Abstract
Phytic acid (myo-inositol 1,2,3,4,5,6-hexakisphosphate; InsP6) is the main storage form of phosphorus in plant seeds, and it decreases the bioavailability of minerals in the feed of monogastric animals. The enzyme myo-inositol-1-phosphate synthase (MIPS, EC 5.1.1.4) catalyzes the conversion of D-glucose-6-phosphate to myo-inositol-1-phosphate, which is the initial step of phytic acid biosynthesis. To date, there is no information about the relationship between the expression of MIPS and the accumulation of phytic acid in developing oat seeds. In the present study, MIPS was isolated by rapid amplification of cDNA ends (RACE)-PCR method from the early developing oat seeds by using three consensus primers which were designed from highly conserved regions in the MIPS sequence from other plants. We examined the changes in the expression of MIPS and accumulation of phytic acid in different seed development stages, as well as the changes in total P (TP), Ca, and Mg concentrations during seed maturation. The results of RACE-PCR and northern blot showed that the maximal MIPS transcription level was observed at 5 days after flowering (DAF), and it was not detected in stems and leaves. Phytic acid was first detected at 25 DAF, when the inorganic P (Pi) level was 8.4-fold lower and the phytic acid P (Phy-P) level was 7.3-fold higher than those after 30 DAF. The TP, Ca, and Mg concentrations increased with the progress of seed maturation. These results indicate that MIPS was only expressed in seeds at the early stage of seed development, after which the seeds started to accumulate phytic acid. In addition, TP, Ca, and Mg are accumulated during seeds maturation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oat (Avena sativa) seeds are considered a healthy food source with high nutritional value. They are the most important livestock feed, containing 11‒15% proteins, 22% slowly digestible starch in the total starch content, 5‒9% lipids, and abundant potassium and phosphorus (Singh et al. 2013; Rasane et al. 2015; Redaelli et al. 2015). However, 75 ± 10% of seed total phosphorus is present in the form of phytic acid (myo-inositol 1,2,3,4,5,6-hexakisphosphate; InsP6), which decreases the bioavailability of minerals in the feed of monogastric animals, such as swine and poultry (Raboy 2020). For this reason, monogastric animal waste contains large quantities of undigested phytic acid and pollutes the water, (Dong et al. 2020). The excessive input of macronutrient, such as phosphorus and nitrogen, to change the nutrient balance of aquatic ecosystem is contributing to eutrophication (Meyer-Reil and Köster 2000). There are two phytic acid biosynthesis pathways, lipid-dependent and lipid-independent pathway. The former occurs in all plant tissues, whereas the latter mainly occurs in seeds (Karmakar et al. 2020). During phytic acid biosynthesis, myo-inositol-1-phosphate synthase (MIPS, EC 5.1.1.4) catalyzes the conversion of D-glucose-6-phosphate (G-6-P) to myo-inositol-1-phosphate (MIP, InsP1) (Ray et al. 2010). This is the initial step of phytic acid biosynthesis and inositol metabolism (Loewus and Murthy 2000). InsP1 is hydrolyzed by myo-inositol monophosphatase, resulting in the formation of inositol, which is a component of many plant compounds. It is vital for many physiological and biochemical processes in plants, including stress signaling, hormonal regulation, and growth regulation (Zhai et al. 2016). In soybean, the transcription levels of MIPS are higher in developing seeds than at later plant development stages, but this gene is minimally (or not at all) expressed in leaves, flowers, and roots (Chappell et al. 2006). MIPS was also detected in the developing seeds of mungbean and yellow passion fruit (Abreu and Aragão 2007; Wongkaew et al. 2010).
There is limited information about the relationship between the expression of MIPS and seed development in oat. Thus, in the present study, we investigated and discussed the changes in MIPS expression and phytic acid accumulation during oat seed maturation, and our findings may contribute to the development of new oat lines with lower phytic acid contents.
Materials and methods
Plant materials and growth condition
Oat (Avena sativa) seeds were sown in plastic pots (diameter 250 mm, height 300 mm) filled with 9 kg of soil mixture containing sandy loam soil and vermiculite in a volume ratio of 1:1. Before sowing, ammonium sulfate, super phosphate, and potassium sulfate (0.66 g, 0.81 g, and 0.27 g kg−1 dry soil, respectively) were thoroughly mixed with the soil mixture. The soil pH (H2O) was adjusted to about 6.5 using dolomitic calcium carbonate. The plants were grown in a glass house under natural light conditions. Buds and flowers were collected before and during the flowering stage, respectively. Pods were harvested 5, 10, 15, 20, 25, 30, 40, and 45 days after flowering (DAF), before seed maturation, and mature seeds were also collected. Leaf and stem samples were obtained at 5, 10, and 30 DAF, these samples were immediately frozen in liquid nitrogen and stored at -80 °C until further analysis.
Total RNA extraction and isolation of MIPS
Total RNA was extracted from 100 mg of buds, flowers, and pods at 5, 15, 20, and 25 DAF using the method described in Dong and Dunstan (1996). The quality and quantity of RNA and cDNA were determined using a spectrophotometer. An RNA sample (1‒3 μL) was used for first strand cDNA synthesis using the SMARTer RACE cDNA Amplification Kit (Clontech, Takara Bio Inc., Japan). MIPS isolation was performed by the rapid amplification of cDNA ends (RACE) PCR method (Doenecke et al. 1997). Three primers (primer 1: 5’-CGACGGCATGAACCTCTCAG.
CTC-3’; primer 2: 5’-GTTCGGAGGATGGGACATCAGCA-3’; primer 3: 5’-CTCCGCCTCGTTCTTGTCCACAG-3’), which were designed from highly conserved regions, were used for MIPS cDNA amplification based on Zea mays (accession. No. AF056326), Oryza sativa (accession. No. AB012107), and Glycine max (accession. No. AY038802) MIPS sequences.
Northern blot
For northern blotting, 20 μg of the total RNA samples from plant tissues were separated by 1% formaldehyde agarose gel electrophoresis in 1 × MOPS buffer (pH 7.0). Then, the gel was transferred to a nylon+ membrane (Amersham, Oakville, Canada) with 20 × SSC overnight. Hybridization was carried out using the 826-bp DNA fragment of the MIPS cDNA labeled with fluorescein-dUTP for 16 h at 60 °C in the prehybridization solution (5 × SSC, 0.1% SDS, 5% (w/v) dextran sulfate and 1/20 dilution of liquid block). A fragment of the MIPS was amplified from MIPS cDNA using the primer 5’-CGACGGCATGAACCTCTCGGCGC-3’. The membrane was washed with the first (1 × SSC, 0.1% SDS) and second washing buffer (1 × SSC, 0.1% SDS) at 60 °C for 15 min, and transferred to the blocking buffer (10% (v/v) liquid block and buffer A (100 mM Tris–HCl and 600 mM NaCl; pH 7.5)) at 25 °C for 1 h. Then, the membrane was treated with 1:5000 anti-fluorescein-HRP conjugate and 0.5% (w/v) BSA in buffer A. Three washes were performed with 0.3% (v/v) Tween 20 in buffer A at 25 °C for 10 min. The membrane was removed from the excess buffer, and 1.5 mL of CDP-Star (Amersham, Oakville, Canada) was added. Finally, the membrane was exposed to X-ray film (FUJI EC-A CASSETTE, FUJIFILM Corporation, Japan) for 4 h.
Measurement of phytic acid P and inorganic P
Phytic acid P (Phy-P) and inorganic P (Pi) were measured using the method described in Raboy and Dickinson (1987). Before measuring, the plant tissue samples were dried at 70 °C for 48 h. In order to measure Phy-P, 250 mg of ground samples were extracted with 12.5 mL 0.4 M HCl containing 0.7 M Na2SO4 and soaked for 16 h at 4 °C. The mixture was centrifuged at 9,000 × g at 4 °C for 10 min. Supernatant (0.1 mL) was used for Pi determination. The rest of the supernatant (10 mL) was mixed with 10 mL of distilled water and 5 mL of 15 mM FeCl3 (with 0.35 M Na2SO4 (w/v) and 0.2 M HCl). The mixture was incubated in a 100 °C water bath for 60 min, after which it was incubated in ice water for 15 min. The sediments were collected by centrifugation at 9,000 × g at 4 °C for 10 min, washed twice with 0.2 M HCl, and collected by centrifugation at 10,000 × g at 4 °C for 10 min. Afterward, they were digested with H2SO4 and H2O2 mixture and diluted to 100 mL. Phy-P and Pi were determined from the dissolved solution and supernatant, respectively, using a colorimetric assay (Chen et al. 1956).
Measurement of total P, Ca, and Mg
Ground samples (50 mg) were digested with 1.25 mL of HNO3. After cooling, the mixture was mixed with 0.5 mL H2O2, heated at 200 ℃ for 2 h, and diluted to 100 mL. The concentrations of total P, Ca, and Mg were measured using ICP-AES (ICAP-575 II, Operation of Nippon Jarrell-Ash, Japan).
Statistical analysis
All statistical analyses including phytic acid P, inorganic P, and total P, Ca, and Mg concentration during seed maturation were performed using SPSS 21 (IBM Inc., Chicago, IL, USA). The data were assessed using analysis of variance (ANOVA). Tukey’s multiple range test was used to determine significant differences (P ≤ 0.05).
Results
RACE-PCR analysis of MIPS in oat seed development
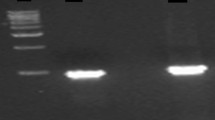
Oat seed development stages are shown in Fig. 1. Comparing the results of MIPS RACE-PCR at 5 and 30 DAF using primer 2 (P2), the expression of MIPS was detected in developing oat seeds at 5 DAF, and RACE-PCR produced approximately 500 bp, but it was not detected at 30 DAF (Fig. 3a). A part of sequence alignment information from which the primers were synthesized is shown in Fig. 2. In developing oat seeds at 5 DAF, RACE-PCR produced approximately 500 bp, 1400 bp, and 900 bp using primer 3 (P3), primer 1 (P1), and primer 2 (P2), respectively (Fig. 3b).
Part of the sequence alignment information of Zea mays, Oryza sativa, and Glycine max. Primer 1 (a), primer 2 (b), and primer 3 (c) (reverse complement) were designed from the MIPS conserved sequence of Zea mays, Oryza sativa, and Glycine max. The arrows indicate the direction of sequence synthesis using these primers
Northern blot analysis of oat seed development
Results of the northern blot analysis showed that the expression of MIPS in flowers and developing seeds was detected at 0, 5, and 10 DAF; however, it was not detected at 15 and 25 DAF (Fig. 4a). In particular, the highest transcription level of MIPS was observed at 5 DAF. Low levels of MIPS expression were recorded in developing seeds at 10 DAF. However, the expression of MIPS in the stem and leaf materials was not detected at 5 DAF (Fig. 4b).
Changes in phytic acid P (Phy-P) and inorganic P (Pi) concentration during oat seed development
Before flowering, Pi concentration was 1.58 mg/g, and Phy-P was not detected. The Pi concentration was significantly increased during seed development until 30 DAF, when it was 3.30 mg/g, after which it decreased until the mature stage (Fig. 5). Phy-P was first detected at 25 DAF at the concentration of 0.24 mg/g, after which its concentration significantly increased until seed maturity, when it was 5.97 mg/g. In particular, compared to that at 25 DAF, Pi was 8.4-fold lower after 30 DAF; however, Phy-P was 7.3-fold higher. The Phy-P concentration of mature seeds was 25-fold higher than that of the seeds at 25 DAF.
Changes in phytic acid P (Phy-P) and inorganic P (Pi) concentration during oat seed development. Data represent the means of multiple replicates (n ≥ 3). Means with different superscript letters in a column are significantly different at P ≤ 0.05, as assessed by Tukey’s range test. DW, dry weight, DAF, days after flowering
Changes in total P (TP), Mg, and Ca concentrations during oat seed development
The TP and Mg concentrations increased with the progress of seed development from 3.25 to 10.15 mg/g and from 1.00 to 2.77 mg/g, respectively (Table 1). Their concentrations were significantly increased during oat seed development compared to that during the flowering period. However, TP concentration did not differ significantly from 10 to 45 DAF, and Mg concentration did not differ significantly from 0 to 30 DAF. Ca concentration reached the peak at 10 and 30 DAF, when it was 6.32 and 6.09 mg/g, respectively. After 30 DAF, Ca concentration decreased until seed maturation.
Discussion
In plants, phosphorous is stored in the form of phytic acid which reduces the bioavailability of minerals in the feed of monogastric animals. In the present study, we evaluated the relationship between the expression of MIPS, which is a gene encoding an enzyme that acts as a catalyst in phytic acid biosynthesis, and seed development in oat (Avena sativa), with the aim to improve the bioavailability of animal feed, as well as to decrease eutrophication in areas where monogastric animals are fed with oat and other crops with high phytic acid content.
Our results showed that MIPS was detected and isolated from the seed pods at 5 DAF; however, it was not expressed after 30 DAF. Results of the northern blot analysis showed that MIPS was expressed from 0 to 10 DAF. Figure 3a shows the maximal transcription levels of MIPS at 5 DAF. Interestingly, similar results were observed in several previous studies, which showed that the peak transcription levels of MIPS were observed 9 days after pollination in yellow passion fruit and mung bean (Abreu and Aragão 2007; Wongkaew et al. 2010). In soybean, MIPS was detected at the cotyledon stage, and its peak transcription level was observed in seeds of 2 to 4 mm in length (Hegeman et al. 2001). In the present study, MIPS was also expressed at the early stage of seed development. These results suggested that glucose-6-phosphate (G-6-P) was converted into MIP at the early stage of seed development (Abreu and Aragão 2007). Moreover, we found that MIPS was not expressed in stems and leaves at 5 DAF, which indicated that in oat, the expression of MIPS only occurs during seed development.
In contrast, MIPS expression was not correlated with the accumulation of phytic acid. MIPS catalyzes the conversion of G-6-P to InsP1, which is the initial step of phytic acid biosynthesis. We found that phytic acid was first detected at 25 DAF, after which its content increased until seed maturation. The mistiming between the expression of MIPS and phytic acid production may be caused by various complicated steps of phytic acid biosynthesis (Loewus and Murthy 2000). We found that during seed development, Pi concentration slowly increased from 0 to 30 DAF; however, when Phy-P significantly increased after 30 DAF, Pi concentration was significantly decreased compared to that after 30 DAF. Moreover, TP concentration did not significantly change from 10 to 45 DAF. The reason for the severe changes between Pi and Phy-P may be related to the storage of P in the period from 30 to 45 DAF. From 40 DAF until maturation, oat plants can transfer P from other organs to seeds in order satisfy the needs of P for producing phytic acid in their seeds. Ca has a vital function in plants. It is a secondary messenger that controls membrane structure and function, as well as plant response to environmental signals (Hepler 2005). The observed increase in Ca concentration may have indicated that the plant Ca requirement increased during seed development compared to that before the beginning of seed development. It is known that phytic acid can chelate positively charged mineral cations and form stable salts (Raboy 2009). In the present study, Mg concentration increased with the increase in phytic acid accumulation, which was in accordance with the previous statement (Lott et al. 1995).
In summary, we showed that there is a relationship between MIPS transcription levels and the accumulation of phytic acid during oat seed development. We confirmed that MIPS was expressed at the early stage of seed development, and the highest transcription level of MIPS was observed at 5 DAF. Phytic acid was detected at 25 DAF, and Phy-P increased with decreasing Pi after 30 DAF. Therefore, if the expression of MIPS could be inhibited at the early stage of seed development, we could develop new oat lines with lower phytic acid content.
References
Abreu EFM, Aragão FJL (2007) Isolation and characterization of a myo-inositol-1-phosphate synthase gene from yellow passion fruit (Passiflora edulis f. flavicarpa) expressed during seed development and environmental stress. Ann Bot 99:285–292. https://doi.org/10.1093/aob/mcl256
Chappell AS, Scaboo AM, Wu X, Nguyen H, Pantalone VR, Bilyeu KD (2006) Characterization of the MIPS gene family in Glycine max. Plant Breed 125:493–500. https://doi.org/10.1111/j.1439-0523.2006.01264.x
Chen PS, Toribara TY, Warner H (1956) Microdetermination of Phosphorus. Anal Chem 28:1756–1758. https://doi.org/10.1021/ac60119a033
Doenecke A, Winnacker EL, Hallek M (1997) Rapid amplification of cDNA ends (RACE) improves the PCR-based isolation of immunoglobulin variable region genes from murine and human lymphoma cells and cell lines. Leukemia 11:1787–1792. https://doi.org/10.1038/sj.leu.2400781
Dong J-Z, Dunstan DI (1996) A reliable method for extraction of RNA from various conifer tissues. Plant Cell Rep 15:516–521. https://doi.org/10.1007/BF00232985
Dong Q, Echigo K, Raboy V, Saneoka H (2020) Seedling growth, physiological characteristics, nitrogen fixation, and root and nodule phytase and phosphatase activity of a low-phytate soybean line. Plant Physiol Biochem 149:225–232. https://doi.org/10.1016/j.plaphy.2020.02.015
Hegeman CE, Good LL, Grabau EA (2001) Expression of D-myo-inositol-3-phosphate synthase in soybean. Implications for phytic acid biosynthesis. Plant Physiol 125:1941–1948. https://doi.org/10.1104/pp.125.4.1941
Hepler PK (2005) Calcium: a central regulator of plant growth and development. Plant Cell 17:2142–2155. https://doi.org/10.1105/tpc.105.032508
Karmakar A, Bhattacharya S, Sengupta S, Ali N, Sarkar SN, Datta K, Datta SK (2020) RNAi-mediated silencing of ITPK gene reduces phytic acid content, alters transcripts of phytic acid biosynthetic genes, and modulates mineral distribution in rice seeds. Rice Sci 27(4):315–328. https://doi.org/10.1016/j.rsci.2020.05.007
Loewus FA, Murthy PPN (2000) myo-Inositol metabolism in plants. Plant Sci 150:1–19. https://doi.org/10.1016/S0168-9452(99)00150-8
Lott JNA, Greenwood JS, Batten GD (1995) Mechanisms and regulation of mineral nutrient storage during seed development. In: Kigel J, Galili G (eds) Seed development and germination. Marcel Dekker, New York, pp 215–235
Meyer-Reil LA, Köster M (2000) Eutrophication of marine waters: effects on benthic microbial communities. Mar Pollut Bull 41(1–6):255–263. https://doi.org/10.1016/S0025-326X(00)00114-4
Raboy V (2009) Approaches and challenges to engineering seed phytate and total phosphorus. Plant Sci 177:281–296. https://doi.org/10.1016/j.plantsci.2009.06.012
Raboy V (2020) Low phytic acid crops: Observations based on four decades of research. Plants 9:140. https://doi.org/10.3390/plants9020140
Raboy V, Dickinson DB (1987) The timing and rate of phytic acid accumulation in developing soybean seeds. Plant Physiol 85(3):841–844. https://doi.org/10.1104/pp.85.3.841
Rasane P, Jha A, Sabikhi L, Kumar A, Unnikrishnan VS (2015) Nutritional advantages of oats and opportunities for its processing as value added foods-a review. J Food Sci Technol 52(2):662–675. https://doi.org/10.1007/s13197-013-1072-1
Ray S, Patra B, Das-Chatterjee A, Ganguli A, Majumder AL (2010) Identification and organization of chloroplastic and cytosolic L-myo-inositol 1-phosphate synthase coding gene (s) in Oryza sativa: comparison with the wild halophytic rice, Porteresia coarctata. Planta 231:1211–1227. https://doi.org/10.1007/s00425-010-1127-8
Redaelli R, Scalfati G, Ciccoritti R, Cacciatori P, De Stefanis E, Sgrulletta D (2015) Effects of genetic and agronomic factors on grain composition in oats. Cereal Res Commun 43:144–154. https://doi.org/10.1556/CRC.2014.0019
Singh R, Subrata D, Asma B (2013) Avena sativa (Oat), a potential neutraceutical and therapeutic agent: an overview. Crit Rev Food Sci Nutr 53(2):126–144. https://doi.org/10.1080/10408398.2010.526725
Wongkaew A, Nakasathien S, Srinives P (2010) Isolation and characterization of D-myo-inositol-3-phosphate synthase from Mungbean (Vigna radiata). Plant Mol Biol Rep 28:122–127. https://doi.org/10.1007/s11105-009-0137-9
Zhai H, Wang F, Si Z, Huo J, Xing L, An Y, He S, Liu Q et al (2016) A myo-inositol-1-phosphate synthase gene, IbMIPS1, enhances salt and drought tolerance and stem nematode resistance in transgenic sweet potato. Plant Biotechnol J 14:592–602. https://doi.org/10.1111/pbi.12402
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by R.N. Chibbar.
Rights and permissions
About this article
Cite this article
Qin, D., Toyonaga, D. & Saneoka, H. Characterization of myo-inositol-1-phosphate synthase (MIPS) gene expression and phytic acid accumulation in oat (Avena sativa) during seed development. CEREAL RESEARCH COMMUNICATIONS 50, 379–384 (2022). https://doi.org/10.1007/s42976-021-00186-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42976-021-00186-6