Abstract
Tropical understory birds have declined due largely to habitat loss and fragmentation. Here, we revisited a study conducted three decades ago and used artificial nests to examine depredation rates in a Costa Rican biological corridor. Using camera trap data, we compared potential nest predator detection rates at experimental tinamou ground nests in La Selva Biological Station and at sites in five local forest fragments. Nest predator detections were positively associated with landscape-scale core forest and distance away from forest edge, as well as with local-scale human trails, and negatively associated with primary forest compared to secondary growth. Twenty-two of 52 artificial nests were depredated, which was similar to previous research in the area. Mammalian and avian predators were common nest predators, but unknown predators (presumably snakes) were responsible for half of nests lost. Nests within La Selva core forest had lower probability of nest loss compared to fragments despite exhibiting higher predator detection rates. Yet other fragmentation covariates such as distance from forest edge, nest occurrence on human trails, or forest age were not associated with nest loss. We suggest that concentrated foraging is the underlying mechanism behind the community interactions that we observed. Community members exist in concentrated use areas within forest fragments, which results in heightened predator foraging rates and thus stronger interactions in fragments despite more predators encountering the nests in core forest. Fragmentation is a global phenomenon and we suspect that concentrated community use of limited resources is driving species to interact more strongly than in natural ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat fragmentation is a global phenomenon affecting most of the world’s biodiversity (Haddad et al. 2015), yet there remains a debate about how edge effects, habitat amount, and patch isolation affect communities and their functionality (Fahrig 2013). Extinction debt, the gradual extirpation of species after habitat loss, further complicates our understanding of community dynamics in continually shifting fragmented systems (Tilman et al. 1994). Prior to species losses associated with extinction debt, animals (particularly forest specialists) become concentrated in their remaining habitat (Cove et al. 2013). Under the “ideal gas” model, animals should experience higher encounter rates in concentrated use areas which could exacerbate predator–prey relationships and lead to strong species interactions (Hutchinson and Waser 2007; Layman et al. 2015).
As with other biodiversity hotspots, Mesoamerica has experienced rapid habitat loss and land use change, relegating the diverse forest taxa to a patchwork of fragmented protected areas (Meyers et al. 2000; Gibson et al. 2011; Boyle and Sigel 2015). In addition, reduction in apex predators coupled with fragmentation and resource provision has promoted top–down and bottom–up release with the variable patterns of species dominance in Neotropical forests (Pardo Vargas et al. 2016). Omnivorous mesopredators such as coatis (Nasua narica) and tayras (Eira barbara), and medium-sized ungulates like collared peccaries (Pecari tejacu) are increasingly abundant and could pose a threat to various species, particularly as nest predators (Romero et al. 2013; Cove et al. 2014).
At the highly protected La Selva Biological Station (hereafter: LSBS), Costa Rica, a 23-year analysis of ‘Christmas Bird Count’ data revealed that understory birds continued to decline while other forest generalists increased (Boyle and Sigel 2015). Because of the large encompassing diversity of tropical avifauna, understory avian communities serve as an excellent proxy for ecosystem health and function (Sekercioglu et al. 2002; Visco et al. 2015). Nest loss and depredation are often considered as one of the underlying mechanisms for bird population declines due to fragmentation across various regions (Small and Hunter 1988; Chalfoun et al. 2002). Ground-nesting birds, in particular, are predisposed to nest loss due to their accessibility to diverse understory predators (Gibbs 1991; Young et al. 2008; Newmark and Stanley 2011). For example, tinamous (Tinamidae) are a basal clade of ground-nesting birds endemic to the Neotropics that are sensitive to habitat loss and fragmentation (Thornton et al. 2012; Prum et al. 2015). Consequently, seven of the 48 (14.5%) listed tinamou species are classified as threatened, yet 40 (83%) of the classified tinamou species currently exhibit decreasing population trends (IUCN 2016). Indeed the ‘Christmas Bird Count’ data suggested that two of the tinamou species (little tinamou [Crypturellus soui] and slaty-breasted tinamou [Crypturellus boucardi]) present in La Selva have exhibited declines, whereas the great tinamou (Tinamus major) exhibited a population increase in the 23-year period (Boyle and Sigel 2015). However, at the global scale, the great tinamou is classified as ‘near threatened’ by the IUCN (BirdLife International 2017). The ground-nesting habits and population trends of tinamous make them an ideal study taxon to evaluate the synergistic effects of fragmented landscapes and predator community dynamics on nest predation rates.
Gibbs (1991) observed that artificial nests varied in their predation rates along habitat gradients suggesting strong edge effects in LSBS and surrounding fragments. However, the relative importance of many of the mechanisms of fragmentation, particularly differences in predator–prey encounter rates, remains enigmatic. We revisited the status of the LSBS understory communities that were examined three decades ago (Gibbs 1991). We used camera traps to explicitly examine the role of nest predators in the decline of ground-nesting birds by quantifying and comparing avian and mammalian predator loads detected at artificial tinamou nests along a gradient of core forest and fragmented habitat edges. We hypothesized that omnivores and mesopredators exert strong effects on ground-nesting birds due to concentrated foraging along fragmentation gradients—which could lead to effects at the ecosystem level.
Methods
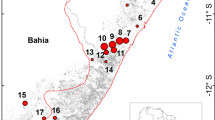
LSBS makes up the southern extent of the San Juan–La Selva Biological Corridor, connecting the lowlands of southern Nicaragua and northeastern Costa Rica to the montane forests of the central volcanic ridge of Costa Rica. This 1600 hectare protected area is bordered by the Sarapíqui and Puerto Viejo rivers and occurs in a region of various plantations, cattle ranches, and growing urban presence. We also visited five nearby forest fragments to evaluate nest loss across the regional landscape.
We followed a similar methodology to Gibbs (1991) to examine nest loss rates of artificial tinamou nests. Each nest consisted of three chicken eggs that were dyed light blue with scentless food coloring to imitate the characteristic color of great tinamou eggs (Gibbs 1991). We stratified sampling points by habitat amount and forest edge effects. We evenly partitioned sites between core forest (LSBS, n = 26 nests) and forest fragments (n = 26 nests) to examine landscape-level effects of fragmentation. These sites corresponded with concurrent amphibian surveys in the region (e.g., Vera Alvarez et al. 2019). Here, we define core forest as large, intact forest that is contiguous and adjacent with additional protected areas that are least impacted by development. Furthermore, we quantified the linear distance of the nest from the forest edge to evaluate landscape-level edge effects. Artificial nests were also randomly assigned to either on or off (> 20 m) human trails to examine local-level edge effects because many predators use trails to travel while foraging (Harmsen et al. 2010). We partitioned sites by primary and secondary forest to account for additional possible habitat differences in nest loss due to differences in cover. Nests were randomly placed in sites and the exact position of each nest was contingent upon the availability of a suitable substrate. Specifically, nest locations occurred at the base of a tree or stump with abundant leaf litter, lianas, and buttress roots for nest concealment. We established camera traps (Reconyx PC800 or PC850, RECONYX, Inc., Holmen WI, USA) 2–3 m away facing the nests to monitor predator–nest encounter rates and detect depredation events. We set cameras not only to take ten rapid-fire photos whenever motion was detected, but also to capture shots every hour in order to detect potential depredation events by snakes and other ectothermic predators. We deployed cameras for a period of six trapnights at each artificial nest. We visited and checked nests partway through the week and recorded any depredated nests, but cameras remained for the full duration of a week.
We identified all potential nest predator species from camera trap photos to determine predator (avian and mammalian) assemblages and nest encounter rates across experimental nest locations. We defined predator–nest encounters as a photographic capture event of any predator at a nest regardless of nest fate. We summed the number of avian predators and mammalian predator encounters separately, and then combined them as a total predator load index. We a priori hypothesized that predator–nest encounter rates would vary among sites due to concentrated foraging in different landscapes. Therefore, we estimated the covariate relationships of predator–nest encounter rates depending on (a) sites occurring in core forest (1 = core forest, 0 = fragment); (b) on (1) or off (0) human trail; (c) distance from habitat edge (continuous); and (d) forest age (1 = primary, 0 = secondary/other) with a global Poisson regression model in R (R Development Core Team 2016).
We considered nests lost if any of the three eggs at a site were depredated or went missing. We combined these data with the habitat and predator load covariates in a logistic regression framework to assess the effects of different landscape features and predator encounter rates on nest survival of ground-nesting birds. We compared 13 biologically reasonable hypotheses (Table 1) to predict nest loss using AICc model selection, Akaike weights (ω), and model averaging in the R package ‘AICcmodavg’ (Burnham and Anderson 2002; Mazerolle 2011). We considered covariates to have significant effects on nest loss and predator–nest encounter rates if their estimated β coefficient 95% confidence intervals excluded 0. We estimated the correlation coefficient (r) between the predictive β coefficients from the Poisson regression for predator–nest encounter rates and the predictive model-averaged β coefficients of nest loss to further examine possible mechanisms behind tinamou nest loss.
Results
Twenty-two of the 52 (42.31%) artificial tinamou nests in the San Juan–La Selva Biological Corridor were lost during our sampling. Eight nest loss events occurred in LSBS core forest, with the remaining 14 nest loss events occurring in forest fragments. Mammals (n = 6 species) and birds (n = 4 species) were responsible for 31.82% and 18.18% of nest loss, respectively, while the remaining 50% of nest loss was caused by unknown predators. Unknown predators were responsible for nest loss in four (50%) of the LSBS core forest nest loss events compared to eight (57%) of the forest fragment nest loss events.
The top-supported model (ω = 0.52) suggested that variation in nest loss was best explained by the binary landscape-scale covariate of fragmentation (e.g., LSBS core forest versus fragments) and total predator encounter rates (Table 1). However, three models received more model support than the null model, so we estimated model-averaged covariate effects. Nests located in core forest had a lower probability of nest loss (β1 = − 1.52 ± 0.73SE) compared to those in forest fragments, which corresponded with our a priori predictions (Table 2). Total predator load was positively related to nest loss with a model-averaged effect of β2 = 0.09 ± 0.06SE, but the 95% confidence interval overlapped 0. Potential avian and mammalian predator counts were both positively associated with nest loss, but confidence intervals strongly overlapped 0 (Table 2). Artificial nest occurrence on trails was negatively related to nest loss, which was the opposite relationship to our a priori predictions, but the effect was not significant. Distance from edge was also in opposition to our a priori predictions, suggesting positive but insignificant associations with nest loss.
We detected 17 potential mammalian and avian nest predators across all sites, with different predator assemblages of 13 species each from LSBS core forest sites (n = 172 total detections) and forest fragment sites (n = 75 total detections—Table 3). The global Poisson model to predict the counts of potential predators encountering nests suggested that all four covariates were significant (Table 2). Total predator counts detected at artificial nests increased (β1 = 1.44 ± 0.17SE) in LSBS core forest sites. Predator detections were also positively associated with artificial nest sites occurring on trails (β2 = 0.44 ± 0.13SE), representing a local edge effect. However, the counts of predators detected were positively associated with increasing distance away from forest edge (β3 = 0.20 ± 0.08SE), which represents a landscape-scale edge effect. Finally, the total predator load was negatively related to mature forest (β4 = − 0.71 ± 0.13SE) compared to secondary and reforestation plots. The estimated covariate effects of (1) LSBS core forest, (2) site occurrence on trails, (3) distance from forest edge, and (4) forest age on nest loss and predator–nest encounters were negatively correlated (r = − 0.81; Fig. 1).
Discussion
Tinamou nest loss was fairly common in our artificial nest experiments. Nest loss was related to landscape-level fragmentation because potential nest predators encountered the nests more frequently in LSBS core forest, but rarely detected and consumed the eggs. The negative correlation between habitat relationships of predators and nest loss probabilities further supports that predator abundance is not the underlying mechanism behind heightened nest loss in forest fragments. We hypothesize that predators reduce foraging time while traveling due to abundant resources in core forest and hence their impacts on tinamou ground nests are reduced. As a result of fragmentation, community members exist in concentrated use areas, which results in heightened predator–prey foraging rates and thus stronger interactions in forest fragments. This hypothesis is closely linked to extinction debt (e.g., Tilman et al. 1994), because potential predators are concentrated in forest fragments before they become locally extirpated after a lag period of competing for limited resources. Several of the mammalian and avian predator species detected in the core forest were not detected in the fragments, signaling that they were already absent. For example, peccaries and tayras were only responsible for nest loss events in LSBS core forest. However, several unexpected species, such as the frugivorous agouti (Dasyprocta punctata) and the keel-billed toucan (Rhamphorus sulfuratus) a canopy dwelling species, consumed ground nests in fragments. Due to concentrated foraging, paired with limited resources, these species are likely interacting strongly with ground-nesting birds in forest fragments compared to core forests (Cove et al. 2017a).
A substantial proportion of nest loss events were caused by unknown predators that were not detected by cameras. These nest losses were conceivably due to snake predators because they are ectotherms and can go undetected by the infrared camera sensors (Visco and Sherry 2015). Common egg-eating snakes, Spilotes pullatus and Phrynonax poecilonotus, are probably responsible for a substantial proportion of observed egg loss where no evidence of egg shells remained and no photos were available to explain the loss (e.g., snakes swallow eggs whole) (Gibbs 1991; Arnold et al. 2012; Visco and Sherry 2015). In addition, snake abundance and their consumption of tinamou eggs might vary between the LSBS core forest compared to forest fragments. Snakes were less commonly detected in control plots compared to peccary exclosures in LSBS (Reider et al. 2013), suggesting that heightened peccary abundance could reduce snake population densities in LSBS core forest. Furthermore, Cove et al. (2017b) found evidence of peccary predation on a coral snake (Micrurus alleni) in LSBS. We recommend that additional research is warranted to better detect snakes in future nest predation studies and further examine the role that snakes play in the loss of avian ground nests.
We followed a similar protocol to a previous survey (e.g., Gibbs 1991) and observed nearly identical nest loss (e.g., 40% versus 42%). These results are encouraging considering that abundance and potential impacts of collared peccaries and other mesopredators have apparently increased during the 30 years between the two surveys (Romero et al. 2013; Pardo Vargas et al. 2016). However, we suspect the differences in our survey study design might make the slight difference in nest loss more substantial because the previous study could have artificially inflated predation rates. Gibbs (1991) placed tinamou nests at 25-m and 50-m intervals along the forest edge, compared to our 250 m distance between nests. We suggest the close proximity of the nests in the previous study could have artificially inflated predation rates due to concentrated foraging by predators along the edge (Chalfoun et al. 2002), particularly because nests were effectively non-independent. We suspect little to no influence of seasonal variation in nest loss between the two studies because we established tinamou nests during the early wet season, whereas Gibbs (1991) established tinamou nests in the late wet season. Our camera trap surveys provided additional insights into the predator guilds responsible for nest loss, because we identified ten mammalian and avian species responsible for nest predation events; whereas previous research was limited to making inferences about five potential predators from diurnal transect surveys (Gibbs 1991).
The average incubation period for tinamou nests is 19–20 days, shortly after which chicks hatch synchronously (Burton and Burton 2002). During the incubation period, males are known to leave nests unattended for anywhere between 45 min and 5 h—enough time for depredation events to occur. Our nest experiment was shorter than typical incubation periods to reduce the influence of olfactory cues if eggs spoiled during extended time in the environment. However, the shortened surveys could bias our estimates of predation rates to be low and we recommend using a similar camera trap protocol on natural tinamou nests in the future. Although there are still gaps in our knowledge of terrestrial ground-nesting birds, this study informs current understanding of nest predation frequency, nest predator detection rates and assemblages, and vulnerability patterns of ground-nesting birds across a fragmented landscape. Building from this understanding, conservation actions can reassess measures to reduce threats to the reproduction of declining birds and other understory species in the Neotropics.
Conclusion
Our nest experiment and camera trap results suggest that fragmentation influences nest predators and their interactions with ground nests in the Neotropics. Nest predators more regularly encountered nests without consuming them in core forest, suggesting that foraging efforts are less concentrated in core forest compared to forest fragments with limited resources. We suspect that concentrated community use of limited resources is driving species to interact more strongly than in natural ecosystems. Fragmentation is a global phenomenon and these concentrated foraging effects could have community-wide impacts and warrant further examination at multiple scales in other fragmented environments.
References
Arango-Vélez N, Kattan GH (1997) Effects of forest fragmentation on experimental nest predation in Andean cloud forest. Biol Cons 81:137–143
Arnold S, Stunkel A, Wasko DK, Visco DM (2012) Factors influencing nest predation rates by bird-eating snakes (Pseustes poecilonotus) on simulated chestnut-backed antbird (Myrmeciza exsul) nests. Organization for Tropical Studies (Unpubl.) REU, pp 32–42
BirdLife International (2017) Tinamus major (amended version of 2016 assessment). The IUCN red list of threatened species 2017: e.T22678148A110915916. http://doi.org/10.2305/IUCN.UK.2017-1.RLTS.T22678148A110915916.en. Downloaded on 27 July 2019
Boyle WA, Sigel BJ (2015) Ongoing changes in the avifauna of La Selva Biological Station, Costa Rica: twenty-three years of Christmas Bird Counts. Biol Cons 188:11–21
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Burton M, Burton R (2002) International wildlife encyclopedia. Marshall Cavendish, Singapore
Chalfoun AD, Thompson FR III, Ratnaswamy MJ (2002) Nest predators and fragmentation: a review and meta-analysis. Conserv Biol 16:306–318
Cove MV, Spínola RM, Jackson VL, Sáenz JC, Chassot O (2013) Integrating occupancy modeling and camera-trap data to estimate medium and large mammal detection and richness in a Central American biological corridor. Trop Conserv Sci 6:781–795
Cove MV, Spínola RM, Jackson VL, Saénz JC (2014) The role of fragmentation and landscape changes in the ecological release of common nest predators in the neotropics. PeerJ. https://doi.org/10.7717/peerj.464
Cove MV, Fernandez CM, Vera Alvarez MD, Bird S, Jones D, Fagan ME (2017a) Toucans descend to the forest floor to consume the eggs of ground-nesting birds. Food Webs 10:2–4
Cove MV, Kuhn KM, Foster P (2017b) Micrurus alleni (Allen’s coral snake) predation and scavenging. Herpetol Rev 48:453–454
Fahrig L (2013) Rethinking patch size and isolation effects: the habitat amount hypothesis. J Biogeogr 40:1649–1663
Gibbs JP (1991) Avian nest predation in tropical Wet forest: an experimental study. Oikos 60:155–161
Gibson L, Lee TM, Koh LP, Brook BW, Gardner TA, Barlow J, Peres CA, Bradshaw CJA, Laurance WF, Lovejoy TE, Sodhi NS (2011) Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478:378–381
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD, Cook WM (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1(2):e1500052
Harmsen BJ, Foster RJ, Silver S, Ostro L, Doncaster CP (2010) Differential use of trails by forest mammals and the implications for camera-trap studies: a case study from Belize. Biotropica 42:126–133
Hutchinson J, Waser PM (2007) Use, misuse and extensions of “ideal gas” models of animal encounter. Biol Rev 82:335–359
IUCN, Species Survival Commission (2016) IUCN red list of threatened species. Prepared by the IUCN Species Survival Commission. <iucnredlist.org>. Downloaded on 27 July 2019
Layman CA, Giery ST, Buhler S, Rossi R, Penland T, Henson MN, Bogdanoff AK, Cove MV, Irizarry AD, Schalk CM, Archer SK (2015) A primer on the history of food web ecology: fundamental contributions of fourteen researchers. Food Webs 4:14–24
Mazerolle MJ (2011) AICcmodavg: model selection and multimodel inference based on (Q) AIC (c). R Package Version 1
Meyers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Newmark WD, Stanley TR (2011) Habitat fragmentation reduces nest survival in an Afrotropical bird community in a biodiversity hotspot. Proc Natl Acad Sci 108:11488–11493
Pardo Vargas LE, Cove MV, Spinola RM, de la Cruz JC, Saenz JC (2016) Assessing species traits and landscape relationships of the mammalian carnivore community in a neotropical biological corridor. Biodivers Conserv 25:739–752
Prum RO, Berv JS, Dornburg A, Field DJ, Townsend JP, Lemmon EM, Lemmon AR (2015) A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 526:569
R Development Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reider KE, Carson WP, Donnelly MA (2013) Effects of collared peccary (Pecari tajacu) exclusion on leaf litter amphibians and reptiles in a Neotropical wet forest, Costa Rica. Biol Cons 163:90–98
Romero A, O’Neill BJ, Timm RM, Gerow KG, McClearn D (2013) Group dynamics, behavior, and current and historical abundance of peccaries in Costa Rica’s Caribbean lowlands. J Mammal 94:771–791
Sekercioglu CH, Ehrlich PR, Daily GC, Aygen D, Goehring D, Sandi RF (2002) Disappearance of insectivorous birds from tropical forest fragments. Proc Natl Acad Sci 99:263–267
Small MF, Hunter ML (1988) Forest fragmentation and avian nest predation in forested landscapes. Oecologia 76:62–64
Thornton DH, Branch LC, Sunquist ME (2012) Response of large galliforms and tinamous (Cracidae, Phasianidae, Tinamidae) to habitat loss and fragmentation in northern Guatemala. Oryx 46:567–576
Tilman D, May RM, Lehman CL, Nowak MA (1994) Habitat destruction and the extinction debt. Nature 371:65–66
Vera Alvarez MD, Fernandez CM, Cove MV (2019) Assessing the role of habitat and species interactions in the population decline and detection bias of Neotropical leaf litter frogs. Neotrop Biol Conserv 14:143–156
Visco DM, Sherry TW (2015) Increased abundance, but reduced nest predation in the chestnut-backed antbird in Costa Rican rainforest fragments: surprising impacts of a pervasive snake species. Biol Cons 188:22–31
Visco DM, Michel NL, Boyle WA, Sigel BJ, Woltmann S, Sherry TW (2015) Patterns and causes of understory bird declines in human-disturbed tropical forest landscapes: a case study from Central America. Biol Cons 191:117–129
Young BE, Sherry TW, Sigel BJ, Woltmann S (2008) Nesting success of Costa Rican lowland rain forest birds in response to edge and isolation effects. Biotropica 40:615–622
Acknowledgements
This research was funded by the National Science Foundation (NSF) and Louis Stokes Alliances for Minority Participation (LSAMP). Thanks to the Organization for Tropical Studies (OTS) and to Selva Verde Lodge, particularly Carlos de la Rosa, Orlando Vargas, Bernal Matarrita, Danilo Brenes, Ivan Castillo, and Gerardo Alvarez for their continued support. Thanks to Carissa Ganong and Adriana Baltodano for managing the REU program. Finally, thanks to S. Bird, D. Jones, and other REUs for assisting with fieldwork, and G. Keating and M. Fagan for GIS assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernandez, C.M., Vera Alvarez, M.D. & Cove, M.V. Heightened nest loss in tropical forest fragments despite higher predator load in core forest. Trop Ecol 60, 281–287 (2019). https://doi.org/10.1007/s42965-019-00032-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42965-019-00032-1