassium fertilization under drought conditions. Ecotoxicol Environ Saf 190:110017
Zehetner F, Wuenscher R, Peticzka R, Unterfrauner H (2018) Correlation of extractable soil phosphorus (P) with plant P uptake: 14 extraction methods applied to 50 agricultural soils from Central Europe. Plant Soil Environ 64:192–201
Zhao X, Meng Z, Wang Y, Chen W, Sun C, Cui B, Cui J, Yu M, Zeng Z, Guo S, Luo D (2017) Pollen magnetofection for genetic modification with magnetic nanoparticles as gene carriers. Nat Plants 3:956–964
Zhao Q, Yu X, Zhou C, Yagoub AEA, Ma H (2020) Effects of collagen and casein with phenolic compounds interactions on protein in vitro digestion and antioxidation. LWT 124:109192
Acknowledgements
The authors would like to thank very much, the international publisher, AbtinBerkeh Scientific Ltd. Company (https://AbtinBerkeh.com), Isfahan, Iran, for revising the manuscript, and formatting it according to the journal format.
Funding
There was not any funding for the present research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they do not have any conflict of interest.
Rights and permissions
About this article
Cite this article
Kazemi, E., Ganjali, H.R., Mehraban, A. et al. Yield and biochemical properties of grain sorghum (Sorghum bicolor L. Moench) affected by nano-fertilizer under field drought stress. CEREAL RESEARCH COMMUNICATIONS 50, 397–405 (2022). https://doi.org/10.1007/s42976-021-00198-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42976-021-00198-2