Abstract
Refined structured tin dioxide gets the amount of attraction because of its low cost and stability. The C@SnO2 nanospheres with mesoporous structures were produced using the hard template method in this work. The C@SnO2 is primarily gained attributed to the dehydration condensation of C6H12O6 and the hydrolysis of SnCl4·5H2O. The morphology of the C@SnO2 was analyzed by physical characterization and the diameter of the obtained C@SnO2 was around 138 nm. When C@SnO2 was applied to lithium-ion batteries as anode material, it performed outstanding electrochemical properties, with a capacity of 735 and 539 mA h g−1 maintained at 1000 and 2000 mA g−1, respectively. Furthermore, it exhibits favorable discharge/charge cycle stability. This is probably because of the more chemically redox active sites provided by C@SnO2 nanocomposites and it also allows fast ion diffusion and electron migration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lithium-ion batteries (LIBs) represent a promising electrochemical energy storage and conversion device due to their good cycle tolerance, high energy density and low cost [1,2,3]. Lithium resource reserves, harsh use environment and safety are the great challenges facing lithium-ion batteries these days. Carbon-based materials are a widely used as anode material owing to their inexpensive price, strong electrical conductivity and high crystallinity. Considerable efforts have been made in the research of carbon substrate anode materials. Kang et al. [4] synthesized the three-dimensional nanostructured material. The results showed outstanding electrochemical properties such as long cycle life and large specific capacity. Gwon and co-workers [5] fabricate the flexible framework material. The graphene-based flexible electrode exhibits excellent electrochemical performances. And this is attributed to the distinctive flexible architecture. However, its poor compatibility with electrolyte affects its wide application [6, 7].
Tin-based materials have the merits of rich resources and high theoretical capacity, and are a favorable candidate for anode materials. However, their structural instability and low electrical conductivity lead to their poor practical performance [8, 9]. Song et al. [10] synthesized a hierarchical SnO2 nanoflower. The results indicated that it showed excellent electrochemical properties. The reversible charge capacity of the sample was maintained at 350.7 mAh g−1 after 30 charge/discharge cycles. Zhang et al. [11] successfully obtained a self-supported tin dioxide anode. The results demonstrate that it exhibits high capacity and favorable cycling tolerance. SnO2 serves as a buffer layer that not only ensures the contact between the active material and the substrate but also allows to contain the volume change during cycling. To overcome these drawbacks, researchers have employed various solutions to address them. In particular, the fabrication of compounds with stable structures is considered as an attractive solution to modify the inherent properties of pristine materials. The porous nano core–shell structure can enhance the battery performance by providing more redox active sites with large surface area, shortening the lithium-ion diffusion distance, and alleviating the volume change during cycling [12, 13].
In this work, C@SnO2 nanocomposites with C as the core and SnO2 as the shell were obtained using the hard template method. When C@SnO2 are applied to a lithium-ion battery, it exhibits excellent electrochemical properties, with C@SnO2 capacity maintained at 735 and 539 mA h g−1 at high current densities of 1000 mA g−1 and 2000 mA g−1. In addition, it shows good discharge/charge cycling stability.
2 Experimental
2.1 Material
Glucose (C6H12O6); tin chloride pentahydrate (SnCl4·5H2O); ethanol (C2H6O, 98%), All chemical reagents were purchased from Aladdin Biochemical Technology Co., Ltd and were used directly without any treatment.
2.2 Synthesis of carbon nanosphere
In a typical synthesis, 3.24 g of C6H12O6 was dissolved into 30 mL of de-ionized water. The solution was then transferred into a 40 mL Teflon-lined stainless steel autoclave, sealed, and kept at 170 °C for 6 h in an oven. The obtained black products was washed three times with de-ionized water and ethanol, respectively. Finally, the C spheres were dried.
2.3 Synthesis of C@SnO2 nanosphere
To prepare the C@SnO2 nanocomposites, the as-prepared C spheres were dispersed in 30 ml of anhydrous ethanol, and 0.41 g of SnCl4·5H2O was added. The solution was then transferred into a 40 ml Teflon-lined stainless steel autoclave, sealed, and kept at 160 °C for 4 h to obtain a white product in an oven. The products were centrifuged and washed three times with deionized water and ethanol at 4500 rpm. The resulting product was gained by drying at 60 °C keeping 4 h.
2.4 Materials characterization
The micromorphology of the C@SnO2 nanoparticles were observed by field emission scanning electron microscopy (FE-SEM, Hitachi S-4800) and field-emission transmission electron microscopy (FE-TEM, JEOL JEM-2100F). The crystal structure of the sample was performed by X-ray diffractometry (XRD, Rigaku MiniFlex600 X-ray generator, Cu Kα radiation, λ = 1.5406 Å). The chemical composition and valence of the C@SnO2 nanocomposites were analyzed by X-ray photoelectron spectrometry (XPS, Perkin Elmer PHI1600 ESCA). The specific surface area and pore diameter distribution were tested by a specific surface and pore size analyzer (JW-BK200B).
2.5 Electrochemical characterization
Electrochemical tests were performed using CR2032-type coin cells. To prepare the C@SnO2 electrodes, active material, acetylene black and polyvinylidene fluoride (PVDF) with a weight ratio of 7:2:1 were mixed with Nmethyl-2-pyrrolidone (NMP) to form a slurry. The slurry is spread evenly on the copper foil. The lithium metal foil was used as a counter electrode. The electrolyte was a 1.0 M solution of LiPF6 dissolved in ethylene carbonate and dimethyl carbonate (volume ratio of 1:1). Coin-type lithium half-cells were assembled with glove boxes in an argon atmosphere with both water and oxygen content below 0.1 ppm. Besides, the cycling performance and rate performance were evaluated by battery-testing system (Land CT4008T). The CHI760E electrochemical workstations are used to record cyclic voltammetry (CV) curves and electrochemical impedance spectroscopy (EIS). All electrochemical tests were performed at room temperature (Fig. 1).
3 Results and discussion
In this experiment, carbon nanospheres were fabricated by the hydrothermal method using glucose as a precursor. Glucose first loses water via the hydrothermal treatment and followed by an aromatization process. The obtained products are black insoluble matters and other substances, including small molecules of aldehydes, acids and gases, etc. (CO2, CH4). The surface of the carbon sphere contains a great amount of oxygen-containing functional groups, such as –OH, C=O and –COOH. At the appropriate temperature, metal cations can be adsorbed onto the surface of the carbon nanospheres by intermolecular forces without surface modification, so that a uniform SnO2 layer can be formed on the surface [14]. This core–shell structure also provides a synthetic strategy for other substances.
Figure 2a, d shows the surface morphology of the carbon spheres (SEM and TEM). As shown in Fig. 2a, d, the carbon nanospheres are approximately 115 nm in diameter. To mitigate the volume expansion of the carbon spheres during cycling, a C@SnO2 core–shell structure was obtained by hydrothermal treatment, as shown in Fig. 2b, c, e. It exhibits the structural integrity of C@SnO2 with a diameter of about 138 nm. The core–shell structure not only shortens the ion diffusion path and accelerates electron transfer, but also alleviates the bulk effect to a degree. Figure 2e indicates SnO2 nanoparticles attached to the surface of the carbon spheres. The HR-TEM in the Fig. 2f illustrates that the lattice spacing is 0.335 nm, which are in consistent with the (110) plane of SnO2.
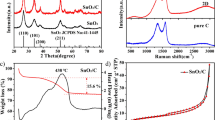
The degree of crystallinity of the C, SnO2 and C@SnO2 were evaluated by X-ray diffraction (XRD) patterns (Fig. 3a). The diffraction peaks located at 26.6°, 33.9°, 37.9° and 51.8° correspond to the typical diffraction peaks of SnO2 (JCPDS No. 41-1445) [15]. The broad peaks at 2θ = 5° to 30° indicate that the carbon is not very crystalline and consists mainly of amorphous carbon [16]. Meanwhile, the absence of other characteristic peaks can be seen in Fig. 3a, demonstrating its high purity.
Figure 4a shows the N2 adsorption–desorption isotherms of C and C@SnO2, it indicates that C@SnO2 is a typical type IV isotherm with a distinct hysteresis loop, which demonstrates that they have a micro/mesoporous structure [17]. In addition, the obtained C@SnO2 has a surface area of 313 m2 g−1, which is superior to porous carbon 152 m2 g−1. Figure 4b shows a pore size for C@SnO2. The porous structure moderates the volume expansion during charging and discharging to a certain extent and also shortens the diffusion distance of ions and the path of electron transfer. In addition, the porous compounds improve the surface area of the electrode material and increase the redox-active sites on the surface, thereby enhancing battery performance.
The chemical composition and valence of the C@SnO2 are investigated by X-ray photoelectron spectrometry (XPS) in Fig. 5a. The peaks of O, Sn and C are shown in Fig. 5b–d, which is in accordance with the XRD results. The high-resolution O 1 s spectrum of the sample (Fig. 5b) consists of three dominant peaks for Sn–O (530.5 eV), C=O (532.6 eV) and -OH of H2O (533.4 eV), demonstrating the successful bridging of C and SnO2. The Sn 3d spectra of the C@SnO2 exhibited the predominant signal of Sn 3d5/2 and Sn 3d3/2 with 486.6 and 495.0 eV of Sn, respectively, suggesting the generation of Sn4+. The peaks at 284.5 and 286.2 eV are assigned to C–C sp2 hybridization and C-O bonding.
The cyclic voltammetry (CV) was tested from 0.01 V to 3.0 V at a scan rate of 0.1 mV s-1, as shown in Fig. 6a. It reveals that the obvious reduction peak occurs at around 0.6 V and the intensity of the peak gradually decreases as the cycle continues. This is largely due to the generation of solid electrolyte interface (SEI) layers as well as the conversion process of tin-containing substances. The reduction peaks of the C@SnO2 at 0.1 V and 0.6 indicate the alloying and dealloying reactions of the Sn. Figure 6a displays that C@SnO2 nanocomposites are in accordance with each other in subsequent cycles, demonstrating the generation of SEI films and the excellent cycling property of the C@SnO2 anodes. The electrochemical reactions that occur during the charge and discharge are shown below [18]:
The galvanostatic discharge/charge profiles in Fig. 6b present that the capacity of the compound at different cycles for 0.5 A g−1. The galvanostatic discharge/charge curves of the corresponding carbon spheres are shown in Figure S1. The initial discharge/charge capacities of the compound are 1503.9 and 853.2 mA h g−1, and its initial Coulomb efficiency was 57%. The greater capacity decay is mostly ascribed to two factors. On the one hand, a stable SEI film is generated on the solid surface, and on the other hand, Li2O is formed. The two charge plateaus at 0.51 and 1.25 V (Fig. 6b) are mainly owing to the dealloying of LixSn and the generation of SnO2. Interestingly, the discharge/charge curves are nearly consistent for the later cycle in the composite electrode, demonstrating its outstanding cycling tolerance. The result is in agreement with the CV.
The good cycling performance of the composite nanoparticles was investigated with a battery test instrument (LAND CT4008T) in the potential range of 0.01–3.0 V (Fig. 6c), and the cycling curves were obtained for 1000 and 2000 mA g−1, respectively. The discharge capacity of the compound anode after cycling is 735 mA h g−1 at 1000 mA g−1. Besides, the composite electrode still possesses a discharge capacity of 539 mA h g−1 at 2000 mA g−1, indicating its excellent cycling properties.
To survey the rate performance of the composite electrode, the rate capability of the C@SnO2 electrode at different current densities was investigated (Fig. 6d). The rate performance for C and SnO2 as shown in Figure S2.The discharge capacities of composite nanosphere electrodes at different current densities are 1045, 933, 852, 798 and 690 mA h g−1 in Fig. 6d, indicating that the C@SnO2 nanosphere electrodes have excellent rate stability. This may be attributed to the mesoporous structure and the refined SnO2 nanocrystals. C can also work as highly conductive substrates. Furthermore, the mesoporous structure can allow fast ion diffusion and electron migration. These results indicate that the C@SnO2 is a promising electrode material due to the good rate performance at different current densities.
The cycling stability of the C/SnO2 was investigated by electrochemical impedance spectroscopy (EIS). The EIS was gained in the frequency range of 106–10−3 Hz with a voltage amplitude of 5 mV. The C/SnO2 nanosphere electrode before cycling for 500 mA g−1 are shown in Fig. 7a. The radius of the composite curve are smaller than after cycles. Here, the C/SnO2 electrode shows a low charge transfer resistance (Rct) of 59.8 Ω, indicating easy electron transfer at the solid–liquid interface. After the cycling test, the Rct increased to 89.6 Ω, which is close to the Rct before cycling. These results indicate that the structure is stable and it is beneficial for the reaction kinetics.
Figure 7b displays the surface morphology of the C/SnO2 nanocomposites after cycling. It indicated that the majority of the original morphology was maintained in the architecture, and only a slight change has occurred in a few samples, which revealed the structural stability. The superior performance is probably attributed to the sufficient deformation capacity of the mesoporous material to moderate the surface and the bulk changes during the charging and discharging process.
4 Conclusions
In summary, C@SnO2 nanocomposites anode material was prepared using the hard template method in this paper. The C@SnO2 nanocomposites were gained in an aqueous solution using carbon as a template and tin dioxide as a precursor, respectively. And the specific surface areas of the C and C@SnO2 samples were 313 and 152 m2 g−1, respectively. When C@SnO2 was applied to lithium-ion batteries as anode material, it performed outstanding electrochemical properties, with a capacity of 735 and 539 mA h g−1 maintained at 1000 and 2000 mA g−1, respectively. Furthermore, it exhibits favorable discharge/charge cycle stability. This may be attributed to the unique features of the porous nanocomposite structure, which not only increases the specific surface area of the anode material but also mitigates the changes in surface and bulk during the electrochemical reaction process. Thus, such a structural design is also an effective strategy to enhance battery properties.
Data availability
No data was used for the research described in the article.
References
Kim D, Kim KH, Lim C (2022) Carbon-coated SiOx anode materials via PVD and pyrolyzed fuel oil to achieve lithium-ion batteries with high cycling stability. Carbon Lett 32(1):321–328
Wang H, Wang Y, Li Y (2015) Exceptional electrochemical performance of nitrogen-doped porous carbon for lithium storage. Carbon 82:116–123
Xie L, Tang C, Bi Z (2021) Hard carbon anodes for next-generation li-ion batteries: review and perspective. Adv Energ Mater 11(38):2101650
Kang C, Lahiri I, Baskaran R (2012) 3-dimensional carbon nanotube for Li-ion battery anode. J Power Sources 219:364–370
Gwon H, Kim HS, Lee KU (2011) Flexible energy storage devices based on graphene paper. Energ Environ Sci 4(4):1277–1283
Landi BJ, Ganter MJ, Cress CD (2009) Carbon nanotubes for lithium ion batteries. Energ Environ Sci 2(6):638–654
Jia X, Chen Z, Cui X (2012) Building robust architectures of carbon and metal oxide nanocrystals toward high-performance anodes for lithium-ion batteries. ACS Nano 6(11):9911–9919
Huang B, Li X, Pei Y (2016) Novel carbon-encapsulated porous SnO2 anode for lithium-ion batteries with much improved cyclic stability. Small 12(14):1945–1955
Chen W, Song K, Mi L et al (2017) Synergistic effect induced ultrafine SnO2/graphene nanocomposite as an advanced lithium/sodium-ion batteries anode. J Mater Chem A 5:10027–10038
Song L, Yang S, Wei W et al (2015) Hierarchical SnO2 nanoflowers assembled by atomic thickness nanosheets as anode material for lithium ion battery. Sci Bull 60(9):892–895
Zhang F, Teng X, Shi W et al (2020) SnO2 nanoflower arrays on an amorphous buffer layer as binder-free electrodes for flexible lithium-ion batteries. Appl Surf Sci 527:146910
Tang Y, Lang L, Zhao H et al (2016) Porous CNT@Li4Ti5O12 coaxial nanocables as ultra high power and long life anode materials for lithium ion batteries[J]. J Mater Chem A 4:2089–2095
Liang J, Yu XY, Zhou H et al (2014) Bowl-like SnO2@carbon hollow particles as an advanced anode material for lithium-ion batteries. Angew Chem Int Edit 53:1–6
Wang Q, Hong L, Chen L et al (2001) Monodispersed hard carbon spherules with uniform nanopores. Carbon 39(14):2211–2214
Pan Q, Zheng F, Ou X et al (2017) MoS2 encapsulated SnO2-SnS/C nanosheets as a high performance anode material for lithium ion batteries. Chem Eng J 316:393–400
Kang S, Li X, Fan J (2012) Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, D-xylose, and wood meal. Ind Eng Chem Res 51(26):9023–9031
Kang WP, Yang X et al (2017) Preparation of porous ZnO/ZnFe2O4 composite from metal organic frameworks and its applications for lithium ion batteries. Chem Eng J 308:340–346
Wen Z, Wang Q, Zhang Q (2007) In situ growth of mesoporous SnO2 on multiwalled carbon nanotubes: a novel composite with porous-tube structure as anode for lithium batteries. Adv Funct Mater 17(15):2772–2778
Acknowledgements
This work is supported by the Shanghai Sailing Program from the Science and Technology Committee of Shanghai (No. 21YF1416200).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, P., Zhang, C., Ding, M. et al. A simple fabrication of porous carbon@tin dioxide as anode material for achieving lithium-ion batteries with high performance. Carbon Lett. 33, 1699–1705 (2023). https://doi.org/10.1007/s42823-023-00494-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-023-00494-3