Abstract
Introduction
Retroperitoneoscopic pyeloplasty is considered as one of the acceptable approaches for pelviureteric junction obstruction (PUJO) in children. Some consider it better than the open and laparoscopic approaches; but it has its own technical challenges.
Objective
To analyse technical aspects in the initial learning curve of retroperitoneoscopic pyeloplasty for pelviureteric junction obstruction (PUJO) in children and to discuss certain tips and tricks.
Study design
We retrospectively evaluated the data of consecutive 10 pelviureteric junction obstruction cases undergone retroperitoneal pyeloplasty in 2 years duration (January 2016 to December 2017). All patients had undergone ultrasound kidney ureter bladder (KUB), intravenous pyelography (IVP) and Ethylene dicysteine (EC) scan. A single surgeon operated on all the patients and placed a DJ stent intraoperatively. Postoperatively, the patients underwent an EC scan and IVP at 6 months. The patient records and operative videos were assessed.
Results
The average patient age was 8.4 ± 2.31 years (5–11 years). Intraoperatively, two patients had crossing vessels and the rest 8 had intraluminal narrowing. The mean operating time was 207.5 ± 36.15 min (150–285 min) and mean hospital stay was 3 ± 1.49 days (2–7 days). The postoperative course was uneventful in almost all except one who developed perinephric collection and had to undergo pigtail drainage. On follow up, all patients showed improved drainage at the PUJ except one.
Conclusion
Retroperitoneoscopic pyeloplasty for pelviureteric junction obstruction can be optimally practiced by understanding the technical difficulties associated with it and the corresponding tips to ease the procedure. The advantages of going retroperitoneoscopically can be achieved and utilised in full for the benefit of the patient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the advent of open dismembered pyeloplasty, the surgical approach to the pelviureteric junction obstruction (PUJO), has undergone a paradigm shift. Various options in the domain of the minimal access surgeon have furthered this fast evolution. The classic open technique is successful in more than 90–95% cases and is routinely practiced by surgeons world over [1,2,3]. The minimally invasive approaches of laparoscopic pyeloplasty (LP) and retroperitoneoscopic pyeloplasty (RP) have well-documented advantages of less pain, shorter hospital stay and quicker recovery have interested more and more surgeons to follow these procedures. The comparison of the two approaches by various authors have a similar conclusion about their success in the outcome being equal; but there are differences of reports with regard to surgical time, ease of surgery, cosmesis, post-operative pain and early complications, hospital stay and early recovery [4,5,6]. This very well shows the possibility of mastering each technique by different surgeons, even though enhanced MIS skills are required for these operations. We looked at the technical considerations of retroperitoneoscopic pyeloplasty based on our early experience.
Materials and methods
This was a retrospective analysis of all the cases diagnosed as PUJO who had undergone RP from January 2016 to December 2017. Institution ethical clearance was taken vide letter no. INT/IEC/2019/001759. The case records and operative videos of 10 patients who had undergone RP for PUJO were reviewed. The patients were diagnosed with ultrasound kidney ureter bladder (KUB), Tc 99 ethylene dicysteine (EC) renal scan, in all cases. Magnetic resonance imaging (MRI) and intravenous pyeloplasty (IVP) was carried out in selected, equivocal cases.
Preoperative baseline evaluation was done complete blood count and renal function tests. The patients were admitted a day before the surgery, and written informed consent was obtained from the caregivers. Preoperative preparation included proctoclysis enema in the night before surgery. At the induction of anaesthesia, all patients were administered similar antibiotics intravenously (Cefotaxime 50 mg/kg) which was continued postoperatively for 72 h. All the patients underwent surgery by a single surgeon (MAM).
RP was performed using a lateral approach with retroperitoneal balloon distention. Anderson Hynes Dismembered pyeloplasty was performed over a stent using 6-0 polyglactin suture. Double J stent as per the age and the length of the remaining ureter was placed in all cases in an antegrade fashion. Patients were discharged on the 2nd or 3rd post operative day if oral intake was adequate and there were no complications. DJ stents were removed on outpatient basis under short general anaesthesia 3 weeks after surgery.
The patients underwent follow up ultrasound and EC scan, six months postoperatively and these were compared with the preoperative findings as per departmental protocol. Parameters studied were patient age, laterality, symptoms, operative time, technical aspects of all steps of surgery, postoperative complications, and hospital stay were assessed.
Surgical technique
The points of interest for discussion can be elaborated under headings including positioning of the patient, retroperitoneal space creation, sites of ports placement, techniques of handling pelvis and ureter, suturing and the DJ stent placement.
-
1)
Patient positioning: flank position, i.e., modified lateral position, keeps the patient in the lateral position with the side to be operated facing up, and the patient is brought close to the edge of the table (Fig. 1a). Adequate padding of pressure points and the lumbar region is done. The flank of the operative side is elevated and made more prominent by placing a roll or breaking the table. The added advantage of this position is to open the space between the costal margin and the iliac crest by 1.5–2 cm, which decreases the overcrowding of ports (Fig. 1b). This position places the patient at a higher height and hence disturbs the ergonomics for the surgeon, especially for a short-statured surgeon. We recommend to keep the operating table at the lowest and tilt the table towards the operating surgeon by approximately 30 degrees after port placement. The usage of a footstep improves the ergonomics of the surgeon.
Fig. 1 a Modified lateral position. b Without flank extension, showing distance of 5 cm from 12th rib to ASIS (anterior superior iliac spine). With flank extension, showing distance of 6 cm from 12th rib to ASIS (anterior superior iliac spine). Positioning of surgeon and assistant with relation to patient and monitor
The surgeon and assistant stand behind the patient with the monitor facing the surgeon. The patient should be strapped to the operating table and tilted as needed during the course of the surgery.
-
2)
Space creation: retroperitoneal space creation is the most important part of the approach. The space is created by a blind method. The anatomy knowledge of the retroperitoneal area is essential to reach the correct plain.
Skin incision 10–12 mm is given just (~ 1–2 cm) posterior to posterior axillary line midway distance between the iliac crest and 11th rib. The incision is deepened in a single plane to reach the retroperitoneal space. This initial step is extremely important to reach the right plane with minimal bleeding. A sterile glove finger and glove palm are tied to the ends of 2 feeding tubes 9 or 10 fr in size with silk sutures snugly as shown in the figure (Fig. 2a). A 50 ml syringe with a three-way stopcock is attached to the end of the glove finger feeding tube (Fig. 2b) after placing it in the retroperitoneal space created and then about 200 ml air is inflated into the glove finger to create the initial space. Then the glove palm is placed into the same space and about 800 ml air is inflated in it to create a larger working space.
a Glove finger and palm tied to infant feeding tubes. b Attachment of syringe 50 ml and stopcock to the customised balloon. Creation of retroperitoneal space with the customised balloon. Intravenous pyelogram showing pelviureteric junction at L5 level. Intravenous pyelogram showing pelviureteric junction at L2–L3 level
It is important to mention that the balloons should be directed superiorly as the peritoneal investment in this area is thicker and takes more pressure and time to separate, as compared to the inferior investment.
Port placement
The area for port placement in RP is limited, decreased by bony restrictions on the three sides (subcostal, spine and iliac crest), in comparison to the abdominal surface for LP hence precise port placement is important for the improved ergonomics. The IVP are useful in this context and serves as a good roadmap. Especially the initial camera port placement is guided well by the IVP (Fig. 3) and MRI images, after visualising the location level of the PUJO area with relation to the vertebra and ribs. This is especially more important for the high and deep PUJs which require the camera port to be placed slightly higher. As shown in the figures, the initial case shows the PUJ area to be at the level of L5 and the second case showed the PUJ area to be around L2–L3 level.
Once inside the retroperitoneal space, the CO2 is insufflated, and under vision the working ports are inserted- one about 1 cm superior to Anterior Superior Iliac Spine and the other between the 11 and 12 ribs. The decision for these ports placement is helped more by putting inside a needle initially to check the manoeuvrability of instruments by moving it in all directions. It is essential to stress upon the fact that in RP there is significant crowding of the ports both outside and inside the body. It, therefore, becomes imperative to have the correct port placement. We use self-retaining ports are used to prevent frequent slippage as well as leakage of gas during the procedure.
Handling pelvis and ureter
It is important to identify pelvis from the colon in the initial dissection. Gentle handling of the pelvis and ureter is of utmost importance. We do a three-port procedure as mentioned above and to be improved with the traction, we initially start with one hitch stitch at pelvis which stays towards the ureter after dismembering. A 2-0 prolene stitch is taken from the abdominal wall towards the lower pelvis. In cases where the pelvis is small two separate hitch stitches may be taken on the pelvis and the PUJ to prevent retraction. It is important to remove ureteric hitch stitch after taking the first bite of anastomosis, otherwise, it entangles with stitching sutures frequently. Rarely in obese patients taking out the needle through the abdominal wall is difficult. In these cases, a knot of the traction suture can be taken and needle left in situ, so that the traction stays maintained till the end of the procedure. Forth port is used only in cases with malrotation kidneys.
Space limitation and also essentiality of fine instruments for gentle handling makes the usage of 3 mm instruments helpful. The availability of 3 mm instruments can be an issue in a resource-challenged system. We thread these 3 mm instruments in longitudinally incised feeding tubes (size 9 or 10 Fr) and place them through 5 mm ports, as an additional sheath over them to prevent air leak from the 5 mm ports. Moreover it reduces instrument clashes in the limited space.
Spatulating the ureter is technically challenging and it is essential to do that in the correct direction of the ureter. It is important to align the ureter to the upper working port and use 3 mm scissors to make the first cut because it is often difficult to insert big 5 mm scissor prongs inside the ureter.
Suturing tips
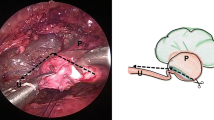
Intracorporeal suturing is the rate-limiting step in RP. The first stitch that puts the distal-most part of the spatulated ureter and the distal-most part of the pelvis together is considered to be one of the most important steps of the anastomosis. This stitch should be aimed to make the anastomosis dependent. Caution must be exercised to take small bites to not compromise the lumen. We use an infant feeding tube with a cut length of 8–9 cm for temporarily stenting the ureter and helps by keeping the ureter rigid and stabilised to make the first stitch easier (Fig. 4). Alternatively, the ureteric area to be sutured may be opened by the jaws of a Maryland forceps.
The length of the suture is usually 10–12 cm but for first stitch the gap length between pelvis and ureter is added to the suture. Interrupted 6-0 polyglactin sutures at apex and two each on both layers create new UPJ to circumvent the purse-string nature of continuous suturing.
The use of a Cinch sliding knot at the beginning of anastomosis avoids the usage of a fourth port and reduces the problem of loosening the first stitch under tension.
DJ stent placement
DJ stent placement in minimally invasive surgery is a demanding task, as the orientation of the DJ with its guide wire has to be aligned well with the stretched ureter along its length. We use a forceps designed for performing intraoperative cholangiogram which places the DJ stent (one end closed) tip just at the ureteric cut edge and with the natural curve of the DJ directed towards the ureteric length, the DJ can be pushed in with the help of the stent pusher from behind. A feel of giving way at ureterovesical junction and reflux of urine through pores of the upper part of DJ stent ensure entry of DJ stent into the bladder.
Rest of the anastomosis is done with continuous suturing using 5-0 Polyglactin. Hitch stitches and excised UPJ and pelvic are taken out before taking out ports and are stitched in two layers.
Results
During the study period a total of 10 patients with diagnosed PUJO were operated. The mean age of the patients was 8.4 ± 2.31 years (5–11 years). The majority of them were males (M:F = 9:1) and the main presenting complaint was pain abdomen, localised usually to the side of the obstruction, except in one patient who had presented with backache and PUJO was incidentally diagnosed on MRI. Before surgery, the blood reports of all the patients were normal.
PUJO was more on the left side (in eight patients)—Out of the other two, one had right PUJO and the other had bilateral involvement. Almost all patients were labelled as PUJO with nuclear scans demonstrating obstructed drainage, except in one patient who was symptomatic but had shown slowed drainage in both EC scan and IVP(done as EC was equivocal), a Retrograde pyelogram was done which showed abrupt cut off at PUJ and difficulty in pushing in contrast to the pelvis, which was dilated.
Intraoperatively, there were crossing vessels seen in two patients and the others had internal PUJ narrowing of varying lengths. The mean operating time was 207.5 ± 36.15 min (150–285 min) with an average hospital stay of 3 ± 1.49 days (2–7 days).
One patient developed an anastomotic leak with a perinephric collection which had to be drained with a percutaneous nephrostomy (PCN) insertion. He gradually improved and was discharged in 7 days. Percutaneous Nephrostogram done two weeks later showed drainage across the PUJO, and PCN was removed following that. Removal of the DJ stent was done after 2 months. In the other patients, DJ stent was removed after 3 weeks duration.
No patients were lost to follow up and the mean follow up period was 15.1 months (6–20 months).
The patients being reviewed clinically in the outpatient department and also EC scan have been repeated at 3–6 months. All the nine patients are doing well clinically with healed scars, except the patient who had presented with backache continues to have a mild backache. The drainage in the follow-up investigations of all the patients show improvement except the patient who had a postoperative anastomotic leak, he continues to have slow delayed drainage (Table 1).
Discussion
PUJO is one of the most common congenital defects of the ureter and also one of the commonest causes of hydronephrosis. The treatment is the surgical correction of the obstruction once the obstruction is confirmed by imaging of the kidney and ureter. The gold standard has been the open dismembered pyeloplasty via a retroperitoneal approach, nearing a success rate of more than 90% [1,2,3].
The advent of minimally invasive surgery over the past few decades with its several advantages including—reduction of flank incision related morbidity, less pain, earlier recovery, lesser hospital stay, better cosmesis, etc. have been successfully achieved over the years. This began with the introduction of endourological approaches which succeeded in achieving improvement in drainage with less invasive techniques but the success rate being lesser, approximately around 80%, did not stay long as the preferred technique [7,8,9]. LP was reported initially in adults by Schuessler et al. in 1993 and then in children in 1995 by Peter et al. and Tan et al. [10,11,12]. The LP has emerged as a feasible and reliable treatment alternative to open surgery because it strictly imitates the open dismembered technique. RP followed, with the initial report given by Janetschek in 1996 [4]. However they struggled with this approach due to the limited space and technical suturing difficulties and commented that it took the longest operative time among their cases approached by certain minimally invasive techniques.
Yeung et al. then reported their collection of PUJO cases approached retroperitoneoscopically with a mean age of 2.7 years (0.25 to 10 years), in which 12 out of 13 cases were successfully operated by this approach. One had to be converted to open due to previous percutaneous nephrostomy tube insertion for pyonephrosis, hence leading to adhesions and bleeding. They emphasised upon various methods and technical pearls including the correct positioning of the patient, peritoneal separation medially and superiorly, keeping a short segment feeding tube to stent the upper cut edge of the ureter and usage of fine 3 mm instruments. With these refinements of techniques, they felt that the success of retroperitoneoscopic approach was successful, even in young patients, as three of their patients were less than 6 months of age, unlike Tan et al., who felt the technique was difficult in < / = 6 months [13]. We believe that focus on the specific technical aspects helps to reduce the learning curve and improve the ergonomics of the procedure.
Bachmann et al. in their publication of 47 cases stressed the advantages of the retroperitoneal approach for PUJO and how they have gone on to use it for all renal surgeries [14]. They successfully completed the procedure in 45 patients, whereas the other two had to be converted to open due to scarring after endopyelotomy in one and difficulty in space due to massive obesity in the other. They concluded that the retroperitoneoscopic approach is comparable to open procedure, but a good knowledge of retroperitoneoscopy is essential. With their experience, they commented on the importance of technical steps which could be followed to ease the procedure. Initial was the importance of positioning wherein an extended flank position was preferred to open up the side of flank to be operated upon. They defined certain landmarks to be identified after entering the right space. Our approach differs as we use air insufflations as compared to their water insufflations.
RPP has been reported to be at par, if not better, than open pyeloplasty by certain studies. Valla et al. in their retrospective comparison of RPP and open dismembered study in 90 patients for PUJO (45 in each group) described the advantages of RPP as—decreased hospital stay, equal complication and success rates as open pyeloplasty; and the disadvantage as the increased operative time as compared to open technique [15]. Wang et al. interestingly reported a comparison between RPP (n = 113) and open pyeloplasty (n = 59) wherein, they found that operative time, as well as hospital stay, was shorter in RPP and success was equivalent in both techniques (RPP 98.1% vs open 98%). They reported a lower complication rate in RPP (4.42% vs 6.78%) [16]. Khoder et al. described the outcome measures from the patients’ perspectives after prospectively evaluating RPP (n = 75) and open pyeloplasty (n = 32). This is an initial study of such a study being done from the patients’ point of view and the two measures that were analysed include—postoperative health-related quality of life (HRQoL) and patients’ subjective evaluation of both procedures through a questionnaire. Their results revealed a complete patient satisfaction with a better HRQoL for RPP [17].
In our study of 10 cases of RP we tried to understand the limitations in each step, which made the procedure difficult and which are key in challenging the surgeon and tried to contribute certain suggestions for easing the technique, accordingly. Starting with the patient positioning, extended flank position exposes the flank better but also increases patient height and thus makes elbow and shoulder angle more acute. To overcome this, we used footstep elevation and tilted table towards surgeon for better ergonomics.
Space creation is vital as unlike other minimally invasive procedures, wherein sufficient space for working already exists, the working space has to be created in here. The entry of the ports—camera, as well as the rest of the working ports, should be well thought of as space limitation can cause clashing of instruments and disturb with the surgery. For this we adopted slightly posterior placement of camera port so that enough space is felt laterally for secondary working ports. The initial camera placement was also guided by the imaging, after having a look at the PUJO area on IVP or MR urography. This became more important in cases of high and deep PUJOs that require the camera port to be placed slightly higher. To increase the distance between ports, the upper working port was placed between 11 and 12th rib space in all the cases, keeping the lung down while putting it inside.
Gentle handling of the delicate tissues in this procedure are helped by using fine instruments (3 mm) Suturing in a limited space is more difficult in comparison to laparoscopy or open procedure. Certain tactics to stabilise the ureter and knotting have been elaborated. DJ stenting has been seen to help in pyeloplasty anastomosis, by draining the pelvis area well to the bladder and decreasing the tension at the anastomotic site. Placing it has been a challenge and the ease of this step by the usage of a cholangiogram forceps has been mentioned.
Follow up of our patients has been satisfactory like other authors, who also find retroperitoneoscopic pyeloplasty, laparoscopic pyeloplasty and open pyeloplasty to have successful outcomes in children of various ages.
Conclusion
Tips and tricks to retroperitoneoscopic pyeloplasty for beginners are essential and not many authors describe their surgical experiences in detail. We believe that the correct positioning, space creation, port placement, Suturing techniques and the insertion of DJ stent by our methods help to reduce the steep learning curve associated with this procedure.
References
Mikkelsen SS, Rasmussen BS, Jensen TM et al (1992) Long-term follow-up of patients with hydronephrosis treated by Anderson-Hynes pyeloplasty. Br J Urol 79:121
Clark WR, Malek RS (1987) Ureteropelvic junction obstruction—observation on the classic types in adults. J Urol 138:276–280
Nguyen DH, Aliabadi H, Ercole CJ, Gonzalez R (1989) Nonintubated Anderson-Hynes repair of ureteropelvic junction obstruction in 60 patients. J Urol 142:704
Janetschek G, Peschel R, Altarac S, Bartsch G (1996) Laparoscopic and retroperitoneoscopic repair of ureteropelvic junction obstruction. Urology 47(3):311–316
Canon SJ, Jayanthi V, Lowe GJ (2007) Which is better—retroperitoneoscopic or laparoscopic dismembered pyeloplasty in children? J Urol 178:1791–1795
Singh V, Sinha RJ, Gupta DK, Kumar V, Pandey M, Akhtar A (2014) Prospective randomized comparison between transperitoneal laparoscopic pyeloplasty and retroperitoneoscopic pyeloplasty for primary ureteropelvic junction obstruction. JSLS 18(3):e2014.00366. https://doi.org/10.4293/JSLS.2014.00366
Motorola JA, Badlani GH, Smith AD (1993) Results of 212 consecutive endopyelotomies: an 8 year follow-up. J Urol 149:453
Cohen TB, Gross MB, Preminger GM (1996) Long-term follow-up of Acucise incision of ureteropelvic obstruction and ureteral strictures. Urology 47:317
Tan HL, Roberts JP, Grattan-Smith D (1995) Retrograde balloon dilatation of ureteropelvic obstructions in infants and children: early results. Urology 46:8
Schuessler WW, Grune MT, Tecuanhuyey LV et al (1993) Laparoscopic dismembered pyeloplasty. J Urol 150:1795
Peters CA, Schlussel RN, Retik AB (1995) Pediatric laparoscopic dismembered pyeloplasty. J Urol 153:1962–1965
Tan HL, Roberts JP (1995) Laparoscopic dismembered pyeloplasty in children/preliminary results. Br J Urol 153:1962–1965
Yeung CK, Tam YH, Sihoe JD, Lee KH, Liu KW (2001) Retroperitoneoscopic dismembered pyeloplasty for pelvi-ureteric junction obstruction in infants and children. BJU Int 87:509–513
Bachmann A, Ruszat R, Forster T et al (2006) Retroperitoneoscopic pyeloplasty for ureteropelvic junction obstruction (UPJO): solving the technical difficulties. Eur Urol 49:264–272
Valla JS, Breaud J, Griffin SJ, Sautot-Vial N, Beretta F, Guana R et al (2009) Retroperitoneoscopic vs open dismembered pyeloplasty for ureteropelvic junction obstruction in children. J Pediatr Urol 5:368–373
Wang X, Zhang Z, Peng N, Liu C (2013) Retroperitoneal laparoscopic versus open dismembered pyeloplasty for ureteropelvic junction obstruction. J X-ray Sci Technol 21:429–439
Khoder WY, Waidelich R, Becker AJ, Karl A, Haseke N, Bauer RM et al (2014) Patients' perception of surgical outcomes and quality of life after retroperitoneoscopic and open pyeloplasty. Urol Int 92:74–82
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Rights and permissions
About this article
Cite this article
Malik, M.A., Yhoshu, E., Peters, N.J. et al. Technical considerations in retroperitoneoscopic pyeloplasty in children: an early experience. J Ped Endosc Surg 2, 131–138 (2020). https://doi.org/10.1007/s42804-020-00056-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42804-020-00056-8