Abstract
Facile process for the fabrication of multi-layer graphene thin film (MLGF) is reported here. Multi-layer graphene dispersion prepared by liquid-phase exfoliation of graphite was sprayed on a glass substrate by spray pyrolysis method. The structural, optical and electrical properties of the deposited MLGF are investigated. The sheets of graphene are deposited uniformly on the substrate and distribution of small graphene sheets with size of 300–500 nm can be observed in SEM image. AFM and micro-Raman results ensured that the spray-coated graphene thin film is composed of multi-layer graphene sheets. Spray coated graphene thin film showed significant optical transparency of 57% in the visible region (400–550 nm). MLGF possessed the electrical conductivity in the order of 744 S/m with surface resistivity of 3.54 k Ω/sq. The prepared liquid-phase exfoliated graphene thin film showed superior photoelectric response. The results of this study provided a framework for fabricating an optimized MLGF using a spray pyrolysis route for optoelectronics devices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
More than a decade, graphene is a fascinating material used in a wide range of applications such as gas sensor, supercapacitors, Li-ion battery, solar cells, flexible electronic devices and photocatalysis, because of its outstanding and attractive properties [1,2,3]. Different physical methods namely mechanical exfoliation, chemical vapor deposition (CVD), epitaxial growth on silicon carbide, arc discharge method, and substrate-free gas-phase synthesis were used for the preparation of high-quality graphene in large scale [4]. But difficulties in the creation of high-temperature control over cooling rate, pressure and requirement of high-quality substrate materials are standup barriers to the above mentioned methods for the production of graphene in large scale.
Later days, large scale synthesis of graphene sheets were achieved by a chemical method called Hummer’s method/modified Hummer’s method to produce reduced graphene oxide (rGO) sheets as a final product [5, 6]. Liquid-phase exfoliation (ultrasonication of pristine graphite powder in suitable organic solvents) is an another simple and low-cost solution process method compared to other methods for the production of a high-quality single layer to few-layer graphene sheets with significant yield and good electrical conductivity [7, 8].
However, the graphene sheets synthesized by the solution process can be effectively used in various devices only after converting them in the form of thin films. The application of the reduced graphene oxide prepared by Hummer’s method was promoted by transferring them as thin film by spray pyrolysis method [9, 10]. Though the production of reduced graphene oxide by Hummer’s method involved various tedious oxidation and reduction processes and the functional materials present in the reduced graphene oxide may have an effect on its electrical and optical properties. Some of the researchers have tried to fabricate the thin film of liquid phase exfoliated graphene by vacuum filtration method using membrane of alumina [7], PTFE [11], nylon [12] and MCE [13], which are having the disadvantages of high cost and less possibility of transferring the film to other substrate for application purposes.
There are few reports on the thin film of liquid-phase exfoliated graphene via spray pyrolysis, dip coating and dielectrophoresis [14,15,16]. Among them, spray pyrolysis technique is the simplest method for the fabrication of thin films since it is cost-effective, involves simple technical apparatus and it is suitable for large-scale production of uniform and high-quality thin films with desirable crystallinity [17]. This method is more reliable for the fabrication of thin films with high electrical and optical parameters as it does not require high vacuum or/and operating temperature [18]. However, the reports on the preparation of graphene thin film by spray pyrolysis of liquid-phase exfoliated graphene and the detailed investigation on their electrical properties are hard to found. Sales et al. [16] have reported the current–voltage characteristics of the spray-coated thin film of graphene exfoliated in ethanol-deionized water solution. The electrical properties of the liquid phase exfoliated multi-layer graphene thin film prepared by dip-coating method is reported by Machunoa [14].
In the present work, we have prepared the graphene dispersion by liquid-phase exfoliation of graphite in N–N′ dimethylformamide and transferred the graphene dispersion as a thin film by spray pyrolysis technique. This report includes the investigation of structural, morphological, electrical as well as photo conducting properties of the fabricated graphene thin film. The main focus of our work is to provide an insight to the electrical and photo conducting properties of the liquid phase exfoliated graphene thin film to provide a platform for the promotion of the liquid phase exfoliated graphene to the application level using facile and cost-effective method.
2 Experimental procedure
2.1 Preparation of graphene dispersion
Graphite powder and N–N′ dimethylformamide (DMF) were used for the preparation of graphene dispersion by liquid-phase exfoliation. 100 mg of graphite powder was mixed with 50 ml of DMF and sonicated for 8 h using an ultrasonic bath sonicator. After ultrasonication, the graphene dispersion was centrifuged with 3000 rpm for 30 min to separate the thin graphene flakes. The 25 ml of supernatant dispersion was collected for the preparation of the thin film.
2.2 Preparation of graphene thin film
The exfoliated graphene dispersion was deposited on a glass substrate (2.5 × 2.5 × 0.15 cm3) by chemical spray pyrolysis technique. Before the deposition of the graphene precursor, the substrate was cleaned by 15 min of successive ultrasonication in double distilled water, acetone, and ethanol baths. The well-cleaned substrate was then kept on the hotplate of the spray pyrolysis unit at 120 °C. The nozzle to substrate distance was carefully maintained as 14 cm. Nearly 50 ml of graphene dispersion obtained by the above-mentioned procedure was sprayed onto the preheated glass substrate at a spray rate of 1 ml/min. The compressed air was used as a carrier gas. The thickness of the deposited thin film is 380 ± 10 nm. Finally, the thin film was dried at 250 °C for 3 h.
2.3 Characterization techniques
Powder XRD pattern of the graphene thin film was recorded using PANalytical Empyrean with Cu-Kα radiation. The thickness of the prepared graphene film was measured using Dektak 3030 Stylus Profilometer. The UV–Vis transmittance spectrum was recorded using SHIMADZU UV-2450 spectrophotometer and the surface morphology of the sample was analyzed using SEM (FESEM; Nova NanoSEM 230), TEM (HRTEM; JEOL, JEM 2100), and AFM (A100 SGS, APE Research). Room temperature micro-Raman spectra were recorded using HORIBA Jobin–Yvon LabRAM HR 800 using a He–Ne laser of wavelength 632.8 nm as an excitation source. Raman spectra of nearly 100 points were collected and the Raman intensity map was constructed for the area of (22 × 22) μm2. The electrical conductivity at room temperature was measured using the Ecopia HMS-3000 Hall measurement setup in Vander Pauw configuration. The photoconductivity measurement was carried out in a vacuum chamber using a Hind High Vacuum cryostat facility instrument at ambient temperature.
3 Results and discussion
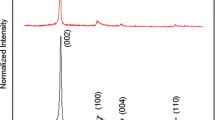
The measured XRD pattern of the graphene thin film and graphite powder are shown in Fig. 1. The X-ray diffraction peak is obtained at 26.53° for graphene thin film and 26.67° for graphite powder. Obtained diffraction peaks of both graphene thin film and graphite powder are well matched with the JCPDS no [75-1621] and other reports [19, 20]. The corresponding inter-planar distance of graphene thin film and graphite powder are 3.36 and 3.34 Å, respectively. The observed increase in inter-planar distance of graphene thin film from that of graphite powder indicates the successful exfoliation of graphene sheets from graphite powder. Sharp diffraction peak of graphene shows that the high-quality graphene crystalline thin films are successfully deposited using spray pyrolysis technique.
Figure 2a, b show the SEM images of the graphene thin film captured at different places on the film surface. Distribution of smaller graphene sheets with a length of 300–600 nm can be seen on the surface of the thin film (Fig. 2a). The surface of the thin film also contains larger size graphene sheets stacked on one another with lateral size varies from 1.2 to 3.2 μm (Fig. 2b). TEM image (Fig. 3a) clearly shows the stacked graphene sheets with a lateral size of ~ 350 nm and a width of 225 nm. In Fig. 3b, thin graphene sheets with a lateral size of 500 nm can be observed.
Further, the surface morphology of the graphene thin film was analyzed using the AFM and its image is shown in Fig. 4. The variation in the thickness of the graphene sheets can be observed through the brightness in the AFM image which also supports the existence of smaller graphene sheets on the surface of the uniformly coated graphene thin film as observed in SEM image. The approximate lateral dimension of the graphene sheet marked in the AFM image is ~ 1.1 μm and the estimated average thickness varies from ~ 11 to 20 nm. This thickness value approximately corresponds to the multilayer graphene with the number of layers varies from 22 to 40 and is comparable with the graphene sheet thickness reported in dip-coated graphene thin film [14].
The transmittance spectrum of the graphene thin film is shown in Fig. 5a. It shows a broad transmittance band from 600 to 350 nm and shows around 57% of transparency in the region 400–550 nm. However, the obtained transmittance is higher compared to that of dip-coated [14] and plasma-treated spray-coated graphene thin films [16] and lesser than the transmission of the single-layer graphene sheet (T ~ 98%) [21]. Hence, it can be inferred that graphene thin film prepared in the present case shows better optical transmission in the middle of the visible spectrum than the previous reports. The reduction of transmittance and increase in absorption than the single layer may be due to the multi-layer nature of the graphene and the higher thickness of the film [22].
In Fig. 5b the observed Raman bands of graphene film at 1319 cm−1 (D band), 1564 cm−1 (G band) and 2647 cm−1 (2D band) are characteristic Raman bands of graphene [23]. The shifting of band positions to the low wavenumber compare to that of graphite and changing of the broad asymmetric 2D band of graphite into a sharp symmetric band in graphene film revealed that the method used for the preparation of thin film from the exfoliated graphene dispersion does not lead to the re-aggregation of graphene into graphite. The increase in the intensity of D band from that of graphite is due to the edge defects created during the exfoliation process [12].
Micro-Raman intensity mapping was performed for a selected area of 20 × 20 μm to study the average structural quality and number of graphene layers. Figure 6a–c shows the integrated intensity image of D, G and 2D bands constructed by scanning the selected area and collecting the intensity data at each spot. The high intensity bright and low-intensity dark regions in D, G, and 2D band indicate the uniformity of the number of layers and defects of graphene over the coated area of the thin film. The bright regions are attributed to the D, G and 2D bands with higher intensity whereas the dark regions are related to their lower intensity. The ID/IG and IG/I2D were calculated from the intensity values of each band collected at 100 different points and their histogram are shown in Fig. 6d, e. The ID/IG ratio gives the evidence of the structural defect in the graphene whereas IG/I2D ratio gives the information about the number of layers of graphene flakes. From the histogram of ID/IG and IG/I2D obtained from the intensity mapping data (Fig. 6d, e), it can be concluded that ID/IG ratio related to the structural defects in the graphene varies from 0.2 to 0.6 and IG/I2D ratio, indicating the number of layers in the graphene sheets, varies from 2 to 3.5. As per the relation reported in Ref. [16], the calculated number of graphene layers (n) using IG/I2D ratio varies from 18 to 33. This result is well agreed with the thickness value measured through AFM image. These results inferred that the spray-coated graphene are multi-layer graphene (MLG) sheets with very fewer edge defects [24].
The electrical conductivity of the graphene thin film is ~ 744 S/m and the surface resistivity is ~ 3.54 kΩ/sq. This value of electrical conductivity is relatively higher than that reported for graphene exfoliated in NMP (~ 5 S/m) [7] and lesser than that of graphene dispersion filtered on Nylon membrane reported in our previous work [25], graphene dispersed in ethanol (~ 1130 S/m) [11] and graphene prepared by ball milling method (1200 S/m) [26]. This decrease in electrical conductivity of multi-layer graphene from that of few-layer graphene/single-layer graphene is attributed to the reduction of surface channels and increase of inter-layer channels, which diminishes the overall mobility of the conduction electron [25]. The sheet resistance is very low compared to that of graphene produced by soft transfer printing method (0.3–17 k Ω/sq) [27] and dip-coated graphene (4.7 k Ω/sq) [14] and rGO (11–37 k Ω/sq) [28]. This appreciable electrical conductivity of spray-coated liquid phase exfoliated graphene thin film, compared to that of graphene thin films prepared by other solution processes, suggested that it can be used in solar cells and fuel cells as a counter electrode instead of platinum/molybdenum electrodes.
The photoconductive response of the graphene thin film was taken in dark and illumination conditions. The electrical contact of the deposited film was prepared with 0.5 cm spacing by silver coating using the thermal evaporation technique. Then it was connected in series with a KEITHLEY 485 Pico ammeter and DC power supply (Fig. 7a). Subsequently, the graphene film is illuminated with a full spectrum of the 60 W xenon lamp. The DC input was increased from − 5 to + 5 volts in steps and subsequent dark currents and photocurrents were measured using the electrometer [29].
The noted linear I–V curve of the film in Fig. 7b discloses the Ohmic nature of the contact and good photoresponse of the graphene film after illumination. This result is due to the stimulation of more quantity of photoelectrons from the liquid phase exfoliated graphene film under illumination. Remarkably, the photocurrent of graphene film is ~ 5 times higher than that of the dark current. This result is well-matched with that of reduced graphene oxide/reduced graphene oxide quantum dots hybrids film [30]. It is observed that the good optical absorbance and electrical properties of the film kindle the good photoresponse of the sample.
4 Conclusion
Homogeneous MLGF prepared by spray pyrolysis technique shows moderate optical transparency of 57%, electrical conductivity of ~ 744 S/m and sheet resistance of 3.54 k Ω/sq. Hence, this simple and cost-effective technique can be applied for large scale production of graphene thin film with high electrical conductivity and optical transparency as a better replacement for ITO in various applications after optimizing the synthesis parameters of graphene dispersion and thickness of the thin film. By increasing the ultrasonication time the thickness of the graphene sheets can be reduced and there by the optical and electrical properties of the graphene thin film can be improved. Deposited liquid phase exfoliated graphene thin film also showed better photoconductive property. Hence, the obtained results implied that graphene thin film prepared using facile chemical spray pyrolysis of liquid-phase exfoliated graphene dispersion can be employed as a reliable candidate in optoelectronics devices.
References
Deng W, Ji X, Mingot MG, Lu F, Chen Q, Banks CE (2012) Graphene electrochemical supercapacitors: the influence of oxygen functional groups. Chem Commun 48:2770–2772
Hu Y, Li X, Geng D, Cai M, Li R, Sun X (2013) Influence of paper thickness on the electrochemical performances of graphene papers as an anode for lithium ion batteries. Electrochim Acta 91:227–233
Wang H, Hu YH (2012) Graphene as a counter electrode material for dye-sensitized solar cells. Energy Environ Sci 5:8182–8188
Guo S, Dong S (2011) Graphene nanosheet: synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem Soc Rev 40:2644–2672
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Shahriary L, Athawale A (2014) Graphene oxide synthesized by using modified hummers approach. Renew Energy Environ Eng 2:58–63
Hernandez Y, Nicolosi V, Lotya M, Blighe FM, Sun ZY, De S, McGovern IT, Holland B, Byrne M, Gunko YK, Boland JJ, Niraj P, Duesberg G, Krishnamurthy S, Goodhue R, Hutchison J, Scardaci V, Ferrari AC, Coleman JN (2008) High-yield production of graphene by liquid-phase exfoliation of graphite. Nat Nanotechnol 3:563–568
Zhu L, Zhao X, Li Y, Yu X, Li C, Zhang Q (2013) High-quality production of graphene by liquid-phase exfoliation of expanded graphite. Mater Chem Phys 137:984–990
Rafiq N, Junaid M, Anwar AW (2017) A low-cost route for synthesis of reduced-graphene oxide thin films for transparent conducting electrodes by spray-pyrolsis. J Ovonic Res 13:195–201
Eluyemi MS, Eleruja MA, Adedeji AV, Olofinjana B, Fasakin O, Akinwunmi OO, Ilori OO, Famojuro AT, Ayinde SA, Ajayi EOB (2016) Synthesis and characterization of graphene oxide and reduced graphene oxide thin films deposited by spray pyrolysis method. Graphene 5:143–154
Zhang X, Coleman AC, Katsonis N, Browne WR, van Wees BJ, Feringa BL (2010) Dispersion of graphene in ethanol using a simple solvent exchange method. Chem Commun 46:7539–7541
Zhou M, Tian T, Li X, Sun X, Zhang J, Cui P, Tang J, Qin LC (2014) Production of graphene by liquid-phase exfoliation of intercalated graphite. Int J Electrochem Sci 9:810–820
Oh SY, Kim SH, Chi YS, Kang TJ (2012) Fabrication of oxide-free graphene suspension and transparent thin films using amide solvent and thermal treatment. Appl Surf Sci 258:8837–8844
Machunoa LGB, Oliveiraa AR, Furlan RH, Limaa AB, Moraisa LC, Gelamoa RV (2015) Multilayer graphene films obtained by dip coating technique. Mater Res 18:775–780
Rouxinol FP, Gelamo RV, Amici RG, Vaz AR, Moshkalev SA (2010) Low contact resistivity and strain in suspended multilayer graphene. Appl Phys Lett 97:1–3
Sales MGC, Shanlene M, Vega DCD, Vasquez MR (2018) Properties of spray-deposited liquid-phase exfoliated graphene films. Jpn J Appl Phys 57:01AF06
Kozhukharov S, Tchaoushev S (2013) Spray pyrolysis equipment for various applications. J Chem Technol Metall 48:111–118
Malik O, Wade FJH, Amador RR (2017) Pyrolysis, chapter 8. IntechOpen, New York, pp 197–219
Chen ML, Park CY, Choi JG, Oh WC (2011) Synthesis and characterization of metal (Pt, Pd and Fe)-graphene composites. J Korean Ceram Soc 48:147–151
Siburian R, Sihotang H, Raja SL, Supeno M, Simanjuntak C (2018) New route to synthesize of graphene nano sheets. Orient J Chem 34:182–187
Nair RR, Blake P, Grigorenko AN, Novoselov KS, Booth TJ, Stauber T, Peres NMR, Geim AK (2008) Fine structure constant defines visual transparency of graphene. Science 320:1308
Bazylewski P, Sharbaf AA, Ezugwu S, Ouyang T, Park J, Fanchini G (2006) Graphene thin films and graphene decorated with metal nanoparticles. In: Mandracci P (ed) Crystalline and non-crystalline solids. Mumbai, Intech, pp 3–16
Ferrari AC (2007) Raman spectroscopy of graphene and graphite: disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun 143:47–57
Morales GM, Schifani P, Ellis G, Ballesteros C, Martınez G, Barbero C, Salavagione HJ (2011) High-quality few layer graphene produced by electrochemical intercalation and microwave-assisted expansion of graphite. Carbon 49:2809–2816
Vasanthi V, Gayathri S, Anitha K, Ramakrishnan V (2017) Exfoliation of high quality graphene in volatile and non-volatile solvents. Graphene Technol 2:29–40
Zhao WF, Fang M, Wu FR, Wu H, Wang LW, Chen GH (2010) Preparation of graphene by exfoliation of graphite using wet ball milling. J Mater Chem 20:5817–5819
Shi L, Yang J, Huang Z, Li J, Tang Z, Li Y, Zheng Q (2013) Fabrication of transparent, flexible conducting graphene thin films via soft transfer printing method. Appl Surf Sci 276:437–446
Zhu Y, Cai W, Piner RD, Velamakanni A, Ruoff RS (2009) Transparent self-assembled films of reduced graphene oxide platelets. Appl Phys Lett 95:1–3
Logu T, Raliya R, Kavidya S, Soundarrajan P, Biswas P, Sethuraman K (2017) Hierarchical architecture of CuInS2 microsphere thin films: altering laterally aligned crystallographic plane growth by Cd and V doping. Cryst Eng Commun 19:6602–6611
Hea X, Suna Y, Tanga N, Du Y (2017) Photoconductivity enhancement of reduced graphene oxide with reduced oxide graphene quantum dots hybrids film. Mater Lett 188:29–32
Acknowledgements
We would like to acknowledge CSIR, Govt. of India (03(1312)/14/EMR-II) for the financial support, UGC-UPE programme of Madurai Kamaraj University, India for providing micro-Raman, DST-PURSE for AFM facilities and DST-SERB for providing Raman mapping accessories.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vasanthi, V., Logu, T., Ramakrishnan, V. et al. Study of electrical conductivity and photoelectric response of liquid phase exfoliated graphene thin film prepared via spray pyrolysis route. Carbon Lett. 30, 417–423 (2020). https://doi.org/10.1007/s42823-019-00111-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-019-00111-2