Abstract
Carbon-based materials have emerged as an excellent class of biomedical materials due to their exceptional mechanical properties, lower surface friction, and resistance to wear, tear, and corrosion. Experimental studies have shown the promising results of carbon-based coatings in the field of biomedical implants. The reasons for their successful applications are their ability to suppress thrombo-inflammatory reactions which are evoked as an immune response due to foreign body object implantation. Different types of carbon coatings such as diamond-like carbon, pyrolytic carbon, silicon carbide, and graphene have been extensively studied and utilized in various fields of life including the biomedical industry. Their atomic arrangement and structural properties give rise to unique features which make them suitable for multiple applications. Due to the specificity and hardness of carbon-based precursors, only a specific type of coating technique may be utilized for nanostructure development and fabrication. In this paper, different coating techniques are discussed which were selected based on the substrate material, the type of implant, and the thickness of coating layer. Chemical vapor deposition-based techniques, thermal spray coating, pulsed laser deposition, and biomimetic coatings are some of the most common techniques that are used in the field of biomaterials to deposit a coating layer on the implant. Literature gathered in this review has significance in the field of biomedical implant industry to reduce its failure rate by making surfaces inert, decreasing corrosion related issues and enhancing biocompatibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Medical devices have become one of the rapidly growing industries globally due to the ever-increasing rate of accidental injuries, vehicle crashes, diseases, and many other fatalities which have helped the medical device industry to become a fundamental part of healthcare systems. According to WHO, there are more than 2 million different types of medical devices being used throughout the world including apparatus, machines, implants, software, and materials. Implantable medical device is a major category of medical devices majorly comprising cardiac, brain, bone, and dental implants whose primary responsibility is to restore injured human body part or function [1]. The market for medical implants was worth 88 billion USD in 2019 and by 2023, it is expected to reach 147.46 billion USD with a growth rate of 19% which was only 7.1% during 2012–2017 [2]. In 2001, about 26 million people in the United States had an implant inside their body [3]. By the virtue of countless efforts of researchers and industrialists, numerous materials, drugs, and polymers have been introduced in medical implants which save millions of lives and extend life expectancy.
Despite many efforts of researchers, medical implants continue to fail due to corrosion [4], material leaching [5], loss of mechanical strength [6], tissue rejection [7], or loss of function [8]. Both short- and long-term implant failure are concerning because it requires the efforts of clinicians and capital for reimplantation. To avoid extra burdens, many strategies and modifications were introduced in biomedical implants to improve the biocompatibility, strength, and topographical properties which may help the implant to perform its function for a longer period. Surface topography plays a crucial role in deciding the fate of foreign body objects inside a human body. The surface characteristics like roughness, nanopores, texture, and wettability are some of the deciding factors for cell adhesion [9], immune response activation [10], degree of biofouling [11], thrombo-inflammatory cascade activation [12, 13], and fibrotic capsule formation [14]. Typically, surfaces of the implants are improved by passive and active coatings. The passive coatings are inert and minimize the excessive attachment of platelets and cells due to their inherent wettability and surface texture. These coatings act as a barrier to protect implants from ionic attacks of body fluids which have the ability to slowly disintegrate the foreign body objects and ultimately cause tissue rejection. In addition, these coatings provide corrosion resistance, reduce material leaching, and strengthen mechanical properties of the implants to increase their endurance and life span. Different materials including calcium, bioactive glass, carbon-based materials, and hydroxyapatite are reported to be used as passive coatings for biomedical implants [15, 16]. In contrast, bioactive coatings are composed of drug loaded polymers which accelerate lesion healing [17], reduce cell proliferation [18], occlude thrombo-inflammatory cascades [19], and stagnate activation of the immune system [20]. Currently, biomedical implants have hybrid coatings consisting of both passive and active layers which help in wound healing and also extends the life span of implants.
Carbon based materials have a myriad of applications due to their excellent chemical, mechanical, thermal, and biological properties. The empty outer shell of carbon makes it suitable for bonding with other materials at an atomic level and produces millions of different compounds by forming four covalent bonds. Carbon based coatings have excellent mechanical and physical properties over a wide temperature range, have chemical inertness, are biocompatible, hemocompatible, and wear, corrosion and erosion resistant which makes them suitable for multiple industries including aerospace, automobile, heavy machinery, textiles, plastics, energy, and food preservatives. All the allotropes of carbon (graphene, diamond, amorphous, fullerenes) have multiple applications in the biomedical industry and many studies have reported their suitability and effectiveness as an inert coating material [21,22,23,24].
Due to hardness of carbon-based materials and requirement of high-temperature conditions, specific coating techniques are utilized including vapor deposition-based techniques, thermal sprays, and laser deposition. There are some other suitable techniques like 3D printing [25, 26], laser cutting [27], electrospinning [28], and dip coating [29] which have been utilized for biomedical applications for decades; however, this article focuses on the various vapor deposition and laser deposition type coating techniques. Previously, many studies in literature solely focused on either carbon-based materials or coating techniques. This paper will be providing insights on both materials and techniques by principally focusing on biomedical implants which could be beneficial for the selection of carbon allotrope and its coating technique for biomedical applications.
2 Carbon-based coatings
Carbon is one of the most important elements of the periodic table which provides the foundations of life, as it is a constituent of 95% of compounds on the earth [30, 31]. It has the ability to not only bind with other elements but also with other carbon atoms as well and also tailor the number of bonds and energies; thus, multiple allotropes of carbon are generated. These allotropes act as excellent coating material and their atomic variations give them specific structures and shapes which are suitable for different medical applications. These allotropic carbon coatings and their applications are explained in the coming sections.
2.1 Diamond like carbon coating
Diamond like carbon (DLC) has been used in multiple fields of life because of its stability, inertness, and excellent tribological, and mechanical properties. DLC is hard and wear-resistant; hence, making it a suitable material to coat industrial tools, batteries, knives, compaction devices, and wear-resistant surfaces [32]. It also has excellent biocompatibility which allows it to be utilized in the biological realm for multiple applications [33]. The structural analysis of DLC demonstrated that it is amorphous in nature and has differing ratios of sp2, sp3 bonded carbon, and hydrogen. The amorphous matrix usually has sp3 nodules in an sp2 bonded matrix. In contrast, graphite has sp2 bonding with a planer configuration in which a single carbon molecule makes a double bond and two single bonds with two different atoms (mostly carbon atoms). The carbon having sp3 bonding possesses a tetrahedral arrangement by making three single bonds [34]. The different combinations of these three elements impart varying properties to DLC coatings and the application depends upon their concentration and arrangement [35].
The properties of DLC betwixt and between diamond and graphite include higher hardness, lower friction coefficient, excellent tribological properties, and lower wear rate [32]. It has many applications in the field of biomedicine due to its hemocompatibility, anticancer, antithrombogenic, and antibacterial properties [36]. DLC is mainly coated on cardiovascular stents to reduce thrombogenicity and to improve the mechanical properties of the stents. The arrangement of carbon atoms in DLC coating makes them inert and hydrophobic which avoids the attachment of blood clots to the surface coating. In addition, the diamond like morphology promotes endothelial healing and tissue repair at the target site. Hasebe et al. administered the adsorption of proteins on DLC coated surfaces since it was the first event of coagulation cascade and thrombus formation. This study also demonstrated the repression of cell proliferation and blood coagulation due to DLC coating [37]. Similar results can be found in many other studies [38,39,40,41]. Coronary stent MOMO® is the commercially available DLC stent that has demonstrated non-inferiority results when compared to MULTI-LINK VISION stent and proved its efficiency and effectiveness in 99 patients from 19 different centers in Japan [42]. In another study, Gorzelanny et al. fabricated a hybrid surface of a bone implant by combining DLC coating with silver nanoparticles to improve the anti-proliferation and anti-bacterial properties. The inert and antiproliferative properties of DLC when complemented by the strong antibacterial properties of sliver material resulted in an excellent mammalian cell compatible surface [43]. Some other studies also demonstrated an improvement in the properties of cardiovascular stents and other medical implants [44, 45]. Overall, DLC coatings are excellent candidates to diminish thrombus and inflammation-related issues of biomedical implants.
2.2 Pyrolytic carbon coating
Pyrolytic carbon is a synthetic material that is made by the thermal deposition (400–2500 °C [46]) of hydrocarbons under a vacuum. It was introduced in the 1960s and it was first used for the coating of nuclear materials. Nevertheless, since then, it has been used for multiple other applications, especially in the field of biomedical devices [47]. Pyrolytic carbon has a turbostratic structure [48] in which carbon atoms are covalently bonded with each other and make a loose hexagonal structure. The crystallites of pyrolytic carbon are randomly oriented and are spaced at 0.348 nm. There are also fine crystalline regions in the pyrolytic carbon in the order of 2.50–4 nm [49]. The good mechanical strength, roughness, durability, physical strength, and biocompatibility of pyrolytic carbon make it a suitable candidate for the coating of biomedical implants and other devices. It has been found compatible with soft and hard tissues due to its inability to evoke thrombo-inflammatory cascades [50].
The major application of pyrolytic carbon was in the field of heart valves in which either the whole artificial heart was made of pyrolytic carbon [51] or only the surface was coated with pyrolytic carbon [52]. In addition, it has also been used for the coating of cardiovascular stents i.e., Cre8 stents. In this stent, the coated surface is called a bio-inducer surface which expedites the stent endothelialization and decreases the risk of thrombosis [53]. The results of Cre8 stent demonstrated better performance than control group and improved clinical results in NEXT and other trials [54, 55]. Other isotopes including low-temperature isotropic pyrolytic carbon (LTIC) have also been extensively investigated and studied and have been used for the coating of medical implants. It is a highly energized and hydrophobic material that is suitable for biomedical applications [56].
2.3 Silicon carbide coating
Silicon carbide is from a diamond like carbon category and is commonly obtained from silica sand and petroleum coke. It possesses excellent stability, hardness, and resistance to chemical attacks. Its common application is to be used as an abrasive material and protective coating for different machines.
SiC has a crystalline structure composed of Si and C atoms. The atoms are covalently bonded by making tetrahedral structures. These structures have electrons in sp3 hybrid orbitals and generally hold 4.5 eV energy. Ogwu et al. found that Si-doped a-C:H coatings (Si-DLC), which lowered the electrical resistivity, effective work function, and surface energy, leading to reduced platelet aggregation and enhanced hemocompatibility. Apart from electrical properties, surface wettability is also a crucial determinant affecting hemocompatibility [57]. Many other studies demonstrated the reduction in platelet aggregation and corrosion by SiC coated surfaces [58, 59]. Furthermore, there are many commercially available cardiovascular stents (i.e., Orsiro drug eluting stent) that are coated with SiC to improve the mechanical strength, reduce thrombo-inflammatory reactions, and improve hemocompatibility [60]. BIOFLOW clinical trials series and BIONYX trial have proven the superior results of Orsiro as compared to other stents i.e., Xience, Biofreedom, and Onyx stents [61, 62].
2.4 Graphene coating
Graphene is a nanomaterial that is synthesized by the isolation of a single layer of graphite. Due to the tunable surface chemistry of graphene, it also exists in the form of graphene oxide and reduced graphene oxide. Both fundamental and applied research attaches great importance to graphene due to its excellent properties i.e., high surface area, high electrical and thermal conductivity, and excellent chemical and mechanical properties. It also has applications in the biomedical industry due to biocompatibility and hemocompatibility. The noteworthy applications of graphene are in the food industry and sensor fabrication.
The structural analysis of graphene showed that carbon is arranged in the form of hexagons by making honeycomb lattices. It has π-electron clouds with sp2 hybridized carbon atoms. Ideally, graphene has 0.14 nm and 0.35 nm C–C bond length and thickness, respectively. A single carbon atom of graphene forms three strong σ bonds with its neighboring atoms making a lattice that may exist in two chiral forms: A and B [63].
Graphene is a miraculous material which is existed in multiple forms i.e., graphene sheets, graphene quantum dots, graphene oxide or pure graphene. Each type of graphene brings different advantages and applications. For instance, graphene quantum dots have smaller size and have functional groups like hydrogen and oxygen molecules incorporated into it; therefore, they are used for scavenging free radicals, biomedical imaging, drug delivery and photoelectric related applications [64]. Graphene oxide is widely used in semiconductors, fuel cells, and energy storage devices. In biomedical sensors, it is used in field-effect transistors (FETs) which helps in chemical and biological sensing. Graphene oxide has also found suitable for gene therapy and gene delivery because it provides the protection to vectors and DNA during delivery [65]. For biomedical implants, graphene coating is used to enhance the surface properties, durability and biocompatibility [66].
The hydrophobic character of graphene sheets makes it an excellent candidate to be utilized in a fluidic environment; therefore, it has been used to coat biomedical implants to reduce the attachment of blood cells and other cellular entities [67]. Many studies have proven the improved lesion healing and reduced thrombosis due to graphene coatings on cardiovascular stents [68, 69]. In conclusion, graphene and its different forms have applications in drug delivery, gene delivery, cancer therapy, bioimaging, and biosensing [70].
2.5 Pure graphite coating
Graphite is an anisotropic compound that is different from diamond on the basis of atomic bonding and hybridization. In contrast to diamond which has both sp2, sp3 bonded carbon, graphite only has sp2 hybridization. This bonding allows the carbon atoms to stack upon each other and form a hexagonal configuration as shown in Fig. 1E. The crystalline structure of graphite is electrically and thermally conductive which makes it suitable to be used in batteries, lubricants, high temperature refractories, and electric vehicles [71].
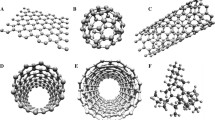
The atomic arrangement of different allotropes of carbon A Tetrahedral arrangement of carbon atoms in pure diamond showing the presence of c–c-c and having 1.544 × 10–10 bond length B Amorphous coating of DLC showing a combination of sp2 and sp3 hybridization C Hexagonal array of pyrolytic carbon having weak interlayer of bonding D Tetrahedral bonding of Si–C–Si and C–C–Si bonds in SiC having up to ~ 1.85 Å bond length E monolayer, planar, and hexagonal lattice structure of graphene having 0.142 nm bond length E sandwich-like structure of graphite having 1.421 Å bond length
Graphite has a slight hydrophobic behavior which can be tweaked as per requirements through structural and configurational modifications. For example, Kozbial et al. reported the absorption of airborne hydrocarbons can decrease the hydrophilic character of graphite [72]. In the biomedical industry, graphite has demonstrated a myriad of applications. In a study, Shahriarinour et al. developed a pencil type graphite sensor for the assessment of cholesterol levels which had sensitivity up to 4.98 nmolL−1 [73]. Similarly, graphite based sensors for heart and glucose monitoring were described by Tang et al. and Lima et al. [74, 75]. In addition, graphite has been reported to be used as a bone cement [76], antimicrobial agent [77], and coating agent for heart valves [78].
3 Nanostructures of carbon
The nanostructures of carbon have opened new avenues of research in the field of biomedical sciences, tissue engineering, automobile, and fuel industry. Carbon itself has a myriad of properties and characteristics which makes it a lifeline of many industries; however, the nanostructures enhance these properties by manifolds and make carbon more desirable and beneficial for industrial applications. The combination of nanotechnology and carbon materials accelerated the natural inclination of forces including covalent bonding, hydrogen bonding, and van der Waals forces which made the material extremely lightweight, ultra-strong, durable, and highly thermally and electrically conductive. The careful investigation of carbon nanostructures demonstrated that each one of the nanostructures had its unique characteristics, properties, and applications. The details of different nanostructures of carbon are explained in the coming sections.
3.1 Carbon nanotubes
Carbon nanotubes (CNTs) are an exquisite type of carbon-based materials that has numerous and divergent applications. It has been widely explored in the biomedical field for its applications in biosensing, drug delivery, tissue engineering, imaging, diagnosis, and cancer therapy. CNTs are formed by the coordination of three carbons which are pyramided from sp3 hybridization towards sp3 hybridization.
These are available in the form of a single cylinder or multiwall tubes. The diameter of single and multi CNTs are ≈ 0.4 nm and > 30 nm, respectively. Both types of CNTs have different applications. The single walled CNTs are ultra-light weight; therefore, these are suitable for electrochemical energy storage, photovoltaics, nanoelectronics and thermoelectric power generation [79]. In biomedical field, there are different applications of single welled CNTs i.e., Yang et al., reported the use of single CNTs as drug carriers in Alzheimer’s disease [80] and Chen et al., described the use of single CNTs for drug delivery to prevent the tumor progression [81]. In comparison, multi walled CNTs are popular for the applications of solar cells, flat panel displays and transistors. Yola & Atar reported the use of multi walled CNTs in immunosensors for tumor detection [82]. In another study, use of multi walled CNTs was reported for detection and analysis of cancer progression and metastatic potential [83]. In addition, different studies have reported the site specific drug delivery through multi walled CNTs [84, 85]. These have also shown potential in the field of biomedical implants as studies have reported applications of multi walled CNTs for corrosion resistance and uncontrolled cell growth suppression through targeted drug delivery [86, 87].
To synthesize CNTs, chemical vapor deposition is used. The commercial uses of CNTs are explained in Table 1. CNTs possess snacking effects which helps them in penetration in membranes and increase their biocompatibility [88] and makes them suitable candidates for the diagnosis of diseases [89], biosensor development [90], drug delivery [91], anticancer therapy [92] and targeted therapy [93]. In the biomedical field. Pahlevanzadeh et al. increased the strength of load bearing bone implants made of PMMA-based cement [94] and Francis et al. reported the suitability of coating of CNT-reinforced chitosan-based ceramic composite on magnesium based biomedical implants to increase the anticorrosion and antibacterial properties [95]. Mondal et al. used the thin films of CNTs as an alternative to induce pluripotent stem cells which proved to be better than stem cells as it maintained pluripotency, and induced the differentiation of ciPSC in regenerative medicine applications [96].
3.2 Fullerenes
Fullerene is a soccer ball shaped allotrope of carbon which is composed of sixty carbon atoms. It has π-electron systems, and the long curvature and hollow spheres of these systems make them suitable for multiple applications. The large surface area of fullerenes makes it appropriate for exo-functionalization which facilitates the attachment of amino acids and peptides for medical applications [97]. Fullerenes have found to be an excellent nanoplatform for cancer detection and management. In a study, the autophagy and anticancer potential of fullerenes was investigated and application of fullerenes in chemotherapy was reported [98]. These have also been found suitable for bioengineering based solution and promotion of osseointegration in bone implantation [99].
3.3 Carbon nanoparticles
Carbon nanoparticles are a widely explored class of materials that have numerous applications in the field of biomedicine, biomedical implants, sensors, automotive mobiles, and many other. fields Carbon black is the most famous example of nanoparticles which is widely used in pigments, cosmetics, inks, coatings, and automobile tyers. Carbon nanoparticles have a diameter of 3–10 nm and are synthesized through carbonization, heating, activation, and grinding. The structural analysis showed that they have an electronic configuration of [He] 2s22p2. Currently, carbon nanoparticles are dominating over metal nanoparticles and are considered an excellent material to be used in various fields due to their stability and performance.
3.4 Carbon nanofibers
Carbon fibers have been extensively used in aircrafts, sports cars, and their equipment due to their hardness, durability, and corrosion resistance properties. Carbon nanofiber is a quasi-one-dimensional carbon material that has a diameter up to 100 nm. According to its structural analysis, it lies in between carbon fiber and carbon nanotube. It is a strong material and has strength up to 8.7 GPa which is the reason for its multiple applications. It is usually synthesized by catalytic decomposition reactions, chemical vapor deposition, and electrospinning. In the field of biomedical engineering, carbon nanofibers are used to increase the strength of biomaterials in tissue engineering, cell regeneration, and cancer treatments. In the field of biomedical implants, Buschbeck et al. described carbon nano fibers as neural implants in insects to assess their activity and reported them as a suitable candidate to replace metal electrodes [100].
3.5 Nanoribbons
Nanoribbons are one of the most fascinating classes of carbon. These are one dimensional, finite and planner structures of graphene which have different types of structural arrangements i.e., zigzag, armchair or intermediate character. The applications of these structures also vary when their structure changes i.e., electronic properties are achieved by using edge structure [101]. In biomedical applications, graphene nanoribbons are used in biosensing and drug delivery [102]. These are also used as therapeutic agents to increases the efficiency and efficacy of treatment regimens [103].
3.6 Carbon nanoflowers
Carbon nanoflowers are a recent class of three-dimensional carbon nanostructures which has similarities to the plant flowers. These nanoflowers have structural and morphological similarities to natural flowers and their petals are excellent sites for the storage. In biomedical industry, nanoflowers have been used for diagnosis and treatment purposes because of their ability to interact with human body at cellular level. The most interesting application of nanoflowers is in cardiovascular disease because of their ability to trigger the regrowth of blood vessels [104, 105]. Nanoflowers have also been found to effective for cancer therapy as in a study, microbial transglutaminase nanoflowers demonstrated effectiveness in treating breast cancer [106]. In a recent study, nanoflowers were used in microneedles to monitor glucose levels in body [107]. Due to lesser toxicity effects of nanoflowers, these are being investigated in the field of biomedical implants; however, a lack of data exists in literature regarding applications of nanoflowers for biomedical implants (Fig. 2).
Nanostructures of carbon A Honeycomb mesh like structure of carbon nanotube which is made by the wrapping of individual layers of carbon around each other B carbon nanoparticles having [He] 2s2 2p2 configuration C Electrospun nanofiber having carbon layers stacked in the form of cones D Quasispherical carbon quantum dots possessing amorphous to crystalline carbon base Quantum dot E Bucky ball structure of fullerenes F Nanoflowers under electron microscope depicting similarities with natural flowers of plants
3.7 Carbon quantum dots
Carbon quantum dots are an emerging class of carbon materials that have polymer-like and fluorescent properties. These are a type of carbon nanoparticle having a discrete and quasi-spherical carbon structure that can be obtained during the purification of single-walled carbon nanotubes. The carbon atoms are arranged as sp2-graphitic carbon which combines with sp3 hybridization resulting in imparting florescence to the quantum dots. Their size is usually less than 10 nm. This class of material is playing a significant role in the field of biomedicine as it has applications in diagnosis, bioimaging, material fabrication, drug delivery, synthetic chemistry, biosensing, and materials science.
4 Toxicity related issues
The traces of carbon could be found in every aspect of life and its nanostructures have made human lives easier. The development of nanostructures of carbon requires different types of processing that modifies the physicochemical and structural properties and increases the toxicity of the material which is a major concern for carbon-based materials [108]. The researchers have worked on optimization of these material and modifying them in such a way that their toxic levels were lessened and fall under the acceptable range [109]. Toxicity of carbon-based nanostructures is witnessed when high concentration of material is used i.e., Delorme et al. reported a persistent inflammation in rats after 90 days’ exposure to carbon nanofibers at 25 mg/m3 concentration [110]. Similarly, another study mentioned the inflammation at 1.5 mg/m3 concentration of multi-welled carbon nanotubes [111]. Despite debatable toxicity, carbon-based materials have been used in bioimaging, diagnostics or different treatment regimens and preclinical studies suggested insignificant or non-toxic effects [112]. There are many factors which help in the reduction of toxicity i.e., type of solvent, size of carbon material, synthesis process and byproducts [113]. Due to advancement in biomedical field, the toxicity levels of these materials have been controlled and reduced; therefore, clinicians can now use these materials without any hesitation.
5 Carbon-coating techniques
Apart from nanostructures of carbon, the coating of carbon materials has garnered much attention due to their excellent properties. Studies have shown that carbon is difficult to coat on target sites through traditional coating techniques like dip coating and spin coating; however, high temperature-based techniques were found to be the most suitable for coating purposes. Therefore, the details of different coating techniques for carbon materials and their applications in the biomedical industry are discussed here.
5.1 Vapor deposition based techniques
The coating techniques are selected on the basis of coating material and the type of substrate because the type of material of target site influences the coating process and adhesion properties. For carbon-based materials, multiple types of coating techniques are used which are selected on the basis of substrate material, intended applications, and thickness of the coating layer. Multiple coating techniques and their process and requirements are explained in the following sections.
5.1.1 Chemical vapor deposition
Chemical vapor deposition (CVD) is a sophisticated technology for materials processing and is utilized to deposit thin films with the help of chemical reaction of gas-phase precursors. It uses chemical reactions from a thermally induced substrate surface to provide a solid coating of reaction product by supplying reagents in gaseous form. Vapor-phased substances are condensed to produce solid phase material [114]. CVD is a lucrative technique because it can accommodate a large number of precursors and coat various types of thin films. Only a few elements from the periodic table, such as noble gases, halogens, and a few actinides and alkali metals cannot be deposited using this method. The chamber of CVD harbors a vapor–solid reaction in which precursor gases move and react with each other in a heated vacuum and deposit multidirectional and assorted coatings.
For decades, CVD has been used to coat the surfaces of different medical devices with various types of materials. Carbon-based materials are the obvious choice of material to be coated through CVD and many studies have reported the improvement in the medical devices after these coatings. Recently, Kheradmandfard et al. reported the exceptional improvement in the corrosion and wear resistance of Ti alloy after coating Si/DLC nanocomposite through CVD [115]. Moreover, Ti and TiN based materials are popularly coated through CVD [116]. In literature, different types of carbon coatings including graphene [117, 118] and pyrolytic carbon [119,120,121] have been successfully applied in the biomedical industry for multiple applications. The CVD based coatings may improve the tribological and performance based properties as well as biocompatibility of biomedical implants.
In addition to innumerable benefits, there are multiple drawbacks of CVD including utilization of high temperature, reactive precursor gasses and requirement of safety gears for operators which results in higher cost and material constraints. To avoid the temperature related issues of CVD, plasma enhanced chemical vapor deposition was introduced which has advantages of low toxicity and substratum constraints such as inorganic materials, in particular, low temperature and chemical stability, solvent and corrosion resistance, and substrate limits due to complicated geometries and composition.
5.1.2 Physical vapor deposition (PVD)
PVD has been used for the coatings of different industrial machines and parts to improve their mechanical surface properties. Initially, it was associated with poor adhesion and uniformity related issues which were resolved by process and machine optimization. Currently, operators have full control over deposition rate, structure, and temperature for substrate coating on the target. It also has computer numeric control which makes it suitable for industrial processes and coating of materials at a larger scale.
PVD uses atomization or vaporization processes that do not involve chemical reactions for material coatings to grow coatings on a surface from the atomic level. These atomic coatings are deposited in the presence of vacuum and plasma or an electrical environment and PVD deposits a line-of-site impingement type of coatings. Chowdhury et al. coated Ti6Al4V alloy with AlTiN and CrN through PVD for aerospace applications and reported the improvement of mechanical properties and wear resistance of the specimens [122]. Similarly, Vega et al. demonstrated the improvement in wear and corrosion resistance by introducing the interlayers of Ti through PVD in TiN coatings [123]. Krella investigated the influence of the deposition process of PVD coatings through cathodic arc evaporation based PVD and reactive magnetron sputtering based PVD on the degradation rate under cavitation erosion. The results exhibited the phase transformation Fe-γ → Fe-α′ of substrate regardless of coating conditions and techniques; however, coatings through reactive magnetron sputtering underwent intense transformation showing that lower density coatings are not suitable for erosion related applications [124].
The coatings of magnesium based implants through PVD had been popular in the field of biomedical industry. Vignesh et al. coated the surface of magnesium with iron and hydroxyapatite to improve the mechanical, biological, and corrosion related properties as well as ameliorate the degradation rate of magnesium [125]. Bahi et al. coated Ti with (Ti/TiN)9 and (Ti/TiN)9/TiO2 through PVD to improve their performance in the biological environment and demonstrated the improvement in biocompatibility, tribological, and corrosion resistance related properties [126]. Generally, PVD based coatings are notorious for their lesser adhesion to the substrate, and an optimization process is needed for better adhesion. Nißen et al. optimized the process of a-C:H:Cu coating on Ti6Al4V alloy using a hybrid of PVD/PECVD coatings and reported that controlling Cu mole fraction and bias voltage of substrate is crucial to achieving better adhesion of film to substrate [127]. Many other studies have also reported the applications of PVD in the biomedical industry.
The PVD has an advantage over CVD in terms of temperature related challenges because it requires a lower temperature as compared to the CVD chamber. In addition, it uses a solid precursor which makes it suitable for many organic and inorganic compounds and elements. However, PVD based processes and coatings face some challenges like limited adhesion, lack of uniformity in the coating’s thickness, and inability to coat inside the implant or tool surface [116]. Scientists are improving PVD processes and machines to increase the favorability of the coating.
5.1.3 Plasma enhanced chemical vapor deposition (PECVD)
PECVD is an advanced version of CVD and was introduced to solve the problem of high coating temperatures because numerous materials could be degraded at higher temperatures making them unable to coat through CVD. Therefore, the thin-film deposition at considerably lower temperatures on organic or inorganic materials was started to be done by using electrical energy to form a plasma that ionizes natural gas and produces free radicals, which are then polymerized to form a deposition layer.
PECVD is a hybrid coating process that combines technologies of CVD and high energy plasma to avoid the high temperature requirements of conventional CVD. In this process, the organic molecules are subjected to electron beams to produce free radicals which ultimately polymerize and stick to the target surface generating a deposition layer. To make the process efficient, later low-intensity electron beams were introduced to create layers on target surfaces and avoid material decomposition related issues [128, 129]. In addition, radio frequency based PECVD became popular for organosilicon based coatings [130, 131].
PECVD has been used in the field of biomedical implants for coating purposes. There were multiple studies in literature that demonstrated the benefits of these techniques which are described here. Eurídicea et al. used PECVD to coat amorphous C:H films on the surface of Ti6Al4V alloy to improve the chemical and biological responses of body against the alloy [132]. The analysis of human peripheral blood mononuclear cells (PBMCs) demonstrated the reduction in inflammatory reaction and immune response activation. In another study, the mechanical properties and hardness of titanium bone implants were improved after SiC deposition through PECVD [133]. J Huran et al. characterized the SiC coating through IR, AES, and RBS. The existence of Si–C, Si–H, and C-H bonds was confirmed through the IR spectra of specimens [134]. Another study demonstrated the analysis of PECVD coated amorphous silicon carbide on vascular stents to improve biocompatibility and mechanical strength. Precursor gases such as Silane (SiH4), methane (CH4), and phosphine (PH3) are employed to deposit n-doped a-SiC:H [135]. Many other studies proved the non-cytotoxicity and biocompatibility of the PECVD based coatings [136, 137]. In summary, PECVD is an effective technique to coat biomedical devices and implants to improve their mechanical and biocompatibility properties (Fig. 3).
Schematic of vapor disposition-based systems A Thermal reactor of CVD having gasses as precursor materials for coating B line-of-sight coating process of PVD which utilizes solid material as a source of material vapors C PECVD chamber having ability to generate plasma and operate at lower temperature and deposit dielectric thin films [138] D Plasma spray having 30 mbar–900 mbar pressure which is used to the formation of plasma jet by a transferring arc between the cathode and wire [139]
5.2 Plasma spraying
Plasma spraying was an amazing technology that emerged after World War II to improve the function of machines and their parts with a thin layer of coating which was done with the help of plasma that could melt ceramics or metal powders. These molten materials were then sprayed over the target surface to deposit a protective coating. Plasma spraying has many advantages including low cost, rapid deposition rate, low probability of thermal damage of material, chemically inertness, and the ability to coat at a relatively low temperature.
Plasma spraying has enormous uses in the field of biotechnology and biomedical devices. It is used to improve the mechanical, material, and corrosion properties of biomedical implants and other devices. The adhesion strength of coatings deposited through plasma spray coating techniques depends on two factors: the nature of the coating material and the nature of the target material. Plasma-sprayed HA or CaP coatings have been reported to have poor adherence with the target in several investigations and scientists are trying to optimize the process conditions to achieve maximum adhesion.
Many studies reported the coating of Ti–6Al–4 V alloy through plasma spraying. The coatings were composed of HA/Titanium or HA/yttrium stabilized zirconia (YSZ)/Ti–6Al–4 V. These coating showed better adhesion to the target due to the inclusion of Ti in HA coating which also reduced the residual stress created during the spraying process. [140, 141] Yang et al. coated Ti and CoCrMo implant materials with ZrO2 coatings and discovered that ZrO2 (4 percent CeO2) had high adhesion. ZrO2 (4 percent CeO2) coatings have average adhesion strengths greater than 68 to titanium and greater than 67.7 MPa to CoCrMo, with failure occurring within the ZrO2 coating [142].
5.3 Thermal spraying
Thermal spraying is a rapidly growing technique that has multiple applications in heavy industry, agriculture industry, and other fields. Many advanced and sophisticated thermal spray machines are used in the automobile industry for the coating of vehicle parts [143]. Thermal spray coatings are formed by the successive impact of a stream of spray droplets in fully molten or partially melted states, followed by flattening, rapid cooling, and solidification. The heating and accelerating of the spray materials are necessary to create fine spray droplets. Chemical reactions such as metal alloy oxidation during heating may occur which may change the chemical compositions and phases of the spray materials and add additional functional properties to the coatings. The parameters of the droplets, including temperature, velocity, and size, which are determined by spraying processes and conditions, influence the interaction of the spray particles with the spray flame, and coating deposition processes [144, 145].
The thermal spray approach is getting a lot of attraction and is being embraced as a new way of coating that improves the biocompatibility and mechanical properties of biomedical implants. Precursors in the form of solution or suspension are used to coat the target substrate and impart desirable qualities in thermal spray. There are three types of plasma sprays: flame, plasma arc, and electrical arc sprays. In addition, a few other techniques use plasma spray to coat their targets including atmospheric plasma spraying (APS), vacuum plasma spraying (VPS), liquid plasma spraying (LPS), suspension plasma spraying (SPS), high-velocity oxy-fuel (HVOF), high-velocity suspension flame spraying (HVSFS), detonation gun spraying, and gas tunnel type plasma spraying (GTPS).
Plasma spray has been used in the biomedical industry to coat the targets for the applications of improved biocompatibility, body response, and mechanical properties. Gadow et al. and Prashar & Vasudev have reviewed many studies and provided strong evidence from literature that plasma spraying is a suitable technique to increase the osteoconductive and biocompatibility of bone implants [146, 147]. In another study, Vilardell et al. studied the effects of three different sprays (thermal, cold, and high velocity oxy fuel spray) and observed their effects on cell adhesion and proliferation. The results indicated the response of cell and grain size hydroxyapatite differs by varying the spray type [148]. Liao et al. reviewed the biocompatibility, anti-infective and anti-corrosive properties, and wear-resistance of cold spray coatings and found them to be suitable for biomedical applications and biological atmospheres. Overall, it may be concluded that thermal spraying is a potentially suitable technique for biomedical applications to improve their microstructure, as well as mechanical, and biological properties [149, 150].
5.4 Laser deposition
Laser deposition is another popular method for coating biomedical implants which uses high power density laser with low frequency bandwidth. In this process, laser beam evaporates the material and deposits a layer of vaporized material on the implant’s surface. The ablated material generates a plasma plume of highly excited atoms, ions, electrons, and molecules because the focused pulse layer has a high energy density. By modifying the deposition rate, thickness of the coating is optimized. Arias et al. experimented with the adhesive characteristics of amorphous and crystalline hydroxyapatite as a coating material, and optimized the coating process and thickness of coating layers. This study concluded that the combination of amorphous and crystalline hydroxyapatite increases the adhesion and strength of coatings [151]. In another study, Blind et al. deposited the hydroxyapatite on titanium alloys to optimize the roughness and adhesion related parameters [152]. These hydroxyapatite based coatings have many applications in the field of biomedical implants which were reported by Hidalgo-Robatto et al. [153], Pradhaban et al. [154], Chen et al. [155] and others [156,157,158] (Fig. 4).
Schematics of thermal spray and laser deposition A melting of source material and formation of thermal spray for coating purpose B Utilization of laser beam to evaporate the source and deposition of material through laser deposition technique [159]
6 Discussion
Diseases are a part of nature and despite huge technological advancements in diagnostic and therapeutic strategies, the elimination of diseases could not be done. Although there had been huge improvements, the complete annihilation of diseases has yet to be achieved. One of the most important parts of the medical industry is biomedical implants which have been used to cure heart clogged/blocked arteries for decades and no one can deny their importance. Apart from their cardiovascular applications, these are widely used in the treatment of bone and dental issues. When an implant is placed inside the human body, it is expected to perform its desired functions without initiating thrombo-inflammatory cascades, immune response, and other undesired reactions which can cause multiple allergic reactions and implant-induced coagulation cascades resulting in implant failure leading towards reimplantation and in the worst case scenario, body part failure. To avoid such drastic consequences, researchers are required to introduce modifications or coatings which have ability to combat corrosion, fracture, toxicity, and poor mechanical strength of the implant. To overcome all these issues, the most suitable way is the deposition of the coating layer on a biomedical implant surface with a suitable material. For successful implant fabrication, there are two main factors that one must keep in mind: one is the material that needs to be coated on the implant and the second technique is to coat material on the implant.
Carbon is an excellent material that is also part of the foundations of life on the earth. It has been popular in the industries like fuel, heavy machinery, automobile, paints, plastics, and even the food industry. In addition, it has excellent hemocompatibility and biocompatibility which makes it a suitable candidate for biomedical applications. Carbon based surgical tools and heart valves have been reported to perform better than other materials. Carbon fiber is found to be an excellent wound healing gauze that has the ability to endure harsh environmental conditions. Carbon has also been used in stem cell technology and tissue regeneration and has shown promising results. The thin films and coatings of carbon may mimic the human body functions favorably and provide a suitable environment for the ibroblasts, osteoblasts, and macrophages at the lesion site without evoking any inflammatory or immune reaction. Because of its durability and hardness, it has been observed to improve the performance and load bearing properties of hip and knee joints which minimizes the rate of corrosion and material leaching, resulting in lesser debris formation. It also acts as a barrier to lock the metal components inside the implant and blocks the release of ions from metallic implants into body fluids. Due to wettability properties, carbon films have a tendency to prevent thrombogenicity by appropriately minimizing the platelet activation and adhesion. Additionally, the hemocompatibility and biocompatibility of carbon films can be improved by the incorporation of materials like silicon, titanium, nitrides, or oxides.
The use of modified carbon-based materials has gained popularity due to the enhancement of properties i.e., structural, electrical, thermal, mechanical, and optical. In addition, the introduction of nanotechnology augmented unique features which sparked the interest of researchers for biological applications. Due to the freedom of manufacturing any type, shape, or nanostructure of carbon, multiple types of functions were obtained; however, a particular focus was on drug delivery systems. The primary function of these systems targeted drug delivery and mimicked protein channels which helped to improve lesion healing. In medical implants, it helped to reduce the failure rate of implants. The adaptability and characteristics of carbon-based nanomaterials and coatings provide the solutions to issues like infections, delayed wound healing, and osseointegration. Furthermore, due to the enhancement of mechanical properties, the life span of implants is also increased.
Selection of technique for the surface modification and coating is an important factor which is selected based on the working environment, type of problem, and materials involved. There are many techniques that can be utilized to coat the material for example sol–gel, electrostatic deposition, thermal spraying, plasma spraying, dip coating, laser coating, physical and chemical vapor deposition, etc. In the current era, the widely used techniques for coating biomedical implants are physical vapor deposition, chemical vapor deposition, and plasma-enhanced chemical vapor deposition. Every technique carries some disadvantages along with many advantages. For example, chemical vapor deposition requires high temperatures to deposit a coating layer on the substrate; however, it can accommodate a large number of precursors and is widely used in coating different materials including TiN, carbon, and silicon. Despite many advantages, it may affect the physical and chemical nature of the substrate due to the utilization of high temperatures. To avoid this issue of high temperature, the new technique was introduced as plasma-enhanced chemical vapor deposition in which the deposition of thin film on organic or inorganic material at a relatively lower temperature than CVD by using electrical energy to produce plasma which ionizes the gas and free radicals are produced which undergo radical polymerization. Table 2 lists all the coating processes commonly utilized in biomedical implant coating. It summarizes all the benefits and drawbacks of employing various ways to deposit layers on biomedical implants. Based on the benefits and drawbacks, it is simple to determine which technique is most appropriate.
Although, carbon has been extensively studied and utilized in various fields of life, its applications are still limited in the field of biomedical devices and implants. Currently, only a handful of products are available in the market which are using carbon based technologies and coatings to improve the results and functions of devices. There is a need to develop cost effective and efficient methods for the production of nanostructures at the industrial level with controlled morphology. CNTs and carbon quantum dots are gaining popularity due to their efficiency and better results. In the future, these may be replacing traditional carbon fiber and drug delivery devices, particularly in vaccine delivery and gene therapy. These are also expected to be used as reinforcing materials to increase the mechanical strength and hardness of materials. In conclusion, carbon-based nanostructures and coatings would be utilized in a myriad of materials to improve the functions of materials and structures.
7 Conclusion
The excellent structural and functional properties of carbon allotropes make them suitable candidates for the passive coatings of biomedical devices and potentially provide a solution to existing problems of medical implants like thrombosis, uncontrolled proliferation, delayed wound healing, and inflammation at the lesion site. Due to its exceptional structural properties, carbon enhances the mechanical strength of materials and makes them able to withstand harsher conditions, both inside and outside the body. In addition, it has the ability to increase the material inertness and corrosion resistance of medical implants which reduces the material ion leaching and prevents the initiation of foreign body object reactions to the body; thus, reducing the chances of implant failure. Despite the availability of suitable material and other conditions, implants fail due to the selection of inappropriate coating techniques; therefore, the selection of a suitable coating process is important for the development of a successful implant. Physical and chemical vapor deposition techniques are considered appropriate for the coating of carbon materials due to their ease, cost-effectiveness, and efficiency. There are many other techniques that are suitable for coating medical implants like laser deposition and thermal sprays; however, each technique is associated with some limitations and drawbacks which effect the performance of medical devices. In a nutshell, the selection of appropriate carbon allotrope, carbon nanostructure, and coating technique are important factors that ultimately improve the hemocompatibility, biocompatibility, mechanical strength, and corrosion resistance of biomedical implants.
Data availability
All relevant data are included in the article and/or its supplementary information files.
References
Grainger DW et al (2013) Critical factors in the translation of improved antimicrobial strategies for medical implants and devices. Biomaterials 34:9237–9243
Arsiwala A, Desai P, Patravale V (2014) Recent advances in micro/nanoscale biomedical implants. J Controll Release 189:25–45
Hassija V, Chamola V, Bajpai BC, Naren, and S. Zeadally, (2021) Security issues in implantable medical devices: Fact or fiction? Sustaina Cities Soc 66:102552
Costa RC et al (2021) Microbial corrosion in titanium-based dental implants: how tiny bacteria can create a big problem? J Bio- Tribo-Corros 7:136
Guo T, Gulati K, Arora H, Han P, Fournier B, Ivanovski S (2021) Race to invade: understanding soft tissue integration at the transmucosal region of titanium dental implants. Dent Mater 37(5):816–831
Kelly CN et al (2021) High-strength, porous additively manufactured implants with optimized mechanical osseointegration. Biomaterials 279:121206
Zhang H, Li Z, Li W (2021) M2 macrophages serve as critical executor of innate immunity in chronic allograft rejection. Front Immunol 12:648539
Alhammadi SH, Burnside G, Milosevic A (2021) Clinical outcomes of single implant supported crowns versus 3-unit implant-supported fixed dental prostheses in Dubai Health Authority: a retrospective study. BMC Oral Health 21(1):171
Kheradmandfard M et al (2018) Significant improvement in cell adhesion and wear resistance of biomedical β-type titanium alloy through ultrasonic nanocrystal surface modification. J Alloy Compd 762:941–949
Abaricia JO, Farzad N, Heath TJ, Simmons J, Morandini L, Olivares-Navarrete RJ (2021) Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomater 133:58–73
Hasan A et al (2020) Surface design for immobilization of an antimicrobial peptide mimic for efficient anti-biofouling. Chem A Eur J 26:5789–5793
Doloff JC et al (2021) The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nat Biomed Eng 5(10):1115–1130
Apte G, Börke J, Rothe H, Liefeith K, Nguyen T-H (2020) Modulation of platelet-surface activation: current state and future perspectives. ACS Appl Bio Mater 3(9):5574–5589
Choi J et al (2022) Micro-textured silicone-based implant fabrication using electrospun fibers as a sacrificial template to suppress fibrous capsule formation. Biomater Adv 135:112687
Ratha I, Datta P, Balla VK, Nandi SK, Kundu B (2021) Effect of doping in hydroxyapatite as coating material on biomedical implants by plasma spraying method: a review. Ceram Int 47(4):4426–4445
Jiang P, Hou R, Zhu S, Guan S (2022) A robust calcium carbonate (CaCO3) coating on biomedical MgZnCa alloy for promising corrosion protection. Corros Sci 198:110–124
Quarterman JC, Geary SM, Salem AK (2021) Evolution of drug-eluting biomedical implants for sustained drug delivery. Eur J Pharma Biopharm 159:21–35
Quarterman JC, Geary SM, Salem AK (2021) Evolution of drug-eluting biomedical implants for sustained drug delivery. Eur J Pharm Biopharm 159:21–35
Manabe K, Nara H (2021) Construction of stable biological albumin/heparin multilayers for elastic coatings on hydrophobic antithrombogenic artificial blood vessels. Tribol Int 156:106843
Zhang B, Su Y, Zhou J, Zheng Y, Zhu D (2021) Toward a better regeneration through implant-mediated immunomodulation: harnessing the immune responses. Adv Sci 8(16):2100446
Srimaneepong V, Skallevold HE, Khurshid Z, Zafar MS, Rokaya D, Sapkota J (2022) Graphene for antimicrobial and coating application. Int J Mol Sci 23(1):499
Gaur M et al (2021) Biomedical applications of carbon nanomaterials: fullerenes, quantum dots, nanotubes, nanofibers, and graphene. Materials 14(20):5978
Cumont A, Pitt AR, Lambert PA, Oggioni MR, Ye H (2022) Properties, mechanism and applications of diamond as an antibacterial material. Funct Diam 1(1):1–28
Rothammer B et al (2021) Amorphous carbon coatings for total knee replacements—part i: deposition, Cytocompatibility, chemical and mechanical properties. Polymers 13(12):1952
Hassan S, Ali MN, Mir M, Ahmed A, Arshad M (2021) Development and evaluation of drug delivery patch for topical wound healing application. SN Appl Sci 3(10):825
Blyweert P, Nicolas V, Fierro V, Celzard A (2021) 3D printing of carbon-based materials: a review. Carbon 183:449–485
Ying B, Park S, Chen L, Dong X, Young EW, Liu X (2020) NanoPADs and nanoFACEs: an optically transparent nanopaper-based device for biomedical applications. Lab Chip 20(18):3322–3333
Maleki H, Azimi B, Ismaeilimoghadam S, Danti S (2022) Poly (lactic acid)-based electrospun fibrous structures for biomedical applications. Appl Sci 12(6):3192
Efe GÇ, Yenilmez E, Altinsoy İ, Türk S, Bindal C (2021) Characterization of UHMWPE-HAp coating produced by dip coating method on Ti6Al4V alloy. Surf Coat Technol 418:127091
Falcao EH, Wudl F (2007) Carbon allotropes: beyond graphite and diamond. J Chem Technol Biotechnol 82(6):524–531
Hirsch A (2010) The era of carbon allotropes. Nat Mater 9(11):868–871
Rajak DK, Kumar A, Behera A, Menezes PL (2021) Diamond-like carbon (DLC) coatings: classification, properties, and applications. Appl Sci 11(10):4445
M. E. Arslan et al. (2022) Structural, biocompatibility, and antibacterial properties of Ge–DLC nanocomposite for biomedical applications. Journal of Biomedical Materials Research n/a, no. n/a
Love CA, Cook RB, Harvey TJ, Dearnley PA, Wood RJK (2013) Diamond like carbon coatings for potential application in biological implants—a review. Tribol Int 63:141–150
Li C, Huang L, Yuan J (2020) Effect of sp3 content on adhesion and tribological properties of non-hydrogenated DLC films. Materials 13(8):1911
Paul R (2017) Diamond-like-carbon coatings for advanced biomedical applications. Global J Nanomed 2(5):555598
Hasebe T et al (2007) Fluorine doping into diamond-like carbon coatings inhibits protein adsorption and platelet activation. J Biomed Mater Res 83A(4):1192–1199
Gutensohn K et al (2000) In vitro analyses of diamond-like carbon coated stents: reduction of metal ion release, platelet activation, and thrombogenicity. Thromb Res 99(6):577–585
Maguire P et al (2005) Mechanical stability, corrosion performance and bioresponse of amorphous diamond-like carbon for medical stents and guidewires. Diam Relat mater 14(8):1277–1288
Polukhina A et al (2022) Cellular and molecular issues of hemo-and biocompatibility of diamond-like carbon films. a brief critical review. Cell Tissue Biol 16(1):1–14
Zhang M, Xie T, Qian X, Zhu Y, Liu X (2020) Mechanical properties and biocompatibility of Ti-doped diamond-like carbon films. ACS Omega 5(36):22772–22777
Ando K et al (2016) Prospective multi-center registry to evaluate efficacy and safety of the newly developed diamond-like carbon-coated cobalt–chromium coronary stent system. Cardiovasc Interv Ther 32:225–232
Gorzelanny C et al (2016) Silver nanoparticle-enriched diamond-like carbon implant modification as a mammalian cell compatible surface with antimicrobial properties. Sci Rep 6(1):22849
Ban M, Tobe S, Takeuchi L (2018) Effects of diamond-like carbon thin film and wrinkle microstructure on cell proliferation. Diam Relat Mater 90:194–201
Zhu W et al (2022) Preparation and characterization of diamond-like carbon (DLC) film on 316L stainless steel by microwave plasma chemical vapor deposition (MPCVD). Diam Relat Mater 122:108820
Zhang X et al (2019) Theoretical strength and rubber-like behaviour in micro-sized pyrolytic carbon. Nat Nanotechnol 14(8):762–769
Wang M, Guo L, Sun H (2019) Manufacture of Biomaterials. In: Narayan R (ed) Encyclopedia of biomedical engineering. Elsevier, Oxford, pp 116–134
Liu F, Shirasu K, Hashida T (2021) Epitaxial pyrolytic carbon coatings templated with defective carbon nanotube cores for structural annealing and tensile property improvement. J Mater Sci 56(34):19015–19028
Stanley J, Klawitter J, More R (2008) 26 - Replacing joints with pyrolytic carbon. In: Revell PA (ed) Joint replacement technology. Woodhead Publishing, pp 631–656
Salkeld SL, Patron LP, Lien JC, Cook SD, Jones DG (2016) Biological and functional evaluation of a novel pyrolytic carbon implant for the treatment of focal osteochondral defects in the medial femoral condyle: assessment in a canine model. J Orthop Surg Res 11(1):155
Bokros J, Gott V, La Grange L, Fadall A, Vos K, Ramos M (1969) Correlations between blood compatibility and heparin adsorptivity for an impermeable isotropic pyrolytic carbon. J Biomed Mater Res 3(3):497–528
Serino G, Gusmini M, Audenino AL, Bergamasco G, Ieropoli O, Bignardi C (2021) Multiscale characterization of isotropic pyrolytic carbon used for mechanical heart valve production. Processes 9(2):338
Mountfort K et al (2014) Cre8™ Unique Technology in Challenging Daily Practice: proceedings of a satellite symposium held at EuroPCR on 20th - 23rd May 2014 in Paris, (in eng). Interv Cardiol (London, England) 9(3):180–183
Carrié D et al (2012) A multicenter randomized trial comparing amphilimus-with paclitaxel-eluting stents in de novo native coronary artery lesions. J Am Coll Cardiol 59(15):1371–1376
Pivato CA, Leone PP, Petriello G, Sanz-Sanchez J, Chiarito M, Stefanini GG (2020) The Cre8 amphilimus-eluting stent for the treatment of coronary artery disease: safety and efficacy profile. Expert Rev Med Devices 17(4):267–275
Goodman SL, Tweden KS, Albrecht RM (1996) Platelet interaction with pyrolytic carbon heart-valve leaflets. J Biomed Mater Res: Off J Soc Biomater Jpn Soc Biomater 32(2):249–258
Ogwu AA, Okpalugo TIT, Ali N, Maguire PD, McLaughlin JAD (2008) Endothelial cell growth on silicon modified hydrogenated amorphous carbon thin films. J Biomed Mater Res 85B(1):105–113
Okpalugo TIT, Ogwu AA, Maguire PD, McLaughlin JAD (2004) Platelet adhesion on silicon modified hydrogenated amorphous carbon films. Biomaterials 25(2):239–245
Li M, Cheng Y, Zheng YF, Zhang X, Xi TF, Wei SC (2012) Surface characteristics and corrosion behaviour of WE43 magnesium alloy coated by SiC film. Appl Surf Sci 258(7):3074–3081
Forrestal BJ, Case BC, Yerasi C, Garcia-Garcia HM, Waksman R (2020) The Orsiro Ultrathin, bioresorbable-polymer sirolimus-eluting stent: a review of current evidence. Cardiovasc Revasc Med 21(4):540–548
Ploumen EH et al (2021) Acute myocardial infarction treated with novel Resolute Onyx and Orsiro stents in the randomized BIONYX trial. Catheter Cardiovasc Interv 98(2):E188–E196
Saito S et al (2019) BIOFLOW-IV, a randomised, intercontinental, multicentre study to assess the safety and effectiveness of the Orsiro sirolimus-eluting stent in the treatment of subjects with de novo coronary artery lesions: primary outcome target vessel failure at 12 months. EuroIntervention 15(11):e1006–e1013
Wang J, Ma F, Sun M (2017) Graphene, hexagonal boron nitride, and their heterostructures: properties and applications. RSC Adv 7(27):16801–16822
Zhao C et al (2020) Synthesis of graphene quantum dots and their applications in drug delivery. J Nanobiotechnol 18(1):142
Jiříčková A, Jankovský O, Sofer Z, Sedmidubský D (2022) Synthesis and applications of graphene Oxide, (in eng). Materials (Basel) 15(3):920
Podila R, Moore T, Alexis F, Rao A (2013) Graphene coatings for biomedical implants, (in eng). J Vis Exp. https://doi.org/10.3791/50276
Reina G, González-Domínguez JM, Criado A, Vázquez E, Bianco A, Prato M (2017) Promises, facts and challenges for graphene in biomedical applications. Chem Soc Rev 46(15):4400–4416
Yang M-C et al (2019) Electrochemical polymerization of PEDOT–Graphene Oxide-heparin composite coating for anti-fouling and anti-clotting of cardiovascular stents. Polymers 11(9):1520
Ge S et al (2019) Inhibition of in-stent restenosis after graphene oxide double-layer drug coating with good biocompatibility. Regen Biomater 6(5):299–309
Shen H, Zhang L, Liu M, Zhang Z (2012) “Biomedical applications of graphene,” (in eng). Theranostics 2(3):283–294
Wu L, Yang H, Cheng J, Hu C, Wu Z, Feng Y (2021) Review in preparation and application of nickel-coated graphite composite powder. J Alloy Compd 862:158014
Kozbial A et al (2014) Understanding the intrinsic water wettability of graphite. Carbon 74:218–225
Shahriarinour M, Rahimi F, Siahbani E, Kochakinejad R, Kaki S (2022) A new electrochemical modified graphite pencil electrode developed for cholesterol assessing. J Irani Chem Soc 19(1):159–171
Tang X, Yang A, Li L (2022) Optimization of Nanofiber Wearable Heart Rate Sensor Module for Human Motion Detection," Computational Mathematical Methods in Medicine 2022
de Lima LF, de Freitas ADS, Ferreira AL, Maciel CC, Ferreira M, WR de, (2022) Enzymeless glucose sensor based on disposable Ecoflex®/graphite thermoplastic composite substrate modified with Au@ GQDs. Sens Actuators Rep 4:100102
Knoell A, Maxwell H, Bechtol C (1975) Graphite fiber reinforced bone cement. Ann Biomed Eng 3(2):225–229
Hung W-C, Wu K-H, Lyu D-Y, Cheng K-F, Huang W-C (2017) Preparation and characterization of expanded graphite/metal oxides for antimicrobial application. Mater Sci Eng: C 75:1019–1025
Oveissi F, Naficy S, Lee A, Winlaw D, Dehghani F (2020) Materials and manufacturing perspectives in engineering heart valves: a review. Materials Today Bio 5:100038
Kharlamova MV, Kramberger C (2021) Applications of filled single-walled carbon nanotubes: progress, challenges, and perspectives, (in eng). Nanomaterials (Basel) 11(11):2863
Yang Z et al (2010) Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease. Nanomed Nanotechnol Biol Med 6(3):427–441
Chen J, Chen S, Zhao X, Kuznetsova LV, Wong SS, Ojima I (2008) Functionalized single-walled carbon nanotubes as rationally designed vehicles for tumor-targeted drug delivery. J Am Chem Soc 130(49):16778–16785
Yola ML, Atar N (2021) Novel voltammetric tumor necrosis factor-alpha (TNF-α) immunosensor based on gold nanoparticles involved in thiol-functionalized multi-walled carbon nanotubes and bimetallic Ni/Cu-MOFs. Anal Bioanal Chem 413(9):2481–2492
Serafín V et al (2019) Graphene quantum dots-functionalized multi-walled carbon nanotubes as nanocarriers in electrochemical immunosensing. Determination of IL-13 receptor α2 in colorectal cells and tumor tissues with different metastatic potential. Sens Actuators B: Chem 284:711–722
Morais RP et al (2020) Naringenin-functionalized multi-walled carbon nanotubes: a potential approach for site-specific remote-controlled anticancer delivery for the treatment of lung cancer cells. Int J Mol Sci 21(12):4557
Ozgen PSO, Atasoy S, Kurt BZ, Durmus Z, Yigit G, Dag A (2020) Glycopolymer decorated multiwalled carbon nanotubes for dual targeted breast cancer therapy. J Mater Chem B 8(15):3123–3137
Sivaraj D, Vijayalakshmi K (2019) Novel synthesis of bioactive hydroxyapatite/f-multiwalled carbon nanotube composite coating on 316L SS implant for substantial corrosion resistance and antibacterial activity. J Alloy Compd 777:1340–1346
Xavier SA, Vijayalakshmi U (2018) Electrochemically grown functionalized-Multi-walled carbon nanotubes/hydroxyapatite hybrids on surgical grade 316L SS with enhanced corrosion resistance and bioactivity. Colloids Surf B: Biointerfaces 171:186–196
Vardharajula S et al (2012) “Functionalized carbon nanotubes: biomedical applications,” (in eng). Int J Nanomed 7:5361–5374
Murugesan R, Raman S (2022) Recent trends in carbon nanotubes based prostate cancer therapy: a biomedical hybrid for diagnosis and treatment. Curr Drug Deliv 19(2):229–237
Wee Y, Park S, Kwon YH, Ju Y, Yeon K-M, Kim J (2019) Tyrosinase-immobilized CNT based biosensor for highly-sensitive detection of phenolic compounds. Biosensors Bioelectronics 132:279–285
Kaur J, Gill GSS, Jeet K (2019) Applications of carbon nanotubes in drug delivery: A comprehensive review. Characterization biology of nanomaterials for drug delivery 113-135
Kiran AR, Kumari GK, Krishnamurthy PT (2020) Carbon nanotubes in drug delivery: focus on anticancer therapies. J Drug Deliv Sci Technol 59:101892
Simon J, Flahaut E, Golzio M (2019) Overview of carbon nanotubes for biomedical applications. Materials 12(4):624
Pahlevanzadeh F et al (2021) CNT and rGO reinforced PMMA based bone cement for fixation of load bearing implants: mechanical property and biological response. J Mech Behav Biomed Mater 116:104320
Francis AA, Abdel-Gawad SA, Shoeib MA (2021) Toward CNT-reinforced chitosan-based ceramic composite coatings on biodegradable magnesium for surgical implants. J Coat Technol Res 18(4):971–988
Mondal T et al (2022) Thin films of functionalized carbon nanotubes support long-term maintenance and cardio-neuronal differentiation of canine induced pluripotent stem cells. Nanomed Nanotechnol Biol Med 40:102487
Li T, Dorn HC (2017) Biomedical applications of metal-encapsulated fullerene nanoparticles. Small 13(8):1603152
Zhang Q et al (2009) Autophagy-mediated chemosensitization in cancer cells by fullerene C60 nanocrystal. Autophagy 5(8):1107–1117
Bhakta P, Barthunia B (2020) Fullerene and its applications: a review. J Indian Acad Oral Med Radiol 32(2):159
Buschbeck EK, Le Duc A, Kelley C, Hoque MA, Alvarez NT (2021) Functionalized carbon nanotube microfibers for chronic neural implants. J Neurosci Methods 364:109370
Bronner C (2018) Bottom-up synthesis and electronic structure of graphene nanoribbons on surfaces. In: Wandelt K (ed) Encyclopedia of interfacial chemistry. Elsevier, Oxford, pp 210–225
Burdanova MG, Kharlamova MV, Kramberger C, Nikitin MP (2021) Applications of pristine and functionalized carbon nanotubes, graphene, and graphene nanoribbons in biomedicine. Nanomaterials 11(11):3020
Zakharova OV, Mastalygina EE, Golokhvast KS, Gusev AA (2021) Graphene nanoribbons: prospects of application in biomedicine and toxicity. Nanomaterials 11:2425
Barui AK et al (2012) Zinc oxide nanoflowers make new blood vessels. Nanoscale 4(24):7861–7869. https://doi.org/10.1039/C2NR32369A
Negahdary M, Heli H (2018) Applications of nanoflowers in biomedicine. Recent Pat Nanotechnol 12(1):22–33
Fatima SW, Imtiyaz K, Alam Rizvi MM, Khare SK (2021) Microbial transglutaminase nanoflowers as an alternative nanomedicine for breast cancer theranostics," (in eng). RSC Adv 11(55):34613–34630
Fang Y, Wang S, Liu Y, Xu Z, Zhang K, Guo Y (2018) Development of Cu nanoflowers modified the flexible needle-type microelectrode and its application in continuous monitoring glucose in vivo. Biosens Bioelectron 110:44–51
Madannejad R, Shoaie N, Jahanpeyma F, Darvishi MH, Azimzadeh M, Javadi H (2019) Toxicity of carbon-based nanomaterials: reviewing recent reports in medical and biological systems. Chemico-Biol Interact 307:206–222
Fedel M, Wong TT, Speranza G, Lohberger B, Nogler M, Awaja F (2019) Hybrid graphene oxide/amorphous carbon coatings and their effect on the viability and toxicity of different cell types. Surf Coat Technol 374:95–102
Delorme MP et al (2012) Ninety-day inhalation toxicity study with a vapor grown carbon nanofiber in rats). Toxicol Sci 128(2):449–460
Pauluhn J (2010) Subchronic 13-week inhalation exposure of rats to multiwalled carbon nanotubes: toxic effects are determined by density of agglomerate structures, not fibrillar structure. Toxicol Sci 113(1):226–242
Holmannova D, Borsky P, Svadlakova T, Borska L, Fiala Z (2022) Carbon nanoparticles and their biomedical applications. Appl Sci 12(15):7865
Maiti D, Tong X, Mou X, Yang K (2019) Carbon-based nanomaterials for biomedical applications: a recent study. Front Pharmacol. https://doi.org/10.3389/fphar.2018.01401
Creighton J, Ho P (2001) Introduction to chemical vapor deposition (CVD). Chem Vap Depos 2:1–22
Kheradmandfard M et al (2022) Exceptional improvement in the wear resistance of biomedical β-type titanium alloy with the use of a biocompatible multilayer Si/DLC nanocomposite coating. Ceram Int 48(12):17376–17384
Song SH, Min BK, Hong M-H, Kwon T-Y (2020) Application of a novel CVD TiN coating on a Biomedical Co–Cr alloy: an evaluation of coating layer and substrate characteristics. Materials 13(5):1145
Anisur MR, Chakraborty Banerjee P, Easton CD, Singh Raman RK (2018) Controlling hydrogen environment and cooling during CVD graphene growth on nickel for improved corrosion resistance. Carbon 127:131–140
Johnson AP, Gangadharappa H, Pramod K (2020) Graphene nanoribbons: a promising nanomaterial for biomedical applications. J Control Release 325:141–162
Zhang Q et al (2021) Fabrication of a gradient hydrophobic surface with parallel ridges on pyrolytic carbon for artificial heart valves. Colloids Surf B: Biointerfaces 205:111894
Li H, Zhang LL, Li K-Z, He YG, Zhao XN, Zhao H (2009) Response of MG63 osteoblast-like cells on pyrolytic carbon coated carbon/carbon composites. Mater Sci Forum 620–622:579–582
Hassan S, Ali MN, Ghafoor B (2022) Evolutionary perspective of drug eluting stents: from thick polymer to polymer free approach. J Cardiothorac Surg 17:65
Chowdhury MSI et al (2020) Wear performance investigation of PVD coated and uncoated carbide tools during high-speed machining of TiAl6V4 aerospace alloy. Wear 446–447:203168
Vega J, Scheerer H, Andersohn G, Oechsner M (2018) Experimental studies of the effect of Ti interlayers on the corrosion resistance of TiN PVD coatings by using electrochemical methods. Corros Sci 133:240–250
Krella AK (2021) Cavitation erosion of monolayer PVD coatings – An influence of deposition technique on the degradation process. Wear 478–479:203762
Velusamy R, Ramakrishna S (2021) An in-vitro evaluation study on the effects of surface modification via physical vapor deposition on the degradation rates of magnesium-based biomaterials. Surf Coat Technol 411:126972
Bahi R, Nouveau C, Beliardouh NE, Ramoul CE, Meddah S, Ghelloudj O (2020) Surface performances of Ti-6Al-4V substrates coated PVD multilayered films in biological environments. Surf Coat Technol 385:125412
Nißen S, Heeg J, Wienecke M, Behrend D, Warkentin M (2018) Enhancing adhesion strength of a-C:H: Cu composite coatings on Ti6Al4V by graded copper deposition in a rf-PVD/PECVD hybrid process. Surf Coat Technol 350:659–671
Christy RW (1960) Formation of thin polymer films by electron bombardment. J Appl Phys 31(9):1680–1683
Baker AG, Morris WC (1961) Deposition of metallic films by electron impact decomposition of organometallic vapors. Rev Sci Instrum 32(4):458–458
Alt LL, Ing SW Jr, Laendle KW (1963) Low-temperature deposition of silicon oxide films. J Electrochem Soc 110(5):465
Mattox DM (2020) Handbook of physical vapor deposition (PVD) processing. Elsevier, pp 287–300
Eurídice WA et al (2020) a-C: H films produced by PECVD technique onto substrate of Ti6Al4V alloy: chemical and biological responses. Appl Surf Sci 503:144084
Fares C et al (2020) Demonstration of a SiC protective coating for titanium implants. Materials 13(15):3321
Huran J, Hrubcin L, Kobzev A, Liday J (1996) Properties of amorphous silicon carbide films prepared by PECVD. Vacuum 47(10):1223–1225
Bolz A, Amon M, Ozbek C, Heublein B, Schaldach M (1996) Coating of cardiovascular stents with a semiconductor to improve their hemocompatibility. Tex Heart Inst J 23(2):162
Frewin CL, Coletti C, Register JJ, Nezafati M, Thomas S, Saddow SE (2015) Silicon carbide materials for biomedical applications. In: Demarchi D, Tagliaferro A (eds) Carbon for Sensing Devices. Springer International Publishing, Cham, pp 153–207
Saddow SE, Frewin C, Reyes M, Register J, Nezafati M, Thomas S (2014) 3C-SiC on Si: a biocompatible material for advanced bioelectronic devices. ECS Trans 61:101–111
Fraga M, Pessoa R, Maciel H, Massi M (2011) Recent Developments on Silicon Carbide Thin Films for Piezoresistive Sensors Applications. M. Mukherjee, Ed.: Intech, pp. 369-388
Lan P, Nunez E, Polycarpou A (2019) “Advanced polymeric coatings and their applications: green tribology,” in Reference Module in Materials Science and Materials Engineering, 2019
Gu Y, Khor K, Pan D, Cheang P (2004) Activity of plasma sprayed yttria stabilized zirconia reinforced hydroxyapatite/Ti–6Al–4V composite coatings in simulated body fluid. Biomaterials 25(16):3177–3185
Zheng X, Huang M, Ding C (2000) Bond strength of plasma-sprayed hydroxyapatite/Ti composite coatings. Biomaterials 21(8):841–849
Yang Y, Ong JL, Tian J (2003) Deposition of highly adhesive ZrO2 coating on Ti and CoCrMo implant materials using plasma spraying. Biomaterials 24(4):619–627
Gérard B (2006) Application of thermal spraying in the automobile industry. Surf Coat Technol 201(5):2028–2031
Kumar S, Handa A, Chawla V, Grover NK, Kumar R (2021) Performance of thermal-sprayed coatings to combat hot corrosion of coal-fired boiler tube and effect of process parameters and post-coating heat treatment on coating performance: a review. Surf Eng 37(7):833–860
Kumar S, Kumar R (2021) Influence of processing conditions on the properties of thermal sprayed coating: a review. Surf Eng 37(11):1339–1372
Gadow R, Killinger A, Stiegler N (2010) Hydroxyapatite coatings for biomedical applications deposited by different thermal spray techniques. Surf Coat Technol 205:1157–1164
Prashar G, Vasudev H (2020) Thermal sprayed composite coatings for biomedical implants: a brief review. J Therm Spray Eng 2(1):50–55
Vilardell AM et al (2020) In-vitro comparison of hydroxyapatite coatings obtained by cold spray and conventional thermal spray technologies. Mater Sci Eng C 107:110306
Jagadeeshanayaka N, Awasthi S, Jambagi SC, Srivastava C (2022) Bioactive surface modifications through thermally sprayed hydroxyapatite composite coatings: a review of selective reinforcements. Biomater Sci 10:2484–2523. https://doi.org/10.1039/D2BM00039C
Prashar G, Vasudev H, Thakur L, Bansal A. Performance of thermally sprayed Hydroxyapatite coatings for biomedical implants: A comprehensive review 0, no. ja, p. null.
Arias JL, Mayor MB, Pou J, Leng Y, León B, Pérez-Amor M (2003) Micro-and nano-testing of calcium phosphate coatings produced by pulsed laser deposition. Biomaterials 24(20):3403–3408
Dailey OBLKB, Jordan L (2005) Characterization of hydroxyapatite films obtained by pulsed-laser deposition on Ti and Ti-6Al-4V. Dent Mater 21:1017–1024
Hidalgo-Robatto BM et al (2018) Pulsed laser deposition of copper and zinc doped hydroxyapatite coatings for biomedical applications. Sur Coat Technol 333:168–177
Pradhaban G, Kaliaraj GS, Vishwakarma V (2014) Antibacterial effects of silver–zirconia composite coatings using pulsed laser deposition onto 316L SS for bio implants. Prog Biomater 3(2):123–130
Chen L, Komasa S, Hashimoto Y, Hontsu S, Okazaki J (2018) In vitro and in vivo osteogenic activity of titanium implants coated by pulsed laser deposition with a thin film of fluoridated hydroxyapatite. Int J Mol Sci 19(4):1127
Li M, Komasa S, Hontsu S, Hashimoto Y, Okazaki J (2022) Structural characterization and osseointegrative properties of pulsed laser-deposited fluorinated hydroxyapatite films on nano-zirconia for implant applications. Int J Mol Sci 23(5):2416
Teghil R, Curcio M, De Bonis A (2021) Substituted hydroxyapatite, glass, and glass-ceramic thin films deposited by nanosecond pulsed laser deposition (PLD) for biomedical applications: a systematic review. Coatings 11(7):811
Joshi P et al (2022) Laser-patterned carbon coatings on flexible and optically transparent plastic substrates for advanced biomedical sensing and implant applications. J Mater Chem C 10(8):2965–2975
Vanalakar SA, Galal A, Singh VN, Min H (2018) A review of nanostructured thin films for gas sensing and corrosion protection. Mediterr J Chem 7:433–451
Acknowledgements
The authors acknowledge Bakhtawar Gafoor and Dr Mariam Mir for their valuable feedback and support during data collection and manuscript preparation.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, S.H. and H.Q.; data curation, S.H. and A.Y.N; writing, original draft preparation, S.H, H.Q. A.N.Y. and K. K.; writing, review and editing, S.H., K.K. A.A, A.S.K. and A.A.; supervision, A.S.K. and A.A. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hassan, S., Nadeem, A.Y., Qaiser, H. et al. A review of carbon-based materials and their coating techniques for biomedical implants applications. Carbon Lett. 33, 1171–1188 (2023). https://doi.org/10.1007/s42823-023-00496-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-023-00496-1