Abstract
A novel approach was presented for deposition of nickel–graphene nanocomposite coating on copper. Unlike conventional methods, graphene and graphene oxide nanosheets were not used. The basis of the method is to synthesize graphene oxide by oxidation of graphite anode during the electrochemical deposition process. The obtained graphene oxide sheets were reduced during the deposition in the cathode and co-formed with the nickel deposition in the coating. The pulsed ultrasonic force was applied during the deposition process. When the ultrasonic force stops, the deposition process begins. Scanning electron microscopy, Raman spectroscopy, atomic force microscopy, X-ray diffraction and X-ray photoelectron spectroscopy confirmed the presence of graphene nanosheets in the coating. The amount of graphene nanosheets increases up to a maximum of 14.8 wt% by increasing the time of applying ultrasonic force to 6 s. In addition, with the presence of graphene in the nickel coating, the wear rate dramatically decreased.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Graphene, a two-dimensional (2D) sheet consisting of carbon atoms with a honeycomb lattice, has been highly regarded by the researchers. Due to the unique properties of graphene, including mechanical, optical, and electrical properties as well as very high chemical stability, this material is a suitable alternative as a catalyst and resistant to corrosion and oxidation [1,2,3,4,5].
Recently, graphene is used as a reinforcing material in the fields of metal matrix composites. Uniform dispersion of graphene inside some metal matrix is one of the main challenges for the development of graphene-reinforced metal matrix composite. To solve the above problems, Dong et al. [6] prepared rGO/Ag nanocomposites using one-step chemical co-reduction route and graphene oxide and Ag+ simultaneously reduced during the process. Also, Chen et al. [7] to overcome the above-mentioned problem used a novel method in which the Cu@Gr/WCu composites were successfully fabricated using an electroless plating process and SPS process. To improve the dispersion and wettability of graphene, Xue et al. [8] using a new and efficient method produced nanocomposite powders based on metal-coated graphene by an in situ co-reduction method.
Ni-based coatings containing nanosized particles of ceramic, metal or organic materials have received extensive attention because of their excellent corrosion resistance, wear resistance, high-temperature oxidation resistance and good catalytic behavior [9,10,11,12,13,14,15,16]. The use of graphene sheets in nickel coatings processed electrochemically with the aim of improving corrosion and wear resistance as well as storage of hydrogen is of great interest [17,18,19,20]. It has been reported that graphene distribution in nickel coatings processed by electrochemical methods due to its inappropriate distribution in solution is non-uniform [21, 22]. Hence, graphene oxide is mainly used. The reason for this choice is the existence of hydrophilic functional groups such as –OH, epoxy and –COOH as well as the more appropriate distribution in solution [1]. The graphene oxides are reduced during the deposition in the cathode and co-formed with the nickel deposition in the coating.
In this research, a new method was developed to form nickel–graphene coatings. In this method, instead of using graphene nanosheets and graphene oxide, graphene was formed in situ during the deposition process.
2 Experimental
The coating solution was obtained by dissolving 0.2 mol of Ni(NO3)2 and 1 Vol. H2O2. High-purity graphite and copper were used as anode and cathode, respectively. The surface area of the anode was ten times larger than cathode surface. They were placed at a distance of less than 5 mm. To form the Ni–graphene coating, the optimized reduction potential of 1.3 V between the cathode and saturated calomel electrode (SCE) as references electrode was applied for 1 h under potentiostatic conditions.
The pulsed ultrasonic force was applied during the deposition process. When the ultrasonic force stops, a constant DC electric potential of 1.3 V was applied between the anode and the cathode for 5 s and the deposition process begins. The pulse-on time of ultrasonic force was changed from 2 to 8 s to study its effect on the amount of embedded graphene nanosheets in the coating. When applying the ultrasonic force, the deposition process was stopped. The schematic diagram of experimental equipment is shown in Fig. 1.
The surface morphology and microstructure of the coatings were characterized using scanning electron microscopy, SEM (XL-30 Philips), and atomic force microscopy (AFM) (DS45-40). Chemical composition of coatings was also examined using X-ray diffraction (XRD, D8 Bruker), micro-Raman spectroscopic (LabRam Jobin–Yvon) and X-ray photoelectron spectroscopy (XPS, PHI-5700 ESCA system using an Al Ka (1486.6 eV) X-ray source). The XRD patterns were collected in the 2θ range of 10°–100° with a Cu-Kα radiation (λ = 0.154 nm) target.
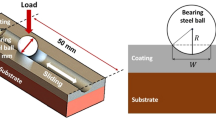
The wear test was done at room temperature without lubricant. Pin made of AISI 52100 steel with a hardness of 62–65 HRC held vertical to the sample surface. The tests were performed under 40-N axial loads at a constant sliding speed of 2 m/s. Wear tests were carried out for a total sliding distance of approximately 2000 m.
3 Result and discussion
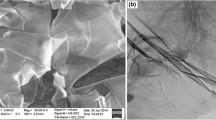
SEM images at various magnifications and elemental mapping of Ni–graphene coating synthesized by electrochemical deposition are presented in Fig. 2. SEM images represent the presence of graphene sheets in nickel coatings. The elemental mapping of the coating also shows a uniform distribution of graphene in nickel coatings. Figure 3 exhibits EDS and XRD analyses of the generated coating.
XRD pattern of the coating (Fig. 3b) reveals Ni and Cu peaks. Also, the wide peak formed at the angle of 26° supports the reduction of the graphene oxide in the coating [19,20,21].
For a closer evaluation of the graphene formed in the existing nanocomposite coating, in another process, graphene sheets were coated with a Ni-free solution on amorphous copper. This coating was analyzed using AFM analysis and Raman spectroscopy (Fig. 4). It is observed that the thickness of graphene sheets is about 2.5 nm. Therefore, it can be said that graphene is multi-layered. Two peaks in the 1340 cm−1 and 1590 cm−1 are observed in the Raman spectrum. These peaks are related to the carbon–carbon bond in diamond and graphite, respectively. The intensity ratio of the diamond peak to the graphite peak (ID/IG) is about 1.53. This refers to a lower amount of graphite than diamonds, which is one of the most important characteristics of graphene [20]. This result shows that the reduced graphene oxide sheets were introduced into the coatings during the electrochemical reduction of nickel ions.
Figure 5 depicts the C 1s and Ni 2p3/2 regions of high-resolution XPS spectra obtained with carbon and Ni in the Ni–graphene nanocomposite coating. The C 1s spectrum of Ni–graphene nanocomposite coating involves five peaks corresponding to the C–Ni, sp2 carbon, C–H defect, C–O–H and the HO–C=O groups. The peak intensity of C=C is noticeably higher in comparison with carbon with oxygen groups. This indicates the effective reduction of the graphene oxide in the deposition process. The very small peak at 284 eV is attributed to the formation of C–Ni bond. The Ni 2p3/2 spectrum of Ni–graphene nanocomposite is characterized by two highly intense bonds at 853.3 and 856.2 eV. These peaks could be related to the Ni and Ni–O groups, respectively [23, 24].
The graph of the amount of embedded graphene nanosheets in the coating in terms of the time of applying the ultrasonic force is given in Fig. 6. The amount of graphene nanosheets increases up to a maximum of 14.8 wt% by increasing the time of applying ultrasonic force to 6 s. Further increase in the time of applying ultrasonic force leads to a decrease in the amount of graphene nanosheets embedded in the coating due to the agglomeration of graphene particles in the solution.
Figure 7 shows schematically mechanism of the graphene formation. It is likely that due to the very close distance between the anode and the cathode, the temperature will increase locally. Hence, it is expected that due to the presence of graphite oxidizing compounds such as hydrogen peroxide and nitric acid, graphite electrode during galvanostatic oxidation in this aqueous solutions converted into graphite oxide [25, 26].
Then, graphene oxide sheets are separated from graphite by ultrasonic force and dispersed in the solution. The obtained graphene oxide sheets move through the electrophoretic force towards the cathode and converted into graphene through the reduction of its oxygen functional groups [27]. Finally, in coincidence with the deposition of nickel, graphene is formed in the coating.
The microhardness diagram of the Ni–graphene nanocomposite coating in terms of the time of applying the ultrasonic force is shown in Fig. 8a. It can be seen that with the increase in the time of applying ultrasonic force, the hardness increases due to the presence of graphene nanosheets in the coating.
The variations of wear rate with the time of applying ultrasonic force are shown in Fig. 8b. As can be seen, the wear rate decreases noticeably as the time of applying ultrasonic force increases. It can be due to the increase of embedded graphene nanosheets in the coating and the subsequent increase in the hardness of the coating. A further increase in the time of applying ultrasonic force from 6 to 8 s causes an increase in the wear rate. It can be related to the agglomeration of graphene nanosheets in the coating.
4 Conclusions
A novel approach was presented for the first time for the formation of nickel–graphene nanocomposite. In this study, to prepare nickel–graphene nanocomposite coating, instead of using graphene particles directly, graphene was produced in situ by reduction of graphite oxide by an electrochemical deposition method. Laboratory experiments by EDS, AFM, XRD, XPS and Raman spectroscopy confirmed the presence of graphene in nickel deposited coating on the copper. It is expected that the high temperature created in the solution due to the high proximity of the cathode and the anode and the presence of oxidizing agents such as hydrogen peroxide and nitric acid in the solution causes graphite oxidation in the anode. Since the solution was under ultrasonic force, thus graphene oxide sheets are separated from graphite and dispersed in the solution. The obtained graphene oxide sheets move through the electrophoretic force towards the cathode and electrochemically converted into graphene. The amount of graphene nanosheets increases up to a maximum of 14.8 wt% by increasing the time of applying ultrasonic force to 6 s. In this condition, the wear rate decreases noticeably.
References
Medvedev AG, Mikhaylov AA, Grishanov DA, Yu DYW, Gun J, Sladkevich S, Lev O, Prikhodchenko PV (2014) Nanocrystalline tin disulfide coating of reduced graphene oxide produced by the peroxostannate deposition route for sodium ion battery anodes. J Mater Chem A 2:8431
Mattevi C, Kim H, Chhowalla M (2011) A review of chemical vapour deposition of graphene on copper. J Mater Chem 21:3324
Szeptycka B, Midzialek AG, Babul T (2016) Electrodeposition and corrosion resistance of Ni–graphene composite coatings. J Mater Eng Perform 25:3134
Badrayyana S, Bhat DK, Shenoy S, Ullal Y, Hegde AC (2015) Novel Fe–Ni–graphene composite electrode for hydrogen production. Int J Hydrogen Energy 40:10453
Dong LL, Chen W, Deng N, Zheng C (2016) A novel fabrication of graphene by chemical reaction with a green reductant. Chem Eng J 306:754–762
Dong LL, Ding YC, Huo WT, Zhang W, Lu JW, Jin LH, Zhao YQ, Wu GH, Zhang YS (2019) A green and facile synthesis for rGO/Ag nanocomposites using one-step chemical co-reduction route at ambient temperature and combined first principles theoretical analyze. Ultrason Sonochem 53:152–163
Chen W, Dong L, Wang J, Zuo Y, Ren S, Fu Y (2017) Synergistic enhancing effect for mechanical and electrical properties of tungsten copper composites using spark plasma infiltrating sintering of copper coated graphene. Sci Rep 7:17836
Xue Y, Chen W, Wang J, Dong L, Zhao Q, Fu Y (2018) Formation mechanism and cohesive energy analysis of metal-coated graphene nanocomposites using in-situ co-reduction method. Materials 11:2071
Nazir MH, Khan ZA, Saeed A, Siddaiah A, Menezes PL (2018) Synergistic wear-corrosion analysis and modeling of nanocomposite coatings. Tribol Int 121:30
Li B, Zhang W, Zhang W, Huan Y (2017) Preparation of Ni-W/SiC nanocomposite coatings by electrochemical deposition. J Alloys Compd 702:38
Hassannejad H, Nouri A (2017) Formation of β-NiAl nanocomposite coating by electrodeposition and effect of cerium ion doping on its oxidation mechanism. Ceram Int 43:9979
Waware US, Hamouda AMS, Pradhan AK (2018) Effect of codeposition of mixed nanoparticles (V2O5 and ZrO2) on the structure and properties of Ni–B nanocomposite coating. J Alloys Compd 752:253
Allahyarzadeh MH, Aliofkhazraei M, Sabour Rouhaghdam AR, Torabinejad V (2016) Gradient electrodeposition of Ni–Cu–W(alumina) nanocomposite coating. Mater Des 107:74
Shafiee Z, Bahrololoom ME, Hashemi B (2016) Electrodeposition of nanocrystalline Ni/Ni–Al2O3 nanocomposite modulated multilayer coatings. Mater Des 108:19
Wasekar NP, Madhavi Latha S, Ramakrishna M, Rao DS, Sundararajan G (2016) Pulsed electrodeposition and mechanical properties of Ni-W/SiC nano-composite coatings. Mater Des 112:140
Hassannejad H, Nouri A, Molavi FK, Barati A (2017) A novel approach to prepare Ni–Al mesoporous powder using electrochemical method in one step. J Alloys Compd 705:226
Jiang J, Feng C, Qian W, Zhu L, Han S, Lin H (2017) Effect of graphene oxide nanosheets and ultrasonic eletrodeposition technique on Ni–Mo/graphene oxide composite coatings. Mater Chem Phys 199:239
Kumar CMP, Venkatesha TV, Shabadi R (2013) Preparation and corrosion behavior of Ni and Ni–graphene composite coatings. Mater Res Bull 48:1477
Tamilarasan TR, Sanjith U, Shankar MS, Rajagopal G (2017) Effect of reduced graphene oxide (rGO) on corrosion and erosion and erosion–corrosion behavior electroless Ni–P coatings. Wear 390–391:385
Zhou C, Szpunar JA, Cui X (2016) Synthesis of Ni/graphene nanocomposite for hydrogen storage. ACS Appl Mater Interfaces 24:15232
Jabbar A, Yasin G, Khan WQ, Anwar MY, Korai RM, Nizam MN, Muhyodin G (2017) Electrochemical deposition of nickel graphene composite coatings: effect of deposition temperature on its surface morphology and corrosion resistance. RSC Adv 7:31100
Berlia R, Kumar MKP, Srivastava C (2015) Electrochemical behavior of Sn–graphene composite coating. RSC Adv 5:71413
Zhang W, Li Y, Zeng X, Peng S (2015) Synergetic effect of metal nickel and graphene as a cocatalyst for enhanced photocatalytic hydrogen evolution via dye sensitization. Sci Rep 5:10589
Johra FT, Lee JW, Jung WG (2014) Facile and safe graphene preparation on solution based platform. J Ind Eng Chem 20:2883–2887
Gurzeda B, Florczak P, Kempinski M, Peplinska B, Krawczyk P, Jurga S (2016) Synthesis of graphite oxide by electrochemical oxidation in aqueous perchloric acid. Carbon 100:540
Low CTJ, Walsh FC, Chakrabarti MH, Hashim MA, Hussain MA (2013) Electrochemical approaches to the production of graphene flakes and their potential applications. Carbon 54:1
Singh BP, Nayak S, Nanda KK, Jena BK, Bhattacharjee S, Besra L (2013) The production of a corrosion resistant graphene reinforced composite coating on copper by electrophoretic deposition. Carbon 61:47
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hassannejad, H., Nouri, A., Farrokhi-rad, M. et al. In situ fabrication of high-percent Ni–graphene nanocomposite coating. Carbon Lett. 30, 63–71 (2020). https://doi.org/10.1007/s42823-019-00071-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-019-00071-7