Abstract
Using accurate and precise species-specific parameters in mechanistic models can lead to better predictions of population dynamics and ecosystem function (e.g. nutrient cycling) across a range of environmental conditions. Zooplankton are important in the aquatic food web and for nutrient cycling but are highly diverse, and there is only limited information on specific species. Knowledge of species-specific attributes is patchy. In particular, tropical species are underrepresented in this regard. Here, we gather all the known information about a wide-spread tropical zooplankton member, Ceriodaphnia rigaudi, and add new information from lab and field experiments. We determine feeding rate across a range of food concentrations and food-dependent population growth rate of C. rigaudi. Additionally, we use 16 years of occurrence data from rock pools in Jamaica to explore environmental characteristics of the habitat in which C. rigaudi live. We compare our data to worldwide records of the species attributes and create a reference map of its occurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functional traits are morphological–physiological aspects of a species that impact fitness (Violle et al., 2007); often functional traits are also traits of the organisms that directly interact with (sensu effect traits Hébert et al., 2017; Lavorel and Garnier, 2002) the environment. Functional traits are important for two reasons: (1) making model predictions involving a specific species of interest (e.g. van derMeer,2006; (2) enabling classifications of species into functional groups for classic trait-based models (e.g. Vogt et al., 2013). Trait-based models may be particularly important in aquatic systems due to high species richness (e.g. phytoplankton) and the desire for species-specific models (e.g. fisheries). Aquatic systems are well-suited to trait-based methods due to a long history of modelling (Robson, 2014) and combining of high species richness into subgroups (e.g. phytoplankton: Litchman et al. 2010; zooplankton: Litchman et al. 2013). Yet, despite advances in data amalgamation for aquatic species (e.g. Hèbert et al., 2016; Robson et al., 2018) more information is always needed for additional species to expand the usefulness of trait-based methods.
Zooplankton represent a large and important part of aquatic systems and yet species specific information is limited. Zooplankton classifications are usually based on size and feeding strategy. However, size may not be a good predictor of traits of interest (e.g. grazing) outside of temperate regions (Pinheiro-Silva et al., 2020) which may be a larger problem than currently realized because tropical species tend to be underrepresented (e.g. Hèbert et al., 2016; Rizo et al., 2017). One challenge is the limited scope of studies on life history that makes it impossible to tell if species traits are consistent throughout their range. With more resolved life history information, more informed and nuanced decisions for trait-based classifications should be easier. Here, we provide additional information for a small, tropical cladoceran, Ceriodaphnia rigaudi.
Cladocerans are a second crucial link between lower trophic levels (microbial) and upper trophic levels (fish) of the freshwater food web (Burns & Schallenberg, 2001), alter algal production and community structure (Sommer et al., 2001), and impact nutrient cycling (e.g. Migal, 2011). However, most cladoceran species have little life history information; a meta-analysis by Hébert et al. (2016) found that the genus Daphnia comprises half of all known cladoceran information. Despite a known 620 unique cladoceran species (Litchman et al. 2010), a meta-analysis found species trait information on less than 70 (Hébert et al., 2016) which is concerning for the ubiquitous use of species traits in aquatic modelling. Gathering more information on smaller cladocerans will help identify when size is or is not a good proxy for traits of interest.
Ceriodaphnia rigaudi is a small cladoceran commonly found throughout tropical freshwater ponds. Originally thought to be distinguished from Ceriodaphnia cornuta by the absence of head, shoulder and tail spikes, this classification turns out potentially to be based solely on predation and not a true physiological characteristic (Rietzler et al., 2008). However, recent genetic tests do in fact place C. rigaudi as a separate species from C. cornuta (Sharma & Kotov, 2013). C. rigaudi have been considered for use as a water quality indicator species and as such have been used in some toxicology experiments (e.g. Raymundo et al., 2019; Mohammed, 2007), and may be important community indicators (Márquez et al., 2016). Because C. rigaudi is also widespread and may play a dominant role in some systems, understanding its traits and distribution could be important for nutrient cycling and food web models in specific locations. However, little is known about their life history traits. Here, we amalgamate all data on the life history and geographical spread of the species and add information from our own laboratory experiments and field observations.
Methods
All analyses were completed in R. Data and codes are available: https://github.com/jwerba14/Ceriodaphnia.
Laboratory conditions
Ceriodaphnia rigaudi has been maintained in our laboratory continuously for several years. All individuals used in our experiments came from our laboratory population and were randomly removed for experiments. The colony originated from Jamaican rock pools. Water temperature of the stock cultures varied from 17–22 \(^{\circ }\text {C}\).
Feeding rates
To determine how quickly Ceriodaphnia rigaudi cleared Haematococcus sp. from the system, we used five treatments of chlorophyll-a concentration (mean ± SD: 0.778 ± 0.0985\(\upmu\)g—Chla/L, 3.11 ± 0.321\(\upmu\)g—Chla/L, 9.43 ± 1.01 \(\upmu\)g—Chla/L, 16.7 ± 1.24 \(\upmu\)g—Chla/L, 20.9 ± 1.27 \(\upmu\)g—Chla/L) with five replicates each. Temperature was kept at (mean ± SD) 20.7 ± 1.1. These chlorophyll-a environments were chosen to span a range of lake trophic states from oligotrophic to eutrophic (Kratzer & Brezonik, 1981). Each replicate had an average of 75 individuals. We measured chlorophyll-a after six hours. We did not expect discernible growth in the Haematococcus sp. population over six hours, but we did include three replicates without any Ceriodaphnia for each chlorophyll treatment to account for any growth or death of algae unrelated to grazing to be used as a reference. We subtracted the average change of the controls, by treatment, from the experimental replicates. We ran a linear model across food treatment to determine average uptake rate.
Population growth
For population growth, we began colonies in 1.1 L of water with 30 individuals of Ceriodaphnia rigaudi each at four chlorophyll-a treatments (mean ± SD: 2.93 ± 0.905\(\upmu\)g—Chla/L, 9.39 ± 22.5\(\upmu\)g—Chla/L, 22.3 ± 6.32\(\upmu\)g—Chla/L, 65.5 ± 10.1 \(\upmu\)g—Chla/L) with six to nine replicates each. Temperature of tanks between (mean ± SD) 21.6 ± 0.5 \(^\circ\)C As in the feeding experiment concentrations was chosen to represent a range of trophic states. Everyday either algae or distilled water was added to bring the tanks back to the chlorophyll concentration level; chlorophyll-a was measured using an AquaFlor fluorometer. Ceriodaphnia rigaudi was counted weekly in eight 50-mL sub-samples. Replicates were ended when C. rigaudi where extirpated or 50 days from initiation had passed. We fit a logistic growth curve (1) to each replicate separately using nlxb (Nash & Murdoch, 2021) and then ran a linear regression of each parameter (r,k) against the food treatment.
where k is the population asymptote, r is the growth rate and \(N_{0}\) is initial population size
Literature search
A Google Scholar search on April 23, 2020, for “Ceriodaphnia rigaudi” resulted in 523 hits. We downloaded those papers that had survey data or had life history information for a total of 196 papers. Of those, we found 94 with either geographical information (92) or life history information (3 papers). After confirming geographical coordinates, we accepted 137 records as sufficiently reliable.
Distribution
We mapped C. rigaudi with the maps package (code by Richard A. Becker et al., 2021); the map includes 138 locations (one is our own data). Whenever possible, we mapped the exact sampling locations (considered exact if the coordinates were reported in the manuscript or we found the body of water mentioned). We did not consider any river an exact location (unless coordinates were given). All coordinates are the closest we could establish based on the location description found in the paper.
Jamaican data and Habitat
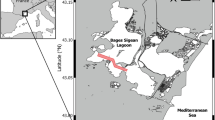
Rock pools are located on the west coast of Discovery Bay, Jamaica. Fifty out of over 200 pools were sampled annually from 1989–2006, (except 2004) (Hammond et al., 2020). We also obtained salinity and oxygen concentrations as well as pH and temperature from each pool. To determine which if any environmental factors were associated with the presence or absence, or level of abundance of C. rigaudi, we ran a hurdle model with a zero inflated negative binomial error distribution in glmmTMB package (Brooks et al., 2017).
Results
Feeding rate Individual C. rigaudi took up marginally more chlorophyll-a per hour as chlorophyll-a availability increased (Estimate: 7.42e−10, p = 0.009, Fig. 1).
Population growth rate We were unable to estimate a nonzero slope across feeding treatments for either r or k (\(p > 0.05\)). Food treatment did not explain much of the variance in either parameter (\(R^2 < 0.1\)). The range of r and k estimates for each treatment was very variable by replicate (Fig. 2).
Environmental correlates We find, in the Jamaican rock pools, that as pH, oxygen and salinity increase, the probability of C. rigaudi being present declines (\(p< 0.05\), Fig. 3). Decreases in pH and salinity increase the abundance of C.rigaudi (\(p < 0.05\), Fig. 3). We are unable to detect an effect (\(p > 0.05\)) of temperature on either presence or abundance of C. rigaudi.
Map of species distribution We were able to find 138 records of C. rigaudi in survey data. We see clusters in southern Brazil. Otherwise, records spread throughout Africa, south Central Asia and parts of central America (Fig. 4), while C. rigaudi is generally thought of as a tropical species; we found records outside the tropics or subtropics, in the United States and southern Europe.
Life history information from literature Three papers provided some measurement of life history information about C. rigaudi. These papers supplied information about either fecundity, clutch size or time to maturity. No papers provided information about feeding or excretion rates (Table 1).
Discussion
We find that feeding rate increases very little as food concentration increases. As far as we are aware, this is the first study of C. rigaudi feeding rate. Estimating population growth rate was challenging, and our fits gave wide possible ranges for k and r. Due to the high variation among replicates, we could not identify a pattern across food concentration for either parameter. Likely some of this variation is due to the fact that experiments contained a range of size and developmental stages of individuals. Interestingly, higher food concentrations did not give reliably higher populations at the end of eight weeks, suggesting that either our algal species (Haematococcus sp. mixed with Scenedesmus sp.) were not favoured food or that other factors were limiting their growth (Fig. 5). Previous studies have shown clutch size, survival and day to first reproduction to vary with different food type and concentrations (all data in our metafile; summary provided in Table 1; Burgis, 1973; Mohammed, 2007; Martinez-Jeronimo and Ventura-Lopez, 2011). Excretion and uptake rates of nutrients for C. rigaudi would be helpful additional traits to measure.
While we find C. rigaudi throughout the tropic and subtropic regions (Fig. 4), some of the records are quite old and may no longer be accurate. For example, we did not include a record from Garças Reservoir, Sao Paulo, Brazil (Di Genaro et al., 2015), because the most recent paper did not find any C. rigaudi, and it appears to have disappeared since 1997. However, it is unclear if this was seasonal and the species will return or if it is in fact extirpated. Many of our records are quite old (e.g. Jones, 1958; Egborge, 1987) and or/ single sightings (e.g. Hart and Boane, 2004) and thus make any extrapolation about continued presence or changes over time impossible.
Tropical freshwater species are understudied. Ceriodaphnia rigaudi is a widespread species that may be useful as a water quality indicator species, but more information about the species is critical. Here, we provide some of the needed information. It is important to continue to learn about tropical species for water quality and toxicity because their responses may be quite different than temperate species (Mohammed, 2007).
Trait-based approaches are powerful tools for understanding community assembly (Cornwell & Ackerly, 2009), species distributions (McGill et al., 2006), and the impact of particular species on the environment (Lavorel & Garnier, 2002). However, trait-based methods require a large database of information about a wide array of species. Furthermore, our experiments indicate that the estimates of crucial parameters prerequisite for the quantitative evaluation of C. rigaudi role in aquatic systems are difficult to obtain even under controlled conditions and considerable time investment. Superimposed on the possible regional variation, this difficulty may limit the range of predictions that trait-based models involving zooplankton species will be able to make. At the same time, our observations highlight a need for further research into the variation of r and k and conditions explaining such variation.
References
Brooks, M. E., Kristensen, K., van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal, 9(2), 378–400.
Burgis, M. J. (1973). Observations on the Cladocera of lake George, Uganda. Journal of Zoology, 170(3), 339–349.
Burns, C. W., & Schallenberg, M. (2001). Calanoid copepods versus cladocerans: Consumer effects on protozoa in lakes of different trophic status. Limnology and Oceanography, 46(6), 1558–1565.
Code by Richard A. Becker, O. S., A. R. W. R. (2021). Version by Ray Brownrigg. Enhancements by Thomas P. Minka, and A. Deckmyn. maps: Draw Geographical Maps. R package version 3.4.0.
Cornwell, W. K., & Ackerly, D. D. (2009). Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecological Monographs, 79(1), 109–126.
Di Genaro, A. C., Sendacz, S., Moraes, M. D. A. B., Mercante, C. T. J., et al. (2015). Dynamics of Cladocera community in a tropical hypereutrophic environment (Garças reservoir, Sao Paulo, Brazil). Journal of Water Resource and Protection, 7(05), 379.
Egborge, A. B. (1987). Salinity and the distribution of Cladocera in Warri River, Nigeria. Hydrobiologia, 145(1), 159–167.
Gu, L., Qin, S., Zhu, S., Lu, N., Sun, Y., Zhang, L., et al. (2020). Microcystis aeruginosa affects the inducible anti-predator responses of Ceriodaphnia cornuta. Environmental Pollution, 259, 113952.
Hammond, M., Loreau, M., De Mazancourt, C., & Kolasa, J. (2020). Disentangling local, metapopulation, and cross-community sources of stabilization and asynchrony in metacommunities. Ecosphere, 11(4), e03078.
Hart, R. C., & Boane, C. (2004). Limnology of southern African coastal lakes-new vistas from Mozambique. African Journal of Aquatic Science, 29(2), 145–159.
Hébert, M.-P., Beisner, B. E., Maranger, R., & de Recherche, G. (2016). Interuniversitaire en Limnologie et en environnement aquatique (GRIL), 2016. A meta-analysis of zooplankton functional traits influencing ecosystem function. Ecology.
Hébert, M.-P., Beisner, B. E., & Maranger, R. (2017). Linking zooplankton communities to ecosystem functioning: Toward an effect-trait framework. Journal of Plankton Research, 39(1), 3–12.
Jones, W. H. (1958). Cladocera of Oklahoma. Transactions of the American Microscopical Society, 77(3), 243–257.
Kratzer, C. R., & Brezonik, P. L. (1981). A Carlson-type trophic state index for nitrogen in Florida lakes 1. JAWRA Journal of the American Water Resources Association, 17(4), 713–715.
Lavorel, S., & Garnier, E. (2002). Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the holy grail. Functional Ecology, 16(5), 545–556.
Litchman, E., de Tezanos Pinto, P., Klausmeier, C. A., Thomas, M. K., & Yoshiyama, K. (2010). Linking traits to species diversity and community structure in phytoplankton. Fifty years after the ‘‘Homage to Santa Rosalia’’: Old and new paradigms on biodiversity in aquatic ecosystems, 15–28.
Litchman, E., Ohman, M. D., & Kiørboe, T. (2013). Trait-based approaches to zooplankton communities. Journal of Plankton Research, 35(3), 473–484.
Márquez, J., Kolasa, J., & Sciullo, L. (2016). Local versus regional processes and the control of community structure. Community Ecology, 17(1), 1–7.
Martinez-Jeronimo, F., & Ventura-Lopez, C. (2011). Population dynamics of the tropical cladoceran Ceriodaphnia rigaudi Richard, 1894 (crustacea: Anomopoda) effect of food type and temperature. Journal of Environmental Biology, 32(4), 513.
McGill, B. J., Enquist, B. J., Weiher, E., & Westoby, M. (2006). Rebuilding community ecology from functional traits. Trends in Ecology and Evolution, 21(4), 178–185.
Migal, M. G. (2011). The cladoceran trophic status in the nitrogen limited ecosystem of Lake Kinneret (Israel). Journal of Environmental Biology, 32(4), 455.
Mohammed, A. (2007). Comparative sensitivities of the tropical cladoceran, Ceriodaphnia rigaudii and the temperate species Daphnia magna to seven toxicants. Toxicological and Environmental Chemistry, 89(2), 347–352.
Mohammed, A. (2009). An approach for assessing the suitability of Ceriodaphnia rigaudii as an indigenous tropical toxicity test species. Toxicological and Environmental Chemistry, 91(1), 79–86.
Nash, J. C., & Murdoch, D. (2021). nlsr: Functions for nonlinear least squares solutions. R package version 2021.8.19.
Pinheiro-Silva, L., Gianuca, A. T., Silveira, M. H., & Petrucio, M. M. (2020). Grazing efficiency asymmetry drives zooplankton top-down control on phytoplankton in a subtropical lake dominated by non-toxic cyanobacteria. Hydrobiologia, 847(10), 2307–2320.
Raymundo, L. B., Rocha, O., Moreira, R. A., Miguel, M., & Daam, M. A. (2019). Sensitivity of tropical cladocerans to chlorpyrifos and other insecticides as compared to their temperate counterparts. Chemosphere, 220, 937–942.
Rietzler, A., Rocha, O., Roche, K., & Ribeiro, M. (2008). Laboratory demonstration of morphological alterations in Ceriodaphnia cornuta Sars (1885) fa Rigaudi induced by Chaoborus brasiliensis theobald (1901). Brazilian Journal of Biology, 68(2), 453–454.
Rizo, E. Z. C., Gu, Y., Papa, R. D. S., Dumont, H. J., & Han, B.-P. (2017). Identifying functional groups and ecological roles of tropical and subtropical freshwater Cladocera in Asia. Hydrobiologia, 799(1), 83–99.
Robson, B. J. (2014). When do aquatic systems models provide useful predictions, what is changing, and what is next? Environmental Modelling and Software, 61, 287–296.
Robson, B. J., Arhonditsis, G. B., Baird, M. E., Brebion, J., Edwards, K. F., Geoffroy, L., et al. (2018). Towards evidence-based parameter values and priors for aquatic ecosystem modelling. Environmental Modelling and Software, 100, 74–81.
Sharma, P., & Kotov, A. A. (2013). Molecular approach to identify sibling species of the Ceriodaphnia cornuta complex (cladocera: Daphniidae) from Australia with notes on the continental endemism of this group. Zootaxa, 3702(1), 79–89.
Sommer, U., Sommer, F., Santer, B., Jamieson, C., Boersma, M., Becker, C., & Hansen, T. (2001). Complementary impact of copepods and cladocerans on phytoplankton. Ecology Letters, 4(6), 545–550.
van der Meer, J. (2006). An introduction to dynamic energy budget (DEB) models with special emphasis on parameter estimation. Journal of Sea Research, 56(2), 85–102.
Violle, C., Navas, M.-L., Vile, D., Kazakou, E., Fortunel, C., Hummel, I., & Garnier, E. (2007). Let the concept of trait be functional! Oikos, 116(5), 882–892.
Vogt, R. J., Peres-Neto, P. R., & Beisner, B. E. (2013). Using functional traits to investigate the determinants of crustacean zooplankton community structure. Oikos, 122(12), 1700–1709.
Map Location Citations
Abdulwahab, S. and A. M. Rabee, 2015. Ecological factors affecting the distribution of the zooplankton community in the tigris river at baghdad region, iraq. The Egyptian Journal of Aquatic Research 41(2), 187–196.
Abu-Gideiri, Y. and M. Ali, 1975. A preliminary biological survey of lake nubia. Hydrobiologia 46(4), 535–541.
Ajeel, S. andM. Abbas, 2012. Diversity of cladocera of the shatt al-arab river, southern iraq. Mesopotamian Journal of Marine Sciences 27(2), 126–139.
Ajeel, S., A. Douabul, and M. Abbas, 2015. Seasonal variations of zooplankton in al-hammar marsh southern iraq. J Ecosys Ecograph 5(173), 2.
Annandale, N., 1907. Port canning, lower bengal. Records of the Zoological Survey of India: A Journal of Indian Zoology, 35.
Arcifa, M. S., 1984. Zooplankton composition of ten reservoirs in southern brazil. Hydrobiologia 113(1), 137–145.
Azoulay, B. and M. Gophen, 1992. Feeding habits of larval mirogrex terraesanctae (steinitz, 1952) in lake kinneret (israel) i. field study. Hydrobiologia 246(3), 243–249.
Bessa, G. F., L. C. G. Vieira, L. M. Bini, D. F. dos Reis, and P. B. de Morais, 2011. Concordance patterns in zooplankton assemblages in the uhe-lu´ıs eduardo magalha˜es reservoir in the mid-tocantins river, to248 cantins state, brazil. Acta Scientiarum. Biological Sciences 33(2), 179–184.
Biswas, S., 1965. Fauna of rajasthan, india. part ii. crustacea: Cladocera. Records of the zoological Survey of India 63(1-4), 95–141.
Biswas, S., 1980. Cladocerans (crustacea: Branchiopoda) from assam and adjacent hill states in north east india. Records of the zoological Survey of India 76(1–4), 93–113.
Bricker, K. S., L.Wongrat, and J. E. Gannon, 1978. Composition and distribution of crustacean plankton in twelve inland water bodies of thailand. Journal of Fisheries and Environment 10, 1–14.
Bromley, H. J., 1993. A checklist of the cladocera of israel and eastern sinai. Hydrobiologia 257(1), 21–28.
Brook, A. and J. Rzo´ ska, 1954. The influence of the gebel aulyia dam on the development of nile plankton. The Journal of Animal Ecology, 101–114.
Burgis, M. J., 1973. Observations on the cladocera of lake george, uganda. Journal of Zoology 170(3), 339–349.
Caleffi, S., 1998. Guarapiranga reservoir: study of the zooplankton community and aspects of its eutrophication. Internationale Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 26(4), 1898–1903.
Campanelli Mortari, R. and R. Henry, 2016. Horizontal distribution of cladocera in a subtropical lake marginal to a river. Journal of limnology, 109–120.
Chandrasekhar, S. and T. Chatterjee, 2004. Freshwater cladocera of dhanbad, jharkhand. Records of the Zoological Survey of India 103(3-4), 79–85.
Chavhan, R., 2014. Limnological studies on talodhi village lake of tahsil chamorshi, district gadchiroli (ms), india, with special respect to plankton species diversity..
Combrink, S., 1994. The zooplankton of zeekoevlei and princess vlei (western cape)-a preliminary assessment. Water SA 20(4), 299–306.
Curtis, B. Freshwater macro-invertebrates of namibia. Madoqua 17(2).
Davidson, N. L., W. E. Kelso, and D. A. Rutherford, 2000. Characteristics of cladoceran and copepod communities in floodplain habitats of the atchafalaya river basin. Hydrobiologia 435(1), 99–107.
Dhua, T. and A. Patra, 2006. Lunar rhythm in the planktonic biomass of the nicco park lake, bhubaneswar. Journal of Environmental Biology 27(4), 739–744.
Dodson, S. I. and D. Silva-Briano, 1996. Crustacean zooplankton species richness and associations in reservoirs and ponds of aguascalientes state, mexico. Hydrobiologia 325(2), 163–172.
Dolley, J. S., 1933. Preliminary notes on the biology of the st. joseph river. American Midland Naturalist, 193–227.
Dube, T., L. DeNecker, J. H. Van Vuren, V. Wepener, N. J. Smit, and L. Brendonck, 2017. Spatial and temporal variation of invertebrate community structure in flood-controlled tropical floodplain wetlands. Journal of Freshwater Ecology 32(1), 1–15.
Egborge, A., 1987. Salinity and the distribution of cladocera in warri river, nigeria. Hydrobiologia 145(1), 159–167.
Eldredge, L. G. and S. E. Miller, 1997. Numbers of hawaiian species: supplement 2, including a review of freshwater invertebrates. Bishop Museum Occasional Papers.
El´ıas-Gutierrez, M., A. A. Kotov, and T. Garfias-Espejo, 2006. Cladocera (crustacea: Ctenopoda, anomopoda) from southern mexico, belize and northern guatemala, with some biogeographical notes. Zootaxa 1119(1), 1–27.
Esteves, K. E., 1996. Feeding ecology of three astyanax species (characidae, tetragonopterinae) from a floodplain lake of mogi-guac¸u´ river, parana´ river basin, brazil. Environmental Biology of Fishes 46(1), 83–101.
Ferraz, H. D. A., G. G. Landa, and H. Paprocki, 2009. Zooplankton of an urban stretch, itapecerica river, divinópolis, minas gerais, brazil. Check List 5(4), 890–894.
Fileto, C., R. Henry, M. S. Arcifa, and R. A. R. Ferreira, 2010. Influence of the mineral content of the seston on tropical cladocerans of a marginal lake. Acta Limnologica Brasiliensia 22(1), 13–22.
Gophen, M., 1979. Bathymetrical distribution and diurnal migrations of zooplankton in lake kinneret (israel) with particular emphasis on mesocyclops leuckarti (claus). Hydrobiologia 64(3), 199–208.
Green, J., 1962. Zooplankton of the river sokoto. the crustacea. In Proceedings of the Zoological Society of London, Volume 138, pp. 415–453.Wiley Online Library.
Gu, L., S. Qin, S. Zhu, N. Lu, Y. Sun, L. Zhang, Y. Huang, K. Lyu, Y. Chen, and Z. Yang, 2020. Micro cystis aeruginosa affects the inducible anti-predator responses of ceriodaphnia cornuta. Environmental Pollution 259, 113952.
Hanazato, T. and M. Yasuno, 1988. Impact of predation of neomysis intermedia on a zooplankton community in lake kasumigaura: With 5 figures in the text. Internationale Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 23(4), 2092–2098.
Harding, D. and N. Rayner, 2001. The zooplankton community of lake kariba in 1962/63 following impoundment of the zambezi river. Southern African Journal of Aquatic Sciences 26(1), 9–15.
Harding, W. R. and S. Wright, 1999. Initial findings regarding changes in phyto-and zooplankton com position and abundance following the temporary drawdown and refilling of a shallow, hypertrophic south african coastal lake. Lake and Reservoir Management 15(1), 47–53.
Hart, R. C., 1997. A limnological profile of the upper okavango delta at low water level. Southern African Journal of Aquatic Science 23(2), 21–33.
Hart, R. C. and C. Boane, 2004. Limnology of southern african coastal lakes—new vistas from mozam bique. African Journal of Aquatic Science 29(2), 145–159.
Havel, J. E., E. M. Eisenbacher, and A. A. Black, 2000. Diversity of crustacean zooplankton in riparian wetlands: colonization and egg banks. Aquatic Ecology 34(1), 63–76.
Havens, K. E., 1999. Comparative analysis of lake plankton structure vs. function. Aquatic sciences 61(2), 150–167.
Havens, K. E., 2002. Zooplankton structure and potential food web interactions in the plankton of a subtropical chain-of-lakes. The Scientific World Journal 2, 926–942.
Henry, R., E. A. Panarelli, S. M. C. Casanova, D. C. Granado, R. C. Mortari, and J. Abra, 2011. Plankton richness and abundance in several different hydrological situations in lakes lateral to a river: a case study in the mouth zone of a tributary into a tropical reservoir. Oecologia Australis 15(3), 537–558.
Hoang, H. T. T., T. T. Duong, K. T. Nguyen, Q. T. P. Le, M. T. N. Luu, D. A. Trinh, A. H. Le, C. T. Ho, 326 K. D. Dang, J. Ne´mery, et al., 2018. Impact of anthropogenic activities on water quality and plankton communities in the day river (red river delta, vietnam). Environmental monitoring and assessment 190(2), 1–18.
Imoobe, T. and O. Akoma, 2008. Assessment of zooplankton community structure of the bahir dar gulf of lake tana, ethiopia. Ethiopian Journal of Environmental Studies and Management 1(2), 26–34.
Jones, W. H., 1958. Cladocera of oklahoma. Transactions of the American Microscopical Society 77(3), 243–257.
Leitao, A., R. Freire, O. Rocha, and S. Santaella, 2006. Zooplankton community composition and abundance of two brazilian semiarid reservoirs. Acta Limnologica Brasiliensia 18(4), 451–468.
Lucena, L. C. A., T. X. d. Melo, and E. S. F. Medeiros, 2015. Zooplankton community of parna´ıba river, northeastern brazil. Acta Limnologica Brasiliensia 27, 118–129.
Martinez-Jeronimo, F. and C. Ventura-Lopez, 2011. Population dynamics of the tropical cladoceran ceriodaphnia rigaudi richard, 1894 (crustacea: Anomopoda). effect of food type and temperature. Journal of Environmental Biology 32(4), 513.
Matsumura-Tundisi, T., J. Tundisi, F. Souza-Soares, and J. Tundisi, 2015. Zooplankton community structure of the lower xingu river (pa) related to the hydrological cycle. Brazilian Journal of Biology 75, 47–54.
Matsumura-Tundisi, T., J. Tundisi,M. Tundisi, and A. Cimbleris, 2006. Carbon content and composition of zooplankton fractions in two reservoirs of central brazil: Serra da mesa and manso. Internationale Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 29(5), 2237–2244.
Mavuti, K. M., 1990. Ecology and role of zooplankton in the fishery of lake naivasha. Hydrobiologia 208(1), 131–140.
McKenzie, K., 1971. Entomostraca of aldabra, with special reference to the genus heterocypris (crustacea, ostracoda). Philosophical Transactions of the Royal Society of London. B, Biological Sciences 260(836), 257–297.
Mohammed, A., 2009. An approach for assessing the suitability of ceriodaphnia rigaudii as an indigenous tropical toxicity test species. Toxicological & Environmental Chemistry 91(1), 79–86.
Montes-Ortiz, L. and M. Elias-Gutierrez, 2018. Faunistic survey of the zooplankton community in an oligotrophic sinkhole, cenote azul (quintana roo, mexico), using different sampling methods, and documented with dna barcodes. J. Limnol 77(3), 428–440.
Nadai, R. and R. Henry, 2009. Temporary fragmentation of a marginal lake and its effects on zooplankton community structure and organization. Brazilian Journal of Biology 69, 819–835.
Nhiwatiwa, T., L. Brendonck, and T. Dalu, 2017. Understanding factors structuring zooplankton and macroinvertebrate assemblages in ephemeral pans. Limnologica 64, 11–19.
Nogueira,M. G., P. C. Reis Oliveira, and Y. Tenorio de Britto, 2008. Zooplankton assemblages (copepoda and cladocera) in a cascade of reservoirs of a large tropical river (se brazil). Limnetica 27(1), 151–170..
Panarelli, E., A. Güntzel, and C. Borges, 2013. How does the taquari river influence in the cladoceran assemblages in three oxbow lakes? Brazilian Journal of Biology 73, 717–725.
Paranaguá, M., S. Neumann-Leitäo, J. Nogueira-Paranhos, T. Silva, and T. Matsumura-Tundisi, 2005. Cladocerans (branchiopoda) of a tropical estuary in brazil. Brazilian Journal of Biology 65, 107–115.
Perbiche-Neves, G. and M. G. Nogueira, 2010. Multi-dimensional effects on cladoceran (crustacea, anomopoda) assemblages in two cascade reservoirs in southeast brazil. Lakes & Reservoirs: Research & Management 15(2), 139–152.
Pérez, L., J. Lorenschat, J.Massaferro, C. Pailles, F. Sylvestre,W.Hollwedel, G.-O. Brandorff,M. Brenner, I. Gerald,M. d. S. Lozano, et al., 2013. Bioindicators of climate and trophic state in lowland and highland aquatic ecosystems of the northern neotropics. Revista de Biolog´ıa Tropical 61(2), 603–644.
Portinho, J. L., G. Perbiche-Neves, and M. G. Nogueira, 2016. Zooplankton community and tributary effects in free-flowing section downstream a large tropical reservoir. International Review of Hydrobiology 101(1-2), 48–56.
Prophet, C. W., T. F. Andrews, and C. E. Goulden, 1959. Annotated check list of the cladocera and copepoda of lyon county, kansas. The Southwestern Naturalist, 185–194.
Prophet, C.W. and S.Waite, 1974. A species list of cladocera and copepoda in kansas. Transactions of the Kansas Academy of Science (1903), 42–47.
Rabee, A. M., 2010. The effect of al-tharthar-euphrates canal on the quantitative and qualitative composition of zooplankton in euphrates river. Al-Nahrain Journal of Science 13(3), 120–128.
Ranae, P., 2011. Cladocera (arthropoda: Crustacea). Zool. Surv. India, Fauna of Madhya Pradesh (including Chhattisgarh), State Fauna Series 15(Part 3), 45–83.
Riato, L., C. Van Ginkel, and J. C. Taylor, 2014. Zooplankton and diatoms of temporary and permanent freshwater pans in the mpumalanga highveld region, south africa. African Zoology 49(1), 113–127.
Riddhi, S., S. Vipul, S. M. Sudan, V. B. Kumar, M. Rachana, and G. K. Singh, 2011. Studies on limnological characteristic, planktonic diversity and fishes (species) in lake pichhola, udaipur, rajasthan (india). Universal Journal of Environmental Research & Technology 1(3).
Robinson, A. and P. K. Robinson, 1971. Seasonal distribution of zooplankton in the northern basin of lake chad. Journal of Zoology 163(1), 25–61.
Rodriguez-Almaraz, G. A. and A. Leija-Tristan, 1995. Cladocerans (branchiopoda: Anomopoda; ctenopoda) of the nuevo leon state, mexico. The Southwestern Naturalist 40(3), 322–324.
Roy, S., R. Roy, A. Prabhakar, A. Pandey, R. Kumar, and L.-C. Tseng, 2013. Spatio-temporal distribution and community structure of zooplankton in the gangetic dolphin sanctuary, 2009. Aquatic Ecosystem Health & Management 16(4), 374–384.
Rumes, B., H. Eggermont, and D. Verschuren, 2011. Distribution and faunal richness of cladocera in western uganda crater lakes. Hydrobiologia 676(1), 39–56.
Saha, S., T. Saha, and P. Basu, 2017. Seasonal changes in zooplankton and macro-fauna populations of the east calcutta wetland fish ponds. In Proceedings of the Zoological Society, Volume 70, pp. 156–164. Springer.
SALAH,M. and G. TAMAS, 1970. General preliminary contribution to the plankton of egypt. Bull. Inst. Oceanography Fish 1, 305–337.
Sampaio, E., O. Rocha, T. Matsumura-Tundisi, and J. Tundisi, 2002. Composition and abundance of zooplankton in the limnetic zone of seven reservoirs of the paranapanema river, brazil. Brazilian Journal of Biology 62, 525–545.
Sartori, L. P., M. Nogueira, R. Henry, and E. M. Moretto, 2009. Zooplankton fluctuations in jurumirim reservoir (sa˜o paulo, brazil): a three-year study. Brazilian Journal of Biology 69, 1–18.
Scharler, U., K. Lechman, T. Radebe, and H. Jerling, 2020. Effects of prolonged mouth closure in a temporarily open/closed estuary: a summary of the responses of invertebrate communities in the umdloti estuary, south africa. African Journal of Aquatic Science 45(1-2), 121–130.
Sendacz, S., 1997. Zooplankton studies of floodplain lakes of the upper parana´ river, sa˜o paulo state, brazil. Internationale Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 26(2), 621–627.
Serafim-Ju´nior,M., F. Lansac-Toˆha, R. Lopes, and G. Perbiche-Neves, 2016. Continuity effects on rotifers and microcrustaceans caused by the construction of a downstream reservoir in a cascade series (iguaçu river, brazil). Brazilian Journal of Biology 76, 279–291.
Sharma, V., B. K. Verma, R. Sharma, M. S. Sharma, and K. S. Gaur, 2012. A report on the freshwater cladocera (crustacea: Branchiopoda) of south rajasthan (india). International Journal of Environmental Sciences 3(1), 275–296.
Silva, E. d. S., O. Rocha, and M. J. d. Santos-Wisniewski, 2018. Diel vertical migration of cladocera in a compartment of a tropical reservoir. Acta Limnologica Brasiliensia 30.
Smirnov, N., 1988. Cladocera (crustacea) from nicaragua. Hydrobiologia 160(1), 63–77.
Soesbergen, M. and J. Sinkeldam, 2019. An annotated checklist of the branchiopoda (crustacea) of the dutch caribbean islands. Zootaxa 4701(1), 25–34.
Tagliarolo,M., F. Porri, C. Garvie, K. Lechman, and U. Scharler, 2019. Zooplankton metabolismin south 424 african estuaries: does habitat type influence ecological strategies? Journal of Plankton Research 41(4), 535–548.
Talling, J. and J. Rzoska, 1967. The development of plankton in relation to hydrological regime in the blue nile. The Journal of Ecology, 637–662.
Tanaka, S. and A. Ohtaka, 2010. Freshwater cladocera (crustacea, branchiopoda) in lake tonle sap and its adjacent waters in cambodia. Limnology 11(2), 171–178.
Tho, N., R. Merckx, and V. N. Ut, 2014. Impacts of saline water irrigation and shrimp pond discharges on the surrounding waters of a coastal district in the mekong delta of vietnam. Environmental earth sciences 71(5), 2015–2027.
Vandekerkhove, J., S. Declerck, L. Brendonck, J. M. Conde-Porcuna, E. Jeppesen, L. S. Johansson, and L. DeMeester, 2005. Uncovering hidden species: hatching diapausing eggs for the analysis of cladoceran species richness. Limnology and Oceanography: Methods 3(9), 399–407.
Yamamoto, H. and H. Seki, 1979. Impact of nutrient enrichment in a waterchestnut ecosystem at takahama-iri bay of lake kasumigaura, japan. Water, Air, and Soil Pollution 12(4), 519–527.
Zago, M. S. A., 1976. The planktonic cladocera (crustacea) and aspects of the eutrophication of americana reservoir, brazil. Boletim de Zoologia 1(1), 105–145.
Acknowledgements
We would like to thank Adit Chokshi and Josephine Huynh for help with data collection. Research was funded by a Natural Sciences and Engineering Research Council of Canada Discovery Grant (Grant No. 10531314).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Werba, J.A., Kolasa, J. The challenge of life history traits: a small cladoceran, Ceriodaphnia rigaudi. COMMUNITY ECOLOGY 23, 377–387 (2022). https://doi.org/10.1007/s42974-022-00115-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42974-022-00115-5