Abstract
Biochar amendment improves the physical, chemical and biological characteristics of different soil types under different climatic and environmental conditions. In this study, effects of biochar or live pasture plants existing alone or co-existing on selected soil properties of sandy loam soil under humid lowland tropical climatic conditions were investigated. The changes measured in the amended soil, with or without plants, were compared to the unamended and unplanted soils. Biochar amendment with or without pasture improved moisture retention, lowered bulk density, increased pH and kept the electrical conductivity within ranges conducive for pasture growth. Generally, contents of all the nutrients increased following biochar amendment, however pasture establishment without amendment resulted in depletion of available potassium and magnesium. Under all treatment conditions, soil organic carbon and soil organic matter were significantly depleted. Cogon grass is invasive under all land use systems and contributes to greenhouse gas emissions through slash-and-burn. Using biomass from the grass instead of burning would mitigate CO2 emissions from the tropics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Under general soil use and management of pasture in the tropics, water retention is poor in sandy soil (e.g. Rijsberman 2006; Basso et al. 2013). Consequently, pasture cultivation in sandy soil requires more frequent irrigation than normal and these soils are often deficient in nutrients needed to support healthy pasture growth (Burrell et al. 2016). Application of inorganic fertilizer is the main option to address the deficiency. However, acquisition and application of inorganic fertilizers is expensive, and affordability is an issue in most small farms in the tropics. Soil fertility issues, buildup of pests (insects and weeds) and diseases are managed through fallowing, a farming practice which allows a soil to revert to its natural vegetation for a number of years (15–30 years) after cropping for certain period (1–3 years). Allowing a soil to fallow results in nutrient replenishment, builds up moisture, breaking of pests and disease cycles, addition of organic matter (OM) and improvements in the physical, chemical and biological characteristics of soils (Aipa and Michael 2018). Fallow is a common practice in rural pasture and crop lands and widely regarded as an effective practice to protect the soils. Pertaining to sustainability, fallow rebuilds the natural vegetation and in doing so prevents biodiversity loss by allowing plants and animals to recolonize the land (Aipa and Michael 2019).
In the Amazon Basin, the indigenous people created islands of rich, fertile soil called “terra preta”, black earth, by amending soil with biochar (a form of charcoal produced by exposing organic materials to high heat in a low oxygen environment) (Sombroek et al. 2003). Like fallow and organic matter turnover which are important to various soil uses, several studies showed that biochar is necessary for soil fertility management, water retention, reducing soil acidity and retaining difficult to hold nutrients like nitrogen and phosphorus (e.g. Karhu et al. 2011; Luo et al. 2011). Because biochar is persistent and holds soil nutrients for a long period of time, it helps reduce fertilizer needs compared to organic matter from fallow which are short-lived. More studies further show that biochar application improves plant growth and yield (Faloye et al. 2019), increases production and enhance sustainability of depleted soils with limited organic resources, water or access to inorganic fertilizers (e.g. Atkinson et al. 2010; Artiola et al. 2012; Basso et al. 2013; Palansooriya et al. 2019). In poor soils (e.g. sandy loam) in the tropics with warm climate and heavy rainfall, biochar application is a potent source of carbon to improve pasture growth and yield yet studies on biochar uses under various land use systems (Gunarathne et al. 2020; Ding et al. 2016), e.g. in croplands or on soil under pasture, are limited. Therefore, this study was conducted to investigate the importance of cogon grass (Imperata cylindrica) biochar amendment in the presence or absence of pasture under humid lowland tropical climatic conditions.
2 Materials and methods
2.1 Description of soil and pasture collection site
The farm from which the soil samples and planting materials were collected is shown in Fig. 1 (Aipa and Michael 2018, 2019; Michael 2019a) in Lae, Morobe Province, PNG (6°42′55.89″ S; 146°59′59.66″ E). The farm (6°41″ S, 146°98″ E) is located at an altitude of 65 m above sea level. The mean annual rainfall is up to 3800 mm, which is distributed throughout the year. Average daily temperature is 26.3 °C, with an average daily minimum of 22.9 °C and an average daily maximum of 29.7 °C. Annual evaporation (US Class A pan) is 2139 mm and rainfall exceeds evaporation in each month. The climate is classified as Af (Koppen), i.e. a tropical rainy climate that exceeds 60 mm rain in the driest month. The soil is well drained of alluvial deposits and is classified as sandy, mixed isohyperthermic, Typic Tropofluents (US Soil Taxonomy) or Eutric Fluvisol (World Reference Base) (Aipa and Michael 2018, 2019; Michael 2019a).
2.2 Soil collection
A stripping method as described by Michael (2019a) was used to collect soil samples at 0–30 cm from the surface. Several buckets of this soil was collected and taken to the greenhouse. Prior to setting, the soil was homogenized by mixing using a spade on the surface of a canvas. This was evenly spread and air dried on the canvas under greenhouse conditions for 3 h. The dry soil was sieved using a 0.5 mm sieve.
2.3 Biochar preparation
To use as biochar, cogon grass leaves were collected from the same site at which the farm soil samples were collected. Cogon grass is an invasive weed and removing it is an important option to manage it invasiveness while at the same time providing abundant and inexpensive plant material for making biochar. Plant materials from this grass were brought to the greenhouse and sun dried on a metal bench for three days, and then chopped into small pieces of equal size (5 cm) and oven dried at 70 °C for 4 days. The brittle plant materials were placed inside a small aluminum pot and burned at 600 °C for 3 h to properly carbonize the biochar.
2.4 Planting material
Several young and intact shoots (with roots and leaves), 5–10 cm tall and 4 weeks old of Panicum coloratum L. were harvested from the farm (described previously) by digging them up from the soil using a small spade. The young shoots were carefully separated from the older plants and cleaned. Excess roots and leaves were trimmed using a sharp scissor. These were kept in a bucket of water to prevent dehydration and brought to the greenhouse to be used as propagules. Panicum coloratum is an important tropical forage for livestock, well adapted to hot climates like the tropics and tolerant to stresses associated with water limitation (Fig. 2).
Soil sampling and sample preparations. a A sample photo showing 6 months old pasture plants in experimental polythene pots, b a core sampler driven into the soil, c taking soil core from the sampler, d a soil core taken out of the sampler, e a soil core laid among a ruler and chopped, f the cut core spread and air dried, g sieving dried core using a 0.5 mm sieve and h dried soil samples packed in small paper bags for analysis
2.5 Experimental treatments
The soil was mixed with biochar (5:1 soil: char w/w) and polythene pots (190 mm in height and 200 mm in diameter) were filled with the mix, henceforth referred to as “amended soil”. A total of four treatments with polythene pots containing approximately 1200 g of the natural (soil without amendment) or the amended soil were prepared. The first treatment was the control using natural soil (no amendment). The second treatment was soil with plants but without amendment. The third treatment was amended soil with no plants. The fourth treatment was the amended soil with plants. In all the planted treatments, a total of three shoots (tillers) each were planted. All the treatments were replicated four times (giving a total of 16 polythene pots) and set in complete randomized design (CRD) under greenhouse conditions. The treatments were watered twice a day using tap water for 6 months after which the pasture plants were fully grown and ready for harvest (Fig. 2a).
2.6 Sampling
Soil sampling for analysis and measurements was done as per Michael (2019a), shown in Fig. 2. A hollow tube (200 mm in height and 50 mm in diameter) was manually driven into the soil in the polythene pots (Fig. 2b) and a core was taken (Fig. 2c, d). A second sample was taken from the adjacent side to ensure enough was obtained. These cores were placed along a rule (30 cm) and cut into small sections of 0–60, 60–120 mm and 120–180 mm, respectively (Fig. 2e). Cut core samples of a profile (e.g. 0–60 mm) of all the treatments were mixed together (Fig. 2f), air dried for 3 h and sieved (Fig. 2g). Triplicate 500 g (wet weight) samples were packed in small paper bags (Fig. 2h) and sent to the laboratory for further processing and analysis.
2.7 Measurements and analysis
Triplicate samples (n = 3) from the 0 to 60 mm and 120 to 180 mm were used. To organize and present the data collected clearly, soil from 0 to 60 mm profile are hereafter referred to as “surface soil” and from the 120 to 180 mm profile as “deep soil” respectively. The data from the 60 to 120 mm profile were not used as they were similar to the deep soil data. pH was measured using a standard dilution (pH meter (1:5 soil: water w/v)) method (Michael et al. 2015) using an Orion pH meter (720SA model). The soil organic carbon (SOC) content (%) was analyzed using the weight loss-on-ignition method (Schulte and Hopkins 1996). Five grams of the samples was placed in a crucible and heated in a muffle furnace for 12 h at 105 °C to remove moisture (Wf) and combusted again at 375 °C for 17 h (Fw), cooled for 2 h and weighed (Michael 2019a). The soil residue in the crucibles was combusted in a muffle furnace at 800 °C for 12 h, cooled for 2 h and reweighed. The SOC content was estimated by multiplying the carbon value by a conversion factor of 1.72 and expressed as percentage:
The conversion factor was used to convert the organic matter content to organic carbon, assuming there was 58% carbon in the organic matter. The size of the carbon stock in each profile was calculated as the sum of the individual carbon fractions (%) × g cm−3 × profile depth (cm) and expressed as percentage as:
The soil organic matter (SOM) contents were estimated using the SOC contents and the conversion factor (1.72) as:
The water holding capacity (WHC) was estimated as per (Michael 2019a) by setting soil samples at 100% WHC after soaking in water and draining through filter paper overnight. The wet weight (Ww) was recorded and the samples dried in an oven at 105 °C for 48 h and reweighed to obtain the oven dry weight (ODw). WHC was determined as:
Bulk density (g cm−3) was calculated by oven drying of the cores at 105 °C for 48 h followed by re-weighing (Aipa and Michael 2018). The oven dry weights were divided by the volume of the core to obtain the bulk density. Total porosity was determined as per Landon (1991):
P is total porosity (%), BD is bulk density and d is particle density equal to 2.65 g cm−3.
Analysis for nitrogen, potassium, phosphorus and magnesium were done at the University Analytical Service Laboratory using standard analytical procedures: Kjeldahl (Buchi K436 speed digester and Buchi K-350 Kjeldahl distillation unit) for nitrogen, OLSEN (Shimadzu 1800 UV/VIS spectrophotometer, Mettler Toledo, Model UV5Bio) for phosphorus and magnesium using ICP-OES (Spectro ARCOS brand) following 1 M NH4Cl extraction. Electrical conductivity was measured using a Direct Soil EC meter (Spectrum Technologies Inc., 12360S Industrial Dr. East Plainfield, IL 60585) using solutions (1:5 sample to water w/v).
The data in milli-equivalent (mEq./100 g soil) were converted to milligram (mg) as:
where Aw is atomic weight of an element (e.g. nitrogen) and V is valence, respectively.
The weight of the SOM to a given depth and area was estimated as:
where SOC is in %, BD is in g cm−3, SP is in m and ha is hectare (10 000 m2).
2.8 Statistical analysis
Statistical analysis was performed as reported in previous studies (e.g. Michael et al. 2015, 2016, 2017). The treatment averages of a profile for each parameter (e.g. pH) were obtained by taking the mean of the three replicates. To compare the treatment means, significant differences (p < 0.05) between treatment means of each profile was determined by two-way ANOVA using statistical software JMPIN, AS Institute Inc., SAS Campus Drive, Cary, NC, USA 27513. If an interaction between the treatments and profile depths was found, one-way ANOVA with all combinations was performed using Turkey’s HSD (honest significant difference) and pairwise comparisons.
3 Results and discussion
3.1 Water holding capacity
The WHC estimations are shown in Fig. 3. In the control, WHC was approximately 28%, and in the surface of the unamended planted soil increased to near 30% and to 36% in the deep soil, respectively. In the amended soil without pasture, the highest WHC was measured in the surface soil which then decreased at the deep soil by nearly 2% (Fig. 3). When biochar and pasture plants co-existed, WHC was almost the same as in the amended treatment without pasture plants, the changes ranging from between 37% at the surface to 36% at the deep soil (Fig. 3). Sandy soils have low organic matter content, hence reduced WHC and the potential to hold soil nutrients. Organic amendments have been shown to improve soil chemical and physical properties and increase yield of crops (e.g. Atkinson et al. 2010; Gomez 2013; Rahim et al. 2019).
Effects of biochar, pasture, or biochar and live pasture co-existing on soil water holding capacity. The values are mean ± standard error of three replicates (n = 3). An asterisk indicates significant difference (p ≤ 0.05) between the control and the treatments at the same depth. The legend “Bio + planted” is the amended and planted treatment
The results presented in Fig. 3 showed biochar amendment and organic matter additions from live plants are important to improve WHC of sandy soil. Compared to the changes caused by biochar amendment, the increase in WHC of the planted soil probably resulted from root exudates of the pasture plants (Michael 2019a; Michael et al. 2017). Soil degradation and poor pasture management in the tropics are concerns for many farmers (Strassburg et al. 2014) and biochar application is important to improve the WHC and pasture productivity. The results further indicated that the presence of pasture is as important as biochar amendment for improvement of the WHC, demonstrating that co-existence of both organic amendment and live pasture plants is important. Biochar amendment increased the WHC by 9% at the surface and by 4% at deep soil (Fig. 3), translating to 1.9% and 0.7% SOC in the same profiles. These results strongly demonstrated that biochar application under general soil use and management condition, especially in the absence of live plants, has the potential to sustain SOC, thereby increasing the surface soil WHC (Michael and Reid 2018). Compared to this, WHC decreased in the planted soils as water was drawn by roots for growth and cellular metabolism and to compensate for evapotranspiration losses (Michael et al. 2017; Michael and Reid 2018). In the amended and planted soil, the opposite happened, that is, even if water was used as described, the inherent porous nature of biochar to retain water was sufficient to increase the WHC. In addition, roots added benefits (e.g. simple organic carbon sources and exudates) capable of establishing microenvironments due to soil microbial respiration (Michael 2018a), sufficient to improve water retention potential and WHC of the sandy soil. This is the most probable reason WHC of the biochar amended deep soil was lower than that of the amended and planted soil at the same profile (Fig. 3).
During drought events, sandy soils lose a lot of moisture and organic amendment conserves it for sustainability of pasture growth. Water retention is also dependent on soil particle composition and porosity. WHC was high (Fig. 3) when porosity was low, in agreement with Prober et al. (2014) and Lewis et al. (2006). Effects of biochar on WHC seem to be dependent on soil type, with the changes being more significant in sandy soil than in other soil types (Lewis et al. 2006).
3.2 Bulk density
The soil BD estimated as described previously is shown in Fig. 4. In the control soil, the changes ranged from between 0.6 and 0.8 g cm−3. Compared to these changes, BD of the unamended soil and planted soil were similar, whereas in the biochar amended soil decreased to 0.5 g cm−3 in the surface soil and 0.4 g cm−3 in the deep soil, respectively (Fig. 4). The highest BD was measured in the surface of the soil when amended and planted, which significantly decreased in the deep soil.
Soil pore spaces are related to the BD; as the latter increases the available pore spaces decrease. The changes in porosity are given in Table 1. Total porosity (P) was much higher in the biochar amended soil compared to other treatments. In the biochar amended and planted soil, P decreased by nearly 3% in the surface soil and increased by 6% at the deep soil, respectively (Table 1), consistent with the findings of Luo et al. (2016). The decrease in porosity in the surface of the amended soil in the presence of plants was probably caused by the combined weight of the plants and the soil held together by the plant roots. Generally, low BD resulted in high porosity. For instance, when BD was lowest at 0.5 g cm−3 within the surface of the amended soil (Fig. 4), P was 82% (Table 1). These results are consistent with the findings of several studies (e.g. Castellini et al. 2015).
3.3 pH
Soil pH influence many biogeochemical processes (Michael and Reid 2018) and was described as the “master soil variable” (Minasny et al. 2016; Brady and Weil 1999). It controls solubility, mobility and bioavailability of nutrients, increases solubility of organic matter and mineralization of carbon and nitrogen as well as other soil nutrients (e.g. Michael et al. 2015). Soil pH in turn is influenced by processes that occur in the rhizosphere where protons (H+) and hydroxyl ions (−OH) are regulated depending on the nutrient status of the soil and plant types present (Hinsinger et al. 2003). Application of organic matter which releases alkalinity, decarboxylation of organic anions, ammonification and nitrification of nitrogen and association and dissociation of organic compounds affect pH (Xu et al. 2006). The changes in soil pH measured are shown in Fig. 5. The control soil remained nearly unchanged at 6 units. Compared to this, amendment increased the pH by 0.5 units within the surface soil and by 0.3 units in the deep soil, respectively. The changes induced are dependent on the residue quality and the results indicate the source as a typical monocot of poor chemical and biochemical composition (Butterly et al. 2011). When we applied raw organic matter of high nitrogen content in acid sulfate soils of varying pH, huge differences between initial and final treatments were observed (e.g. Michael et al. 2015, 2017), indicating the initial pH and organic matter type affect the changes.
When pasture was established without amendment, small increases in pH were measured, ranging from 0.2 to 0.1 units (Fig. 5), confirming that roots have the tendency to increase pH (Bravin et al. 2012; Shi et al. 2011). When pasture was established in the amended soil, pH also increased but the changes were smaller than those without pasture. The results strongly indicated that amendment increases soil pH but co-existence with live plants lowers it. The probable reason for this is that some of the organic carbon and anions released into the soil from the biochar were used by plants for growth and development. This is supported by the observation where SOC content of the amended soil and planted was smaller compared to the changes in all the treatments (Fig. 5). We have reported that plant roots facilitate oxygen penetration into the soil by cracking which then leads to oxidation reactions, lowering the pH (e.g. Michael et al. 2016, 2017). The alkalinizing effect measured most likely came from mineral carbonates and basic-charged groups in the biochar (Yuan and Xu 2011). Soil exchangeable base cations and base saturation are other characteristics of biochar that have alkalinizing effects on soil pH (Dai et al. 2017).
3.4 Electrical conductivity
In agricultural soil, salinity is related to concentration in soil pore water of sodium, magnesium, calcium, chloride, sulfate, bicarbonate, and potassium and nitrate ions. As salinity increases, the potential pore water decreases, requiring plants to overcome a high energy gap for water uptake from the soil. Under certain conditions, nutrient toxicity results as imbalance sets in, resulting in low biomass or crop productivity (Friedman 2005). In the control soil, electrical conductivity (EC) was higher at the surface soil then decreased to 0.01 dSm−1 in the deep soil (Fig. 6). The opposite happened when pasture was planted. The EC decreased within the surface but rose in the deep soil to 0.04 dSm−1. In the amended soil with or without plants, the changes in EC were smaller at the surface than in the deep soil and the changes were nearly the same at each profile (Fig. 6). A soil is considered safe when EC is within a range of 0.0–0.75 dSm−1 (Lane 1985), demonstrating the sandy soil was reasonably safe for the plants. The results indicate also that the influence of either biochar or plants alone or co-existing is variable, and this may depend on the particular soil and plant types. The variability in EC in the planted soils with and without biochar and low EC in the unamended planted soil and high when co-existing, tend to suggest that plants had minimal to no effect, and that the increase was due to biochar.
3.5 Soil organic carbon
In sandy soil, SOC is important to improve WHC, rain infiltration, nutrient availability and plant growth (Milne et al. 2015). The SOC was high in the control soil throughout the profiles (Fig. 7), even compared to the SOC of 2.5% (i.e. 0.3 g C) from the site where the samples were taken as reported in Michael (2019a). This significantly decreased to near 6% (0.7 g C) in all the treatments in the surface soil. In the deep soil, the changes were variable; the lowest being measured in the amended soil with plants. The decrease in SOC measured in all the planted soils showed C was used by plants. The native OM content [estimated using (7)] decreased under all the treatment conditions except that the contents were high in the planted soils (Table 2). This demonstrated that no OM was added in the soil from the amendment except from the live plants.
Biochar amendment resulted in a decrease in SOC which can probably be explained by plant uptake of labile SOC. In agricultural soil, continuous use, poor land use management practice such as short fallow and tillage causes C fractions to decrease. In such soils, the recalcitrant C fractions (e.g. particulate or humus) are unable to establish a functional microbial ecology to enhance decomposition unless the labile fraction becomes available. Decomposable products (simple sugars) of biochar and root exudates of plants enhance the capacity of the established functional microbial ecology to act on the recalcitrant fraction, lowering its content. This is supported by the fact that C sourced from biochar is difficult to decompose, contributing to the recalcitrant C pool in a soil environment (Lorenz and Lal 2014) and not readily available in the soil or to pasture.
3.6 Total nitrogen
Nitrogen is an essential nutrient for all living things and required in large quantities in many soil types. Most of the nitrogen comes from mineralization and bacterial fixation, with ammonium and nitrate being the available forms to plants (Ma et al. 2016). In the humid tropics, nitrogen retention is low because of heavy rainfall, leaching, denitrification and volatilization, making management of this nutrient a challenge. The challenges of knowing the quantity of nitrogen available in the soil, the right type of nitrogen source to add and to minimize the losses under the prevailing environmental and climatic conditions. The sandy soil used in this study had 0.4% nitrogen, a strong indication of need of the nutrient. As shown, amendment, planting or amendment followed by pasture establishment increased nitrogen by nearly 0.11% throughout the profiles (Fig. 8). In the deep soil with plants, even with amendment, a decrease in nitrogen content was measured; indicating nitrogen use by pasture. The nitrogen content of cogon grass is relatively low and the contribution by 0.7% is proportionate to its content, compared to nitrogen contributions from biochar originating from legumes or animal wastes (Shinogi 2004).
The implication for these findings is that in the humid tropics, establishing pasture for large-scale livestock production is still an issue and limited to a few farms. The results showed that biochar amendment to sandy soil prior to establishing pasture plants improves the nitrogen content, which is important for pasture productivity. The biggest challenge is the amount of plant material that is needed to make the char to be applied on big farms because of the costs and labor requirement associated with collection and preparation. If making biochar becomes expensive, the results indicated that there is potential for establishing pasture without biochar amendment, at least in the surface soils, however the need for nitrogen by pasture will eventually lead to depletion. This reduction in nitrogen is manageable by pasture-legume rotations such that after a period of time, a suitable legume is established and rotated with the same pasture or another pasture species (Vinod et al. 2016; Hocking and Reynolds 2012). Consistent with these findings, Rahim et al. (2020) reported biochar-legume interactions significantly increased total nitrogen, an important implication for general soil use and management.
3.7 Available phosphorus
As shown in Fig. 9, pasture establishment alone increased phosphorus content by 89 mg kg−1 in the deep soil (Fig. 9). In the amended soil, phosphorus content increased further by 2 mg kg−1 consistent with Ali et al. (2015) where biochar application significantly increased total phosphorus but decreased when amended and planted. In the surface soil, phosphorus contribution from co-existence of biochar and pasture was high, compared to that of the deep soil with plants. Comparatively, increase in content by biochar amendment alone was smaller than when planted (Fig. 9). These results demonstrated that co-existence of biochar and live pasture is important, contributing to significant availability. In the tropics, heavy rainfall leads to leaching and resultant contamination of water sources, dependent on the source and types of phosphorus, soil types and pasture species (Blum et al. 2013; Townsend et al. 2015; Vendramini et al. 2007; Aguiar et al. 2013). Biochar amendment followed by planting lowered phosphorus in deep soil, an important indication that biochar availability enhances uptake by live pasture or supports functions of soil microbes. Uptake of phosphorus depends on pasture species, underground biomass, rhizosphere microbial ecology and general climatic and environmental conditions (Duubel and Merbach 2005). The plant available phosphorus, unless removed by livestock, returns to the soil and is used again by the pasture, sustaining phosphorus cycling in the pasture ecosystem.
3.8 Available potassium
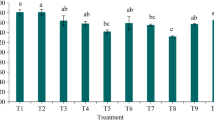
Planting pasture without amendment nearly depleted the potassium (K) content (Fig. 10). For example, in the surface soil of the control, 3 mg K was present which decreased to 1.4 mg when planted. Existence of biochar alone or co-existing with pasture increased K content but the changes were much higher in the absence of plants. This indicated that even though biochar addition increased the K content, some was used by the live pasture (Fig. 10). For example, K content in the amended soil was 3.3 mg which decreased to 2.9 mg in the surface soil. Similarly, K content of the deep soil in the same treatment was 2.8 mg in the absence of plants, which decreased to 2.5 mg (89%). The overall results showed amendment alone or amendment followed by planting was important for improving K content. Comparing the changes, no clear relationship between BD and EC was found. Potassium availability was influenced by pH. When pH was high, the available K was high, especially in the amended soil. Nutrient retention in sandy soil is poor and the results indicated amendment improves K retention and nutrient accumulation (Biederman and Harpole 2013).
3.9 Available magnesium
Presence of pasture in the unamended soil significantly reduced the Mg content to 4.1 mg and only increased it by 0.2 mg in the deep soil (Fig. 11). When amended alone or amended and planted, available Mg increased but more so in the deep soil. It was shown in Fig. 10 that pasture depleted surface soil K. Depletion of surface soil Mg was evident too (Fig. 11). The deep soil data shown in Fig. 9 indicated that the turnover of OM and possibly organic compound from the roots of pasture helped build available K. The same processes increased Mg content at the same profiles (Fig. 11). The OM content contributed by the roots in the deep soil, estimated using (2), was 11.9% (6.9 × 1.72) compared to 11.9% in the control. This suggested that no OM was contributed by roots, pointing out that the increase in Mg was from sources other than the roots, translating to a profile-specific SOM stock of 166.15 t cm−3, estimated as per (3). In the amended soil, Mg content estimated using (6) was 0.05 g kg−1 (i.e. 50 mg) throughout the soil. This is considered to be moderate as Mg content of sandy soil ranges from 31 to 90 mg kg−1 (Staugaitis and Rutkauskiene 2010) and from 3 to 25 g kg−1 in most soils (Yan and Hou 2018).
4 Management implications
Sustainable production and management of pasture is not only important for livestock production for food and nutritional security but also helps to address impacts of climate change on factors that affect productivity (Michael 2019b). This study showed biochar amendment is important for water retention (WHC) and improvement of BD, soil EC and pH. On the other hand, SOC was significantly reduced even when biochar co-existed with pasture. The sole reason for this is that carbon from biochar was not readily available either in the soil or to pasture. Establishment of pasture alone or following amendment on sandy soil increased nitrogen and phosphorus contents, whereas K and Mg contents were lowered instead. Consistent with these findings, Novak and Busscher (2013) reported biochar improved cation exchange capacity of soil. These contradicting results call for strategic management of SOC, K and Mg in sandy soil under pasture in the humid tropics. The native SOM content decreased under all the treatment conditions (Table 2), indicating SOC contributed by biochar and pasture alone or co-existing was insignificant. The amount of carbon added to the soil from amendment or by the live pasture was less except that a significant amount of SOM was added by the pasture, even if smaller than the native SOM content. Addition of OM to any soil type by live pasture in the form of shed leaf litter or root exudates is important as it affects important soil characteristics like BD, pH and WHC (Michael 2018a, b; Vasbieva 2019). Assuming an increase in SOM of 0–1% in sandy soil results in retention of 67, 350 L of water (Brassard et al. 2016); a decrease in water retention was evident. The decrease in SOM in the surface of the planted soil was 91.5%, equating to an estimated loss of 3.2 million liters of water under the pasture system (Table 3). Compared to this, biochar amendment resulted in 37.5% decrease in the native SOM content whilst the changes in the amended soil planted was 5.8% (Table 2), respectively.
The implication for these findings is that a lot of water is lost in sandy soil under pasture in the humid tropics and this need to be managed. Since amendment resulted in a decrease in native SOM by 37.5% (Table 3), biochar amendment followed by establishment of pasture is an important option not only to manage the soil characteristics but also to improve SOC, SOM and soil water retention (Gusev and Dzhogan 2019). In the surface soil, an estimated 3.2 million liters of water is lost under pasture, equating to 47.7 tonnes of SOM (Table 3). Amendment of the surface soil resulted in retention by 62.6% of water (that is 1.3 million liters). In the deep soil of planted, 0.6 million liters loss was estimated. This loss increased by 80% when amended (Table 3). When planted in the amended soil, 26.5 tonnes of native SOM was lost, resulting in 1.8 million liters of water loss in the deep soil (Table 3). These are disastrous under conditions of water scarcity and irregular supply (Singh et al. 2019).
In the light of climate change, the global temperature is projected to change (IPCC 2013) and water loss management is important to mitigate the impacts (de Gerenyu et al. 2018; Michael 2019b; Pokharel et al. 2020). These results showed more water is lost from the surface soil when pasture is present compared to loss from the deep soil when amended. In the tropics, the slash-and-burn system of land invaded by cogon grass is a dominant practice for land use or management of its invasiveness. This system is a potent source of carbon dioxide emission contributing to greenhouse gases. Sourcing plant material for biochar from the invasive grass instead of burning to be used under pasture would reduce the emission and improve management of its invasiveness (Lehmann et al. 2006). The carbon sequestered by the biochar would last for decades, thereby reducing emissions and mitigating climate change impacts.
5 Conclusions
Application of biochar in sandy loam soil under humid lowland tropical climatic conditions has a number of benefits, including higher pH, reduced bulk density, increased water retention and ideal electrical conductivity. Application of biochar resulted in improved nutrient status of the sandy loam soil when planted. The SOC and SOM did not improve when biochar was applied; plants existed alone or co-existed with biochar, implying that native SOC and SOM contents become limited in sandy soil in the humid tropics under pasture. Cogon grass is highly invasive and its use for biochar shows promise for improvement of sandy loam soil for pasture productivity while at the same time contributing to the management of its invasiveness in the tropics.
References
Aguiar ACF, Candido CS, Carvalho CS, Monroe PHM, Moura EG (2013) Organic fraction and pools of phosphorus as indicators of the impact of land use in the Amazonian periphery. Ecol Indic 30:158–164
Aipa J, Michael PS (2018) Poultry manure application and fallow improves peanut production in a sandy soil. Agric J 4:68–75
Aipa J, Michael PS (2019) Different land use system improves soil fertility status of a sandy soil and increases the yield of rice under rain-fed wet tropical lowland conditions in Papua New Guinea. Int J Agric Environ Res 5:19–27
Ali K, Arif M, Jan MT, Khan MJ, Jones GL (2015) Integrated use of biochar: a tool for improving soil and wheat quality of degraded soil under wheat-maize cropping pattern. Pak J Bot 47:233–240
Artiola JF, Rasmussen C, Freitas R (2012) Effects of a biochar-amended alkaline soil on the growth of romaine lettuce and Bermudagrass. Soil Sci 177:561–570
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Basso AS, Miguez FE, Laird DA, Horton R, Westgate M (2013) Assessing potential of biochar for increasing water-holding capacity of sandy soils. GCB Bioenergy 5:132–143
Biederman LA, Harpole WS (2013) Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy 5:202–214
Blum J, Melfi A, Montes CR, Gomes TM (2013) Agric Water Manag 117:115–122
Brady NC, Weil RR (1999) Nature and property of soils. Prentice Hall, Upper Saddle Hall
Brassard P, Godbout S, Raghavan V (2016) Soil biochar amendment as a climate change mitigation tool: key parameters and mechanisms involved. J Environ Manag 181:484–497
Bravin M, Garnier C, Lenoble V, Gérard F, Dudal Y et al (2012) Root-induced changes in pH and dissolved organic matter capacity after copper dynamic speciation in the rhizosphere. Geochim Cosmochim Acta 84:256–268
Burrell LD, Zehetner F, Rampazzo N, Wimmer B, Soja G (2016) Longterm effects of biochar on soil physical properties. Geoderma 282:96–102
Butterly CR, Kaudal BB, Baldock JA, Tang C (2011) Contribution of soluble and insoluble fractions of agricultural residues to short-term pH changes. Eur J Soil Sci 62:718–727
Castellini M, Giglio L, Niedda M, Palumbo AD, Ventrella D (2015) Impact of biochar addition on the physical and hydraulic properties of a clay soil. Soil Tillage Res 154:1–13
Dai ZM, Zhang XJ, Tang C, Muhammad N, Wu JJ, Brookes PC, Xu JM (2017) Potential role of biochars in decreasing soil acidification—a critical review. Sci Total Environ 581–582:601–611
de Gerenyu VOL, Kurganova IN, Khoroshaev DA (2018) The Effect of contrasting moistening regimes on CO2 emission from the gray forest soil under a grass vegetation and bare fallow. Eurasian Soil Sci 51:1200–1213
Ding Y, Liu YG, Liu SB, Li ZW, Tan XF, Huang XX, Zheng GM, Zhou L, Zheng BH (2016) Biochar to improve soil fertility: a review. Agron Sustain Dev 36:36
Duubel A, Merbach W (2005) Influence of microorganisms on phosphorus bioavailability in soils. In: Varma A, Buscot F (eds) Microorganisms in soils: roles in genesis and functions. Springer, Berlin, pp 177–191
Faloye OT, Ajayi AE, Alatise MO et al (2019) Nutrient uptake, maximum yield production, and economic return of maize under deficit irrigation with biochar and inorganic fertiliser amendments. Biochar 1:375–388
Friedman PS (2005) Soil properties influencing apparent electrical conductivity: a review. Comput Electron Agric 46:45–70
Gomez SM (2013) Recycling agricultural by-products to grow sugarcane on sandy soils in South Florida. MS thesis. University of Florida
Gunarathne V, Senadeera A, Gunarathne U et al (2020) Potential of biochar and organic amendments for reclamation of coastal acidic-salt affected soil. Biochar. https://doi.org/10.1007/s42773-020-00036-4
Gusev YM, Dzhogan LY (2019) Soil mulching as an important element in the strategy of using natural water resources in agroecosystems of the steppe crimea. Eurasian Soil Sci 52:313–318
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59
Hocking MD, Reynolds JD (2012) Nitrogen uptake by plants subsidized by Pacific salmon carcasses: a hierarchical experiment. Can J For Res 42:908–917
IPCC (2013) Climate change 2013: the physical science basis. In: Qin STFD, Plattner GK, Tignor M, Allen SK, Boschung K, Midgley PM (eds) Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Karhu K, Mattila T, Bergström I, Regina K (2011) Biochar addition to agricultural soil increased CH4 uptake and water holding capacity results from a short-term pilot field study. Agric Ecosyst Environ 140:309–313
Landon RJ (1991) Booker tropical soil manual: a handbook for soil survey and agricultural land evaluation in the tropics and subtropics. Wiley, New York
Lane M (1985) Salt damage in container plants. Aust Hortic 96–100
Lehmann J, Gaunt J, Rondon M (2006) Biochar sequestration in terrestrial ecosystems—a review. Mitig Adapt Strat Glob Change 11:403–427
Lewis SA, Wu JQ, Robichaud PR (2006) Assessing burn severity and comparing soil water repellency, Hayman Fire, Colorado. Hydrol Process 20:1–16
Lorenz K, Lal R (2014) Biochar application to soil for climate change mitigation by soil organic carbon sequestration. J Plant Nutr Soil Sci 177:651–670
Luo Y, Durenkamp Q, Lin M, Nobili M, Brookes PC (2011) Soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol 43:2304–2314
Luo Y, Yu ZY, Zhang KL, Xu JM, Brookes PC (2016) The properties and functions of biochars in forest ecosystems. J Soils Sediments 16:2005–2020
Ma F, Ma H, Qiu H, Yang H (2016) Effects of water levels and the additions of different nitrogen forms on soil net nitrogen transformation rate and N2O emission in subtropical forest soils. J Appl Ecol 26:379–387
Michael PS (2018a) Comparative analysis of the ameliorative effects of soil carbon and nitrogen amendment on surface and subsurface soil pH, Eh and sulfate content of acid sulfate soils. Eurasian Soil Sci 51:1181–1190
Michael PS (2018b) Effects of live plants and dead plant matter on the stability of pH, redox potential and sulfate content of sulfuric soil neutralized by addition of alkaline sandy loam. Malays J Soil Sci 22:1–18
Michael PS (2019a) Roles of Leucaena leucocephala on sandy loam soil pH, bulk density, water-holding capacity and carbon stock under humid lowland tropical climatic conditions. Bull J Soil Sci 4:33–45
Michael PS (2019b) Current evidences and future projections: a comparative analysis of the impacts of climate change on critical climate-sensitive areas of Papua New Guinea. J Soil Sci Agroclimatol 16:229–253
Michael PS, Reid JR (2018) The combined effects of complex organic matter and plants on the chemistry of acid sulfate soils under aerobic and anaerobic soil conditions. J Soil Sci Plant Nutr 18:542–555
Michael PS, Fitzpatrick R, Reid R (2015) The importance of organic matter on amelioration of acid sulfate soils with sulfuric horizons. Geoderma 225:42–49
Michael PS, Fitzpatrick R, Reid R (2016) The importance of soil carbon and nitrogen in amelioration of acid sulphate soils. Soil Use Manag 32:97–105
Michael PS, Fitzpatrick WR, Reid JR (2017) Effects of live wetland plant macrophytes on acidification, redox potential and sulfate content in acid sulphate soils. Soil Use Manag 33:471–481
Milne E, Banwart SA, Nollemeyer E et al (2015) Soil carbon, multiple benefits. Environ Dev 13:33–38
Minasny B, Hong SY, Hartemink AE, Kim YH, Kang SS (2016) Soil pH increase under paddy in South Korea between 2000 and 2012. Agric Ecosyst Environ 221:205–213
Novak J, Busscher W (2013) Selection and use of designer biochars to improve characteristics of southeastern USA coastal plain degraded soils. Advanced biofuels and bioproducts. Springer, Berlin
Palansooriya KN, Wong JTF, Hashimoto Y, Huang L et al (2019) Response of microbial communities to biochar-amended soils: a critical review. Bochar 1:3–22
Pokharel P, Ma Z, Chang SX (2020) Biochar increases soil microbial biomass with changes in extra- and intracellular enzyme activities: a global meta-analysis. Biochar. https://doi.org/10.1007/s42773-020-00039-1
Prober SM, Stol J, Piper M, Gupta VVSR, Cunningham SA (2014) Enhancing soil biophysical condition for climate-resilient restoration in mesic woodlands. Ecol Eng 71:246–255
Rahim HU, Mian IA, Arif M, Rahim ZU, Ahmad S, Khan Z, Zada L, Khan MA, Haris M (2019) Residual effect of biochar and summer legumes on soil physical properties and wheat growth. Pure Appl Biol 8:16–26
Rahim HU, Mian IA, Arif M, Ahmad S, Khan Z (2020) Soil fertility status as influenced by the carryover effect of biochar and summer legumes. Asian J Agric Biol 8:11–16
Rijsberman FR (2006) Water scarcity: fact or fiction? Agric Water Manag 80:5–22
Schulte EE, Hopkins BG (1996) Estimation of soil organic matter by weight loss-on-ignition. In: Magdoff RF, Tabatabai MA, Hanlon EA (eds) Soil science Society of America. Wiley, New York, pp 21–31
Shi S, Richardson AE, O’Callaghan M, DeAngelis KM, Jones EE et al (2011) Effects of selected root exudate components on soil bacterial communities. FEMS Microbiol Ecol 77:600–610
Shinogi Y (2004) Nutrient leaching from carbon products of sludge. ASAE/CSAE annual international meeting. Paper No. 044063; Ottawa (ON), Canada
Singh R, Singh P, Singh H et al (2019) Impact of sole and combined application of biochar, organic and chemical fertilizers on wheat crop yield and water productivity in a dry tropical agro-ecosystem. Biochar 1:229–235
Sombroek W, Ruivo ML, Fearnside PM, Glaser B, Lehmann J (2003) Amazonian dark earths as carbon stores and sinks. In: Lehmann J, Kern DC, Glaser B, Woods WI (eds) Amazonian dark earths: origins, properties, management. Kluwer Academic Publishers, Dordrecht, pp 125–139
Staugaitis G, Rutkauskienė R (2010) Comparison of magnesium determination methods as influenced by soil properties. Žemdirbystė Agric 97:105–116
Strassburg BBN, Latwiec AE, Barioni LG et al (2014) When enough should be enough: improving the use of current agricultural lands could meet production demands and spare natural habitats in Brazil. Glob Environ Change 28:84–97
Townsend AR, Asner GP, Cleveland CC, Lefer ME (2002) Unexpected changes in soil phosphorus dynamics along pasture chronosequences in the humid tropics. J Geophys Res Atmos 107:D20
Vasbieva MT (2019) Effects of long term application of organic matter and mineral fertilizers on organic carbon content and nitrogen regime of soddy-podzolic soil. Eurasian Soil Sci 52:1422–1428
Vendramini JMB, Silveira MLA, Debeux JCB Jr, Sollenberger LE (2007) Environmental impacts and nutrient cycling of pastures grazed by cattle. Revist Bras Zootec 36:139–149
Vinod K, Rawat AK, Rao DLN (2016) Influence on soil carbon and nitrogen content in vertisols of Madhya Pradesh under different crop rotation. J Anim Plant Sci 31:108–113
Xu JM, Tang C, Chen ZL (2006) The role of plant residues in pH change of acid soils differing in initial pH. Soil Biol 38:709–719
Yan B, Hou Y (2018) Effects of soil magnesium on plants: a review. IOP Conf Ser Earth Environ Sci. https://doi.org/10.1088/1755-1315/170/2/022168
Yuan JH, Xu RK (2011) The amelioration effects of low temperature biochar generated from nine crop residues on an acidic ultisol. Soil Use Manag 27:110–115
Acknowledgements
There is no conflict of interest. The study was supported by Final Year Project Funds from the PNG University of Technology to the Department of Agriculture. The 2019 final year project students (Amon Muri, Elijah Pakne, Tikio Patrick and Israel Koakisnon) in my group were instrumental and acknowledged for their efforts. I’m grateful to Prof. Robert J. Reid, University of Adelaide, Adelaide, South Australia, Australia, for technical and editorial assistance. The anonymous reviewers whose input improved the quality of the manuscript are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michael, P.S. Cogon grass biochar amendment and Panicum coloratum planting improve selected properties of sandy soil under humid lowland tropical climatic conditions. Biochar 2, 489–502 (2020). https://doi.org/10.1007/s42773-020-00057-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42773-020-00057-z