Abstract
The biodegradation of mycotoxins has become a specific, efficient, and environmentally protective way to reduce the adverse effects of mycotoxins in both foods and feeds. In the current study, the effectiveness of dietary administration of Bacillus subtilis on health parameters and regulated gene expression in mice receiving zearalenone zearalenone-contaminated diet was explored. In this trial, a total of twenty-four white balb/c mice were randomly assigned to three treatments. Dietary treatments were as follows: T1: The control (fed non-zearalenone-contaminated diet), T2: fed zearalenone-contaminated diet, T3: fed zearalenone-contaminated diet + Bacillus subtilis ARKA-S-3 (1 × 109 cfu/kg) for 28 days. The results showed, B. subtilis notably degraded zearalenone in cultured media during 18 h incubation (p < 0.05). It significantly improved average daily weight gain and feed intake. Dietary B. subtilis notably reduced the adverse effects of zearalenone on serum antioxidant indices (GSH-Px, SOD, ) and saved mice from oxidative stress. Also, treatments with B. subtilis improved morphometric characteristics of the ileum ((Villus Height (µm), Villus Width (µm), and Crypt Depth (µm)) in the mice received zearalenone-contaminated diet (p < 0.05). The molecular analysis illustrated that B. subtilis has also improved the mRNA expression levels and antioxidant-related gene expression of SOD and CAT in the jejunum tissue. Moreover, it alleviated the IL-2 and IFN-γ gene profiling in the jejunum tissue. These findings illustrate that dietary administration of B. subtilis by having a degraded effect on zearalenone, possesses a protective effect on the health parameters and gene expression regulation in mice receiving a zearalenone-contaminated diet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycotoxins are secondary fungal metabolites that contaminate almost all agricultural stuff worldwide, and they cause a main risk to animal and human health [1]. In this sight, the Food and Agricultural Organization of the United Nations (FAO) reported that rather than 25% of all agricultural food crops (foodstuff and feeds) are contaminated with various mycotoxins [2]. In general, mycotoxins have severe economic losses through clinical changes with reducing feed intake and growth, alteration in intestinal morphology index and nutrient absorption, metabolism, and suppression of the immune system [3].
Zearalenone (ZEN), a resorcylic acid (a stable toxin), is the most significant nonsteroidal toxin related to the incidence of global toxicity [3]. The sense that various mycotoxins, aflatoxins, and zearalenone can induce oxidative stress, which leads to obvious damage in certain organ tissues, especially the kidney and liver, and changes in cell function and enzymatic parameters such as DNA lesions, protein cleavages, and lipid peroxidation in human and animal [4]. Various researchers have detected that oxidative stress could be caused by ZEN toxicity [5,6,7]. Moreover, ZEN can increase the production of ROS, resulting in increasing lipid peroxidation in tissue organs like the testis, spleen, liver, and kidney [8], leading to the induction of apoptosis [9] and oxidative DNA damage [10]. Recently studies in mice, emphasized that exposure to low-dose ZEA mycotoxin in the long term causes impairment in vital organs and also leads to immunosuppression and triggers autoimmune responses in animals [11]. Also, Zearalenone (ZEA) and its metabolite, α-zearalenol (α-ZOL) are stable toxins that could reduce fertility, create hyperestrogenic, increase the number of dead and absorbed fetuses in sheep, cattle, and pigs; and induce extremely toxicity to young children in the primary phases of growth [12, 13].
The biodegradation of mycotoxins has received a considerable deal of notice in recent years and has become a major procedure in fungal toxin research. This way is the special process of transforming mycotoxin into less toxic or non-toxic compounds, by absorption of mycotoxins on the walls of microbial cells, and the degradation of mycotoxins by microbial metabolites [14] which is also more efficient, specific, and environmentally friendly. The ZEA could be degraded by several microorganisms, such as B. licheniformis CK1 [15], B. pumilus ES-21 [16], B. amyloliquefaciens ZDS-1 [17] and Bacillus velezensis A2 [18]. Previous studies reported the degradation of ZEA by B. subtilis to various extent 66.3%, 84.6%, and 83.0% in the commercial pig feed [19]. In addition, this study reported that B. subtilis ANSB01G could alleviate the negative effects of ZEA on reproductive performance in the prepubertal gilts [20]. Similar to this observation, a recent study showed the biological detoxification of DON by B. licheniformis YB9 up to 82.67% degradation of 1 mg/L DON upon 48 h incubation at 37 ◦C in the laboratory condition (Wang et al. 2020). Therefore, this study aimed to explore the effectiveness of dietary administration of B. subtilis ARKA-S-3 on the health parameters and the expression of antioxidant, anti-inflammatory, and immunomodulation-related gene patterns in the mice who received zearalenone-contaminated diet.
Material and method
All reagents and chemicals in this study were provided by Merck Company in Germany and were in high-level purity. The methanol and acetonitrile were HPLC grade, and others were set based on analytical grade.
Bacterial culture preparation
In the current study, Bacillus subtilis ARKA-S-3 strain with the NCBI accession number OP954653 was used which was previously isolated and characterized by our research team in the Industrial and Mineral Research Center, Arka Industrial Cluster, Mashhad, Iran. The fresh culture of bacteria was prepared by transferring 100 µl of fresh bacterial culture into 10 ml of nutrient broth (Merck, Germany) and incubated in an incubator (24 h, 37oC). After determining the bacterial counts (by using the normal agar plating method), bacterial counts were presented in the form of colony unit (cfu) per milliliter of culture. Next, after centrifuging (at 6000×g for 10 min at 4oC) of growth culture, the cell pellet was re-suspended in 10 ml of normal saline (0.9% NaCl). The bacterial count was adjusted at the cell density of ~ 1 × 1011 cfu/ml before use.
Production and extraction of zearalenone
A mixture of soybean meal (20%), corn flour (45%), wheat flour (15%), and wheat bran (20%) were weighed and transferred to flask (2000 ml). Next, 250 ml of distilled water was spilled and the cap was covered by a cotton stopper. In this trial, ten individual flasks were prepared. The flask was incubated at 120 °C for 15 min. After sterilization, a fresh culture of Fusarium graminearum was prepared on potato, dextrose agar, and 1 × 1 cm size of the agar which was covered by the fungus was added to the fermentation media (solid-state). The fungal isolation, characterization, and identification were previously conducted by the University of Tarbiat Modarres, Tehran, Iran. The fungal isolation was able to produce zearalenone [21]. The flask was maintained at 25oC for 20 days. Next, 2000 ml of methanol (80% v/v) and 400 g of fermented product were mixed in the backer (5000 ml), and filtered. To increase the concentration, the mixture was evaporated by a rotary evaporator. The zearalenone concentration was measured in the extract by using LC-MS [22].
Zearalenone detoxification assay
To prepare the zearalenone at the concentration of 30 µg/ml zearalenone, the extract including zearalenone was mixed with nutrient broth. Then this nutrient broth was cultured by the Bacillus subtilis to obtain a certain bacterial count (109 cfu/ml) and incubated (37oC, 12 h, 120 rpm). Finally, after centrifuging culture media (4oC, 5 min, 14000 rpm), the zearalenone amount was measured by LC-MS. To provide negative control, we used the same protocol of nutrient broth medium with no bacteria inoculation [23].
Animal trails
The study was carried out based on the law of the Institutional Animal Care and Use Committee of the Islamic Azad University of Mashhad, Iran. A total of 24 white balb/c mice (with bodyweight 20–25 gr) were housed in individual cages in 12-hour light/dark periods at 23 °C ± 1 °C and 58%±10% humidity and were randomly assigned to three treatments [24]. Dietary treatments were as follows: T1: The control diet (fed non-zearalenone-contaminated diet), T2: fed zearalenone-contaminated diet, T3: fed zearalenone-contaminated diet + B. subtilis (1 × 109 cfu/kg). All treatments had free access to water and food. The dry matter intake and weight were measured at the end of every week. At the end of the trial (Day 28), pentobarbital-HCL (50 mg/kg, i.p.) was injected for sacrificing mice. The blood samples, liver, and ileum were sampled and stored for further analysis.
Liver enzymes and blood parameters determination
The liver enzymes status including alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), and serum immunoglobulin (IgA, IgG, IgM) were assessed by automated chemistry analyzer (Roche, Hitachi 902 analyzer, Japan) [25].
Lipid peroxidation assay
For this purpose, the spectroscopic assay has been carried out for the assessment of lipid peroxidation. Briefly, the malondialdehyde (MDA) was reacted with Thiobarbituric acid (TBA) and produced a reddish color that was recorded at 532 nm. The MDA was applied as the lipid peroxidation marker and reflected in the intensity of the color [25]. The lipid peroxidation results were reported as a percentage of MDA value changes relative to the control group.
Histopathology and morphometric analyses
After sacrificing mice (Days 28), the intestine, liver, and kidney tissues were gathered and washed with physiological serum for the histopathological studies. Samples were imbrued in a prepared buffered formalin (10% formalin in 0.1 M sodium phosphate buffer, pH7). Then, samples were sliced, paraffinized, and sustained at hematoxylin/eosin [25]. At the end, slides were evaluated by a light microscope (magnification of 20X). The intestinal morphology features, including villus width, villus height, and crypt depth, were evaluated.
Gene expression analysis
At the end of the trial, to profile gene expression, jejunum, and liver tissues were sustained in liquid nitrogen and kept in -80 until analysis. The RNA was extracted from tissues according to the instructions provided by the RNeasy Mini kit from Qiagen in Hilden, Germany. Next step, the sample’s cDNA was generated by a Reverse Transcription kit (Qiagen, Hilden, Germany). The real-time PCR program was performed in three as follows: step 1 at 95oC for 5 min (1X), step 2: at 95oC for 20s, step 3: at 60oC for 30s, and step 4: at 72oC for 30s (35X). The expressions of antioxidant-related genes (CAT, SOD), inflammation-related genes (iNOS), and immunomodulation-related genes (IFN-γ, IL-2) were determined. Table 1 illustrates the list of primers used in this study.
Quantification of B. subtilis in the jejunum digesta
The major site of nutrient absorption in the monogastric is the jejunum, which is why in this study the population of B. subtilis was determined only in the jejunum section. The entire jejunum digesta was taken and mixed and 100 mg of the digesta was used for the DNA extraction using QIAamp DNA Stool Mini Kit (Germany). The quantitative real-time PCR was applied using the SYBR Green PCR Master Mix (Biofact, Korea). The fold changes for the B. subtilis in the jejunum digesta were determined using the 2−∆∆Ct method as described earlier [26,27,28]. The primers used in this study are B. subtilis ( F: ttgatcttagttgccagcattc; R: acagatttgtgggattggctta) [28] and Total bacteria (F: cggcaacgagcgcaaccc; R: ccattgtagcacg tgtgtagcc) [29].
Statistical analysis
The data of this study are expressed as the data arithmetic mean (with three repeats) and mean standard deviation. All statistical analyses were carried out by one-way ANOVA analysis, with a significant level of p < 0.05. To compare the means, Duncan multiple-test rage was also performed.
Results and discussion
Zearalenone disappearance
In the recent decade, the interest has increased in finding effective and practical procedures to reduce the risk of contamination of ZEN in the food and feed industry [30, 31]. The biodegradation of mycotoxins is an efficient approach to control ZEN in contaminated food [31]. Various studies have reported that several strains of Bacillus spp. were able to degrade the toxicity of ZEN [31, 32]. As illustrated in Table 2, B. subtilis has a visible degraded effect on zearalenone (p < 0.05). Importantly, we detected that B. subtilis could degrade 58.1%, of ZEN during 18 h. Previous reports illustrated that B. subtilis ANSB01G degraded 65.13%, 92.57%, and 100.00% of ZEN at 6 h, 24 h, and 48 h, respectively [33]. Also, Gonzalez Pereyra et al. [34] reported that different Bacillus strains could degrade ZEA during 72 h at 30 C to various extents.
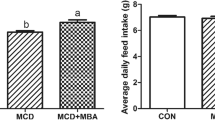
Weight gain and feed intake changes
In recent years, researchers have vast investigations on the toxicity and detoxification of mycotoxins. Table 3 illustrates the effectiveness of adding B. subtilis (1 × 109 cfu/kg) into a zearalenone-contaminated diet on the feed intake and body weight gain in mice. The administration of B. subtilis was able to significantly (p < 0.05) improve the body weight and the feed intake of mice as compared to the group that fed with a zearalenone-contaminated diet. Aligned with the results of this study, Guo et al. [35] reported that the presence of mycotoxin in the diet of mice could cause a decrease in the average daily weight gain.
Liver enzyme and lipid peroxidation results
Furthermore, to the reproductive performance, the kidney and liver are other important organs for ZEA toxicity. ZEA can alter the content of liver protein, the liver enzyme’s function, the amount of lipid peroxidation, the inflammatory response, and the antioxidant index which results in hepatotoxicity [36]. As presented in Table 4, dietary B. subtilis notably reduced adverse effects of zearalenone on serum antioxidant indices (GSH-Px, SOD, ) and saved mice from oxidative stress. The treatment with B. subtilis had no effects (P > 0.05) on serum IgA, IgG, IgG and meanwhile improved (p < 0.05) the liver enzymes concentration including ALP, ALT, and AST. Abbès et al. [37] reported that a feeding diet with 40 mg/kg bw ZEA raised serum AST, ALP, and ALT content in the mice. These findings were also observed in other studies, displaying ZEA’s liver toxicity [38]. Van et al. [39] expressed that adding ZEN to piglets’ feed led to induced oxidative stress in piglets, this was discernible with an increased level of activity of SOD and MDA [29].
Morphometric analysis
Based on the funding in Table 5 the mice administrated with B. subtilis significantly (p < 0.05) improved the morphometric of ileum parameters including villus height, villus width, and crypt depth (T2 group) as compared to the mice receiving zearalenone-contaminated diet. The small intestine is the main site where nutrient absorption, structural integrity, and intestinal morphology are essential to maintaining natural function [40]. ZEN exposure reduced the villus height, villus width, and crypt depth, showing intestinal obstacle injury led to reduced nutrient digestion and absorption. The intestinal morphology including crypt depth, villi height, and the villi height/crypt depth relativity were indispensable indicators to assess, absorb and digest nutrients [41]. Thus, the loss or shortening of the intestinal villus commonly causes a reduction in nutrient absorption, consequence, diarrhea, malnutrition, and reduction in disease resistance [42].
Histopathological analysis
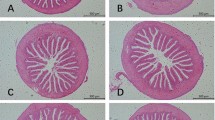
Histopathological features including intestine, liver, and kidney tissues that received different treatments have been shown in Fig. 1. The overall results manifested that the mentioned organs except the intestine indicated no prominent changes. These results were in agreement with Ma et al. [43] who reported that ZEN led to injured integrity the small intestinal morphology changes and the reduced digestibility of nutrients in the piglets. The observation of no clear histopathological changes in the liver and kidney could be associated with the notable mycotoxin detoxification potential of these two organs which reduced the effects of ZEN to alter the histology of tissue in these two organs or the short duration of exposure to ZEN [44,45,46].
Gene expression analysis
Table 6 displays the antioxidant, inflammatory, and immunomodulatory related genes profiling. The mice treated with B. subtilis, improved mRNA expression levels of SOD, and CAT and alleviated IL-2, and IFN-γ genes in the jejunum notably. Researchers have reported that ZEN probably elevates the expression and synthesis of pro-inflammatory indicators via JNK signaling pathway activation [47]. Shen et al. [33], reported that the presence of ZEN in the feed notably increased the amount of TNF and IL-2, in the serum of gilts. An elevated amount of TNF is the most powerful pro-inflammation indicator, which probably creates a risk of a more drastic inflammatory response [48]. These inflammatory responses (elevated in inflammatory cytokines IL-2, IL-8, and IL-10) have been shown in previous studies [20]. Moreover, the study of Shen et al. [33] showed that B. subtilis ANSB01G had a protective effect against ZEN toxicosis in gilts (Table 6).
Quantification of B. subtilis in the jejunum digesta
The effects of probiotics on mycotoxin toxicity in animals have garnered significant attention due to their potential to mitigate the harmful consequences of mycotoxin exposure. Probiotics such as B. subtilis offer a multifaceted approach to addressing mycotoxin toxicity by modulating gut microbiota, detoxifying mycotoxins, preserving gut barrier function, and exerting immune-modulatory and anti-inflammatory effects. Through these mechanisms, probiotics show promise in reducing mycotoxin production and absorption, strengthening the gut barrier, boosting immune responses, and minimizing inflammatory damage caused by mycotoxins [49]. The Fig. 2 shows the population of B. subtilis in the jejunum digesta. The population of B. subtilis in the jejunum digesta increased considerably (p < 0.05) upon dietary administration to mitigate the adverse effects of ZEN. The increase in the population of mycotoxin-detoxifying microbes in the intestine could help in the biotransformation and biodegradation of ZEN in the intestine. Consistent with these results, Wang et al. [16], also reported the association between an increase in the population of probiotics with improvement in reducing the toxic effects of ZEN in mice fed ZEN contaminated diet.
Conclusion
Clear interactions between ZEA and Bacillus subtilis illustrated that Bacillus had a very effective degraded effect on zearalenone. Also, Bacillus reduces the damage induced by zearalenone by improving mRNA expression levels and antioxidant-related gene expression of SOD, CAT, IL-2, and IFN-γ in the jejunum. Moreover, by improving health and food intake, B. subtilis improved the average weight gain in mice feeding with a zearalenone-contaminated diet. Our funding suggests that dietary administration of B. subtilis is one of the feasible approaches to mitigate the adverse effects of certain toxins such as zearalenone on performance and health parameters.
Data availability
The datasets applied during the current study are available upon reasonable request.
References
Luo S et al (2021) Contamination status of major mycotoxins in agricultural product and food stuff in Europe. 127:p108120
Marin DE et al (2013) Effects of zearalenone on oxidative stress and inflammation in weanling piglets. 58:408–415
Wu F et al (2021) Effects of zearalenone on genital organ development, serum immunoglobulin, antioxidant capacity, sex hormones and liver function of prepubertal gilts. 189:39–44
Virk P et al (2020) Protective effect of resveratrol against toxicity induced by the mycotoxin, zearalenone in a rat model. 146:111840
Zhou J et al (2020) Bacillus subtilis ANSB01G culture alleviates oxidative stress and cell apoptosis induced by dietary zearalenone in first-parity gestation sows. 6(3):372–378
Kowalska K et al (2019) Estrogen receptor β plays a protective role in zearalenone-induced oxidative stress in normal prostate epithelial cells. 172:504–513
AbuZahra HM, Rajendran P, Ismail MBJA (2021) Zerumbone exhibit protective effect against zearalenone induced toxicity via ameliorating inflammation and oxidative stress induced apoptosis. 10(10):1593
Bai J et al (2022) Roles of stress response-related signaling and its contribution to the toxicity of zearalenone in mammals. 21(4):3326–3345
Zhu W et al (2022) Hyperoside attenuates zearalenone-induced spleen injury by suppressing oxidative stress and inhibiting apoptosis in mice. 102:108408
Rai A et al (2020) Occurrence and toxicity of a fusarium mycotoxin, zearalenone. 60(16):2710–2729
Kraft S, Buchenauer L, T.J.I.J.o.M S, Polte (2021) Mold, Mycotoxins and a Dysregulated Immune System: A Combination of Concern? 22(22): p. 12269
Hennig-Pauka I et al (2018) Current challenges in the diagnosis of zearalenone toxicosis as illustrated by a field case of hyperestrogenism in suckling piglets. 4(1):1–9
Liu J, Applegate TJT (2020) Zearalenone (ZEN) in livestock and poultry: dose, toxicokinetics, toxicity and estrogenicity. 12(6):377
Possibilities for the Biological Control of Mycotoxins in Food and Feed. Toxins 2021,13, 198. 2021, s Note: MDPI stays neutral with regard to jurisdictional claims in published…
Fu G et al (2016) Effect of degradation of zearalenone-contaminated feed by Bacillus licheniformis CK1 on postweaning female piglets. 8(10):300
Wang Y et al (2018) Isolation and characterization of the Bacillus cereus BC7 strain, which is capable of zearalenone removal and intestinal flora modulation in mice. 155:9–20
Xu J et al (2016) Isolation and characterization of Bacillus amyloliquefaciens ZDS-1: exploring the degradation of zearalenone by Bacillus spp. 68:244–250
Wang N et al (2018) Bacillus velezensis A2 fermentation exerts a protective effect on renal injury induced by zearalenone in mice. 8(1):1–14
Lei Y et al (2014) Degradation of zearalenone in swine feed and feed ingredients by Bacillus subtilis ANSB01G. 7(2):143–151
Shi D et al (2018) Alleviation of mycotoxin biodegradation agent on zearalenone and deoxynivalenol toxicosis in immature gilts. 9(1):1–11
Safaei N et al (2005) optimization of a bioassay method for evaluation of zearalenone production in fungi and its application to iranian isolates of fusarium graminearum.
Hsu T-C et al (2018) Probiotic characteristics and zearalenone-removal ability of a Bacillus licheniformis strain. 13(4):e0194866
Møller COdA et al (2021) Effect of lactic acid bacteria strains on the growth and aflatoxin production potential of aspergillus parasiticus, and their ability to bind aflatoxin B1, ochratoxin A, and zearalenone in vitro. 12:655386
Sanei S et al (2023) Microcapsules loaded with date seed extract and its inhibitory potential to modulate the toxic effects of mycotoxins in mice received mold-contaminated diet. 30(20):58654–58662
Poorbagher MRM et al (2022) Hepatoprotective effect of nanoniosome loaded Myristica fragrans phenolic compounds in mice-induced hepatotoxicity. 26(21):5517–5527
Feng Y et al (2010) Identification of changes in the composition of ileal bacterial microbiota of broiler chickens infected with Clostridium perfringens. Vet Microbiol 140(1–2):116–121
Oskoueian E, Abdullah N, Oskoueian A (2013) Effects of flavonoids on rumen fermentation activity, methane production, and microbial population. BioMed research international, 2013
Whelan RA et al (2019) The impact of Bacillus subtilis DSM 32315 on the pathology, performance, and intestinal microbiome of broiler chickens in a necrotic enteritis challenge. Poult Sci 98(9):3450–3463
Denman SE, McSweeney CS (2006) Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS microbiology ecology. 58(3):572–582
Wu N et al (2021) Recent advances in detoxification strategies for zearalenone contamination in food and feed. 30: pp. 168–177
Mahato DK et al (2021) Occurrence, impact on agriculture, human health, and management strategies of zearalenone in food and feed: A review. 13(2): p. 92
Zhang J et al (2021) Effects of Bacillus subtilis ZJ-2019-1 on Zearalenone Toxicosis in female gilts. 13(11):788
Shen W et al (2021) Comparison of Ameliorative effects between Probiotic and biodegradable Bacillus subtilis on Zearalenone Toxicosis in Gilts. 13(12):882
Pereyra MG et al (2019) Presence of aiiA homologue genes encoding for N-Acyl homoserine lactone-degrading enzyme in aflatoxin B1-decontaminating Bacillus strains with potential use as feed additives. 124:316–323
Guo Z et al (2019) Quantitative assessment of zearalenone in maize using multivariate algorithms coupled to Raman spectroscopy. 286:282–288
Han X et al (2022) Res Progress Saf Zearalenone: Rev 14(6):386
Abbès S et al (2006) The protective effect of hydrated sodium calcium aluminosilicate against haematological, biochemical and pathological changes induced by Zearalenone in mice. 47(5):567–574
Čonková E et al (2001) The effect of zearalenone on some enzymatic parameters in rabbits. 121(3):145–149
Van Le Thanh B et al (2016) The potential effects of antioxidant feed additives in mitigating the adverse effects of corn naturally contaminated with Fusarium mycotoxins on antioxidant systems in the intestinal mucosa, plasma, and liver in weaned pigs. 32(2):99–116
Zhong G et al (2021) Arsenic exposure induces intestinal barrier damage and consequent activation of gut-liver axis leading to inflammation and pyroptosis of liver in ducks. 788:147780
Lei X et al (2015) Effect of Bacillus amyloliquefaciens-based direct-fed microbial on performance, nutrient utilization, intestinal morphology and cecal microflora in broiler chickens. 28(2):239
Liu M et al (2014) Toxic effects of maternal zearalenone exposure on intestinal oxidative stress, barrier function, immunological and morphological changes in rats. 9(9):e106412
Ma L et al (2022) Quantitative proteomic analysis of Zearalenone-Induced intestinal damage in weaned piglets. 14(10):702
Li P et al (2020) Detoxification Mycotoxins through Biotransform 12(2)
Liu L, Xie M, Wei D (2022) Biological Detoxification of mycotoxins: current status and future advances. Int J Mol Sci, 23(3)
Oskoueian E et al (2015) Cytoprotective effect of palm kernel cake phenolics against aflatoxin B1-induced cell damage and its underlying mechanism of action. BMC Complement Altern Med 15:1–12
Pistol GC et al (2015) Zearalenone mycotoxin affects immune mediators, MAPK signalling molecules, nuclear receptors and genome-wide gene expression in pig spleen. 10(5):e0127503
Salah-Abbès JB et al (2020) Zearalenone nephrotoxicity: DNA fragmentation, apoptotic gene expression and oxidative stress protected by Lactobacillus plantarum MON03. 175:28–35
Liew W-P-P (2018) S.J.F.i.c. Mohd-Redzwan, and i. microbiology, Mycotoxin: Its impact on gut health and microbiota. p. 60
Acknowledgements
The authors would like to acknowledge the contribution of the Islamic Azad University of Mashhad, Iran, and the Industrial and Mineral Research Center, Arka Industrial Cluster, Mashhad, IRAN in providing the facilities to conduct this research.
Funding
There has been no financial support for this work.
Author information
Authors and Affiliations
Contributions
SM, PM, SGH, SGH, AZ, HT: Study design, experimental work, formal analysis, and writing original draft; PSH and MFJ.: formal analysis, and writing original draft; E.K. and E.O.: project administration, supervision, review, and editing of the original draft; All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All animal handling methods were accomplished as per the Islamic Azad University of Mashhad, IRAN regulations with their prior approval for using the animals. All methods are reported in accordance with ARRIVE guidelines.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Rita de Cássia Mendonça de Miranda.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marashi, S., Mostarshedi, P., Ghorbanikalateh, S. et al. Dietary administration of Bacillus subtilis improves the health parameters and regulates the gene expression in mice receiving zearalenone-contaminated diet. Braz J Microbiol (2024). https://doi.org/10.1007/s42770-024-01501-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42770-024-01501-z