Abstract
Background
Bronchogenic cyst (BC) of the mediastinum in infancy is a rare lesion. They sometimes produce severe recurrent bouts of respiratory distress that may have leathal outcomes if not recognized. BCs are benign congenital malformations of the primitive ventral foregut, that can present at any age. The diagnosis is often suspected by clinical symptoms and later on confirmed by endoscopic and radiologic evaluation.
Patient report
A 6-month-old infant with persistent respiratory distress had recurrent admissions to Paediatric Intensive Care Unit. The infant was managed as a case of bronchiolitis, which led to ineffective treatment on numerous occasions. Radiological work-up revealed unusual findings of asymmetrical hyperinflation, with hyperinflation occurring in both lungs but it is more noticeable in right one by a ball-valve effect. Bronchoscopy, was performed with the primary intention to retrieve a possible foreign body, showed a non-pulsatile external mass compressing the entry of the main bronchi with more pressure on the right main bronchus. A bronchogenic cyst was suspected and confirmed by computed tomography. Bronchoscopic management with laser was performed, with no reported complications or recurrence after a 10 month follow-up.
Conclusions
In infants presenting with respiratory distress and no signs of infection, the differential diagnosis of BC should be considered. These pathologies are commonly treated by open or minimal invasive surgery. This is a report of an infant presenting with a BC that was successfully treated by rigid bronchoscopy and laser as a novel alternative approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bronchogenic cysts (BCs) represent a spectrum of bronchopulmonary malformations that result from an abnormal budding of the tracheobronchial tree during fetal development [1,2,3]. They may be detected at any age. The cysts are usually solitary and thin-walled. Most BCs are located in the mediastinum, while an intrapulmonary localization is also possible. BCs in the mediastinum, are most frequently located in the pericarinal area followed by the right trachea and hilum; whereas intrapulmonary BCs are commonly localized in the lower lobes [4,5,6]. The cysts contain bronchial cartilage, smooth muscle, elastic tissue, and mucous glands. Histopathologically, the internal cystic wall is covered with a ciliary columnar or cuboidal epithelium [7, 8]. BCs exhibit a variety of clinical and radiologic presentations, posing a diagnostic challenge, especially in areas with endemic hydatid disease. Endoscopic drainage has emerged as a diagnostic and potentially therapeutic option [9]. BCs may be symptomatic or asymptomatic. The symptoms vary depending on the location and adjacent structures, as they can cause infection and other complications such as compression of the tracheobronchial tree leading to stridor, bronchospasms, respiratory distress, or air trapping [5, 10]. BCs are rarely detected during prenatal sonography, and Computed Tomography (CT) is highly useful in diagnosis. In suspected cases, Magnetic Resonance Imaging (MRI) provides additional information in differentiating cystic from solid lesions. Barium studies can also be helpful to show the abnormal relation between the respiratory tract and esophagus or stomach. On differential diagnosis, esophageal duplication cysts, neuroenteric cysts, congenital pulmonary adenoid malformation (CPAM) and cystic teratoma, or thymic cyst should be kept in mind as differntials [4, 5]. The management of patients with uncomplicated bronchogenic cysts has evolved over the last decade with the development of more precise diagnostic techniques and a better understanding of the variable natural history of these lesions. Although a surgical approach is still indicated for infants and children, there is disagreement with this invasive approach [11]. Minimally invasive surgical methods, such as thoracoscopy [12], mediastinoscopy [13], percutaneous drainage approach [14] and endobronchial ultrasound-transbronchial needle aspiration for carinal or paratracheal cysts have been safely used to manage these lesions [15]. An air-fluid level in the cyst, the rare presence of malignant cells in the aspirate or biopsy, or enlargement or recurrence of the cyst on follow-up examination mandates complete surgical removal [16,17,18,19].
Patient report
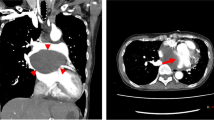
A 6-month-old male infant with severe recurrent respiratory distress had multiple admissions to the Pediatric Intensive Care Unit. Clinical exam revealed diminished breath sounds over the right chest, and bilateral, moist rales and wheezing. The initial chest x-ray showed a hyperaerated right lung and a mediastinal shift to the left. A diagnosis of bronchiolitis was suspected, and the patient was managed accordingly. However, subsequent x-rays continued to show overdistension of the right lung. CT of the chest revealed a mediastinal mass with fluid density, located within the sub-carinal area (Fig. 1b). The clinical suspicion now moved to foreign body aspiration or tracheo-esophageal fistula, especially due to recurrent respiratory infections, respiratory distress during breastfeeding, and resultant hyperinflation of the right lung. A rigid bronchoscopy under general anesthesia was performed and a dome-shaped carina with obliteration of the sharp angle and significant external non-pulsatile compression on both entrance of the main bronchi, especially the right side, were noticed (Fig. 1a). A fine needle aspiration (Fig. 2a)was performed under direct visualization removing 6 ml of fluid after which the protruding impression of the lesion regressed. A holmium laser (Fig. 2b) was used to create a window at the site of the puncture (Fig. 1c). The cystic cavity was then enetred by the bronchoscope that confirmed the integrity of the cystic wall. The infant improved after the procedure and was discharged after 3 days. Follow up for 10 months has shown complete recovery from respiratory symptoms, except an occurrence of intussusception in the ninth month of follow-up.
Discussion
BCs are frequently incidental findings on ultrasound or chest radiograph in the neonatal period [20]. Symptoms in neonates are usually related to mass effect on the involved structure or infection [21]. Major bronchus obstruction is rare but has been reported in the setting of a sub-carinal cyst [22]. More commonly, the presentation mimics centrilobular emphysema secondary to air trapping of the smaller airways [23].
A retrospective 20 years report from a tertiary pediatric center on 45 BCs pointed out that 82.2% BCs were located in sub-carinal and paratracheal area (Group I), and the rest in Group II with intrapulmonary presentation. In Group I, a partial resection was done because of adherence to the airway, and the majority of these (90%) were approached by thoracoscopy. while Group II, a lobectomy was done. Complications occurred in seven patients (15%): subcutaneous emphysema, extubation failure, reoperation due to bleeding, surgical site infection, bronchopleural fistula, and pneumothorax [9].
Maturu et al. reported the role and safety of endobronchial ultrasound-guided transbronchial needle aspiration [24]. Complete surgical excision on the other hand ensures a decreased chance of recurrence and eliminates the theoretical risk of malignancy, as the wall of the cyst is removed [16]. Most of the cysts are in the mediastinum and require at least VATS for complete removal, if not thoracotomy [26]. These procedures are major operations, with the latter approach more associated with significant mortality and morbidity [25]. There are multiple reports of recurrence after surgery especially if complete excision is not performed [27,28,29]. The risk and benefit of this approach needs to be discussed with the patient's family. If complete excision is not possible, surgical approach is probably not justified since BCs have been reported to recur in these instances [27,28,29]. In addition, incomplete resections do not eliminate the theoretical risk of malignancy and offers no advantage in our opinion over the less invasive bronchoscopic approach. The use of sclerosing agent is rare in the literature and should be attempted if recurrence occurs and patient is not a surgical candidate [27]. Our procedure also offers a solution for difficult-to-manage intraluminal cases, an example of which is a published case that required thoracoscopic surgery that resulted in a recurrence, later requiring open surgery with removal of some parts of the trachea [30]. There is no known optimal duration of follow-up, and because of presence of multiple reports of recurrence and rare risk of malignancy, there is need for extended follow-up. Follow up should probably be continued for at least 36 months since there are currently no reports that suggest recurrence can occur after this time [31,32,33].
Conclusion
The optimal strategy for management of mediastinal BCs remains unknown. Bronchoscopic laser fenestration is a safe option and can be both diagnostic and therapeutic for the management of paratracheal and sub-carinal BCs. This technique initially cannot be an alternative for surgical excision, because of microspillage of cystic content triggers strong inflammatory reaction and hence the experts opinion is to removing BCs completely. So this technique at least should be reserved for hemodynamically unstable children who cannot tolerate a thoracotomy or thoracoscopy. If this technique is used an extended follow-up for at least 36 months is recommended.
Data availability
The data that support the findings of this study are available within the article and publicly on Dryad. https://doi.org/10.5061/dryad.tx95x6b5x.
References
Ahrens B, Wit J, Schmitt M, Wahn U, Niggemann B, Paul K (2001) Symptomatic bronchogenic cyst in a six-month-old infant: case report and review of the literature. J Thorac Cardiovasc Surg 122:1021–1023. https://doi.org/10.1067/mtc.2001.11374
Stewart B, Cochran A, Iglesia K, Speights VO, Ruff T (2002) Unusual case of stridor and wheeze in an infant: tracheal bronchogenic cyst. Pediatr Pulmonol 34:320–323. https://doi.org/10.1002/ppul.10129
Kiralj A, Vuckovic N, Mijatov I (2015) Congenital cervical bronchogenic cyst: a case report. Srp Arh Celok Lek 143:317–321
Ashizawa K, Okimoto T, Shirafuji T, Kusano H, Ayabe H, Hayashi K (2001) Anterior mediastinal bronchogenic cyst: demonstration of complicating malignancy by CT and MRI. Br J Radiol 74:959–961. https://doi.org/10.1259/bjr.74.886.740959
Turkyilmaz A, Aydin Y, Erdem AF, Eroglu A, Karaoglanoglu N (2009) Congenital cystic pulmonary malformations in children: our experience with 19 patients. Eur J Med 41:15–21
Bailey PV, Tracy T Jr, Connors RH, de Mello D, Lewis JE, Weber TR (1990) Congenital bronchopulmonary malformations. Diagnostic and therapeutic considerations. J Thorac Cardiovasc Surg 99:597–603
Aktoğu S, Yuncu G, Halilçolar H, Ermete S, Buduneli T (1996) Bronchogenic cysts: clinicopathological presentation and treatment. Eur Respir J 9:2017–2021. https://doi.org/10.1183/09031936.96.09102017
Limaïem F, Ayadi-Kaddour A, Djilani H, Kilani T, El Mezni F (2008) Pulmonary and mediastinal bronchogenic cysts: a clinicopathologic study of 33 cases. Lung 186:55–61. https://doi.org/10.1007/s00408-007-9056-4
Gross DJ, Briski LM, Wherley EM, Nguyen DM (2023) Bronchogenic cysts: a narrative review. Mediastinum 7:26. https://doi.org/10.21073/med-22-46
Jeffries JM 3rd (1987) Asymptomatic bronchogenic cyst of the mediastinum. Postgrad Med 81:235–240. https://doi.org/10.1080/00325481.1987.11699830
Ponn RB (2003) Simple mediastinal cysts: resect them all? Chest 124:4–6. https://doi.org/10.1378/chest.124.1.4
Sugarbaker DJ (1993) Thoracoscopy in the management of anterior mediastinal masses. Ann Thorac Surg 56:653–656. https://doi.org/10.1016/0003-4975(93)90942-b
Urschel JD, Horan TA (1994) Mediastinoscopic treatment of mediastinal cysts. Ann Thorac Surg 58:1698–1701. https://doi.org/10.1016/0003-4975(94)91664-0
Whyte MK, Dollery CT, Adam A, Ind PW (1989) Central bronchogenic cyst: treatment by extrapleural percutaneous aspiration. BMJ 299:1457–1458. https://doi.org/10.1136/bmj.299.6713.1457
Singh A, Singh S, Malpani A, Devgarha S, Singh V (2011) Treatment of bronchogenic cyst surgical versus transbronchial drainage? J Bronchol Interv Pulmonol 18:359–361. https://doi.org/10.1097/LBR.0b013e31823575c5
Bolton JW, Shahian DM (1992) Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg 53:1134–1137. https://doi.org/10.1016/0003-4975(92)90412-w
Whooley J, White A, Soo A (2022) Bronchogenic cyst: a rare case of malignant transformation. BMJ Case Rep 15:e248916. https://doi.org/10.1136/bcr-2022-248916
Read CA, Moront M, Carangelo R, Holt RW, Richardson M (1991) Recurrent bronchogenic cyst. An argument for complete surgical excision. Arch Surg 126:1306–1308. https://doi.org/10.1001/archsurg.1991.01410340148022
Cuypers P, De Leyn P, Cappelle L, Verougstraete L, Demedts M, Deneffe G (1996) Bronchogenic cysts: a review of 20 cases. Eur J Cardiothorac Surg 10:393–396. https://doi.org/10.1016/s1010-7940(96)80103-5
Di Lorenzo M, Collin PP, Vaillancourt R, Duranceau A (1989) Bronchogenic cysts. J Pediatr Surg 24:988–991. https://doi.org/10.1016/s0022-3468(89)80199-x
Haller JA, Shermeta DW, Donahoo JS, White JJ (1975) Life-threatening respiratory distress from mediastinal masses in infants. Ann Thorac Surg 19:365–370. https://doi.org/10.1016/s0003-4975(10)64035-0
Funakoshi Y, Takeda S, Kadota Y, Maeda H (2007) Mediastinal bronchogenic cyst with respiratory distress from airway and vascular compression. Thorac Cardiovasc Surg 55:53–54. https://doi.org/10.1055/s-2006-924002
Lucaya J, Strife JL (2007) Pediatric chest imaging: chest imaging in infants and children. Springer, Berlin, Heidelberg
Bukamur HS, Alkhankan E, Mezughi HM, Munn NJ, Shweihat YR (2018) The role and safety of endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis and management of infected bronchogenic mediastinal cysts in adults. Respir Med Case Rep 24:46–49. https://doi.org/10.1016/j.rmcr.2018.04.002
Granato F, Voltolini L, Ghiribelli C, Luzzi L, Tenconi S, Gotti G (2009) Surgery for bronchogenic cysts: always easy? Asian Cardiovasc Thorac Ann 17:467–471. https://doi.org/10.1177/0218492309343855
Cioffi U, Bonavina L, De Simone M et al (1998) Presentation and surgical management of bronchogenic and esophageal duplication cysts in adults. Chest 113:1492–1496. https://doi.org/10.1378/chest.113.6.1492
Alraiyes AH, Shaheen K, Reynolds J, Machuzak M (2015) Recurrent bronchogenic cyst after surgical resection. Ochsner J 15:176–179
Rice DC, Putnam JB Jr (2002) Recurrent bronchogenic cyst causing recurrent laryngeal nerve palsy. Eur J Cardiothorac Surg 21:561–563. https://doi.org/10.1016/s1010-7940(01)01145-9
Chamberlain MH, Wells FC (2000) Recurrent bronchogenic cyst in a Jehovah’s witness. J Cardiovasc Surg (Torino) 41:785–786
Cuestas G, Rodríguezab V, Doormann F et al (2017) Tracheal bronchogenic cyst: a rare cause of cyanosis in the neonate. J Pediatr Surg Case Rep 10:56–59
Patel SR, Meeker DP, Biscotti CV, Kirby TJ, Rice TW (1994) Presentation and management of bronchogenic cysts in the adult. Chest 106:79–85. https://doi.org/10.1378/chest.106.1.79
Nakajima T, Yasufuku K, Shibuya K, Fujisawa T (2007) Endobronchial ultrasound-guided transbronchial needle aspiration for the treatment of central airway stenosis caused by a mediastinal cyst. Eur J Cardiothorac Surg 32:538–540
Liu HS, Li SQ, Cao ZL, Zhang ZY, Ren H (2009) Clinical features and treatment of bronchogenic cyst in adults. Chin Med Sci J 24:60–63. https://doi.org/10.1016/s1001-9294(09)60061-4
Funding
No financial or proprietary interests in any material discussed in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No funding was received.
Ethical approval
None.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El-Mefleh, N. Treatment of mediastinal bronchogenic cyst by rigid bronchoscopy and laser. J Ped Endosc Surg 6, 147–150 (2024). https://doi.org/10.1007/s42804-024-00227-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42804-024-00227-x