Abstract
The Bluetongue disease is an infectious and non-contagious viral disease mainly transmitted through hematophagous vector of the Culicoides genus, to domestic and wild ruminants. The aim of this study was to evaluate the antibodies occurrence, persistence and potential risk factors associated with bluetongue virus infection in sheep flocks in the state of Parana, Brazil. The competitive ELISA test was applied to evaluate 690 blood serum samples from 22 farms in eight mesoregions of Parana in 2014, and 270 sheep blood serum samples from 10 of the 22 previously studied farms in 2017. A questionnaire was applied to evaluate the risk factors associated with BTV infection. In 2014 and 2017, the numbers of seroreactive sheep were found to be 28.26% (195/690) and 41.11% (111/270), respectively, representing 95.45% (21/22) and 90% (9/10) of the flocks. The significant variables considered as risk factors were Culicoides presence (P < 0.0001; OR = 8.83 and 95% CI 4.28–18.22); genealogical record (P < 0.0001; OR = 0.23 and 95% CI 0.12–0.45) and use of sheepfold (P = 0.0208; OR = 0.36 and 95% CI 0.15-0 0.86). It was determined that BTV infection is endemic in Parana and persists in the mesoregions where the climate is favorable to vector proliferation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bluetongue is an infectious, non-contagious, vector-borne viral disease that affects wild and domestic ruminants, it is listed under the OIE Terrestrial Animal Health Code and must be reported to the World Organization for Animal Health, implying animals and products exportation restrictions [1]. The infection ranges from in apparent to fatal, involving respiratory, hemorrhagic, mucosal and reproductive disorders [1].
The bluetongue virus (BTV) is an RNA virus, genus Orbivirus, family Reoviridae, subfamily Sedoreovirinae, with twenty-eight BTV distinct serotypes [2]. The BTV has genetic diversity, with differences between serotypes and viral strains. This is due to it’s viral genome, which consists of 10 double-stranded RNA segments which encode four non-structural proteins (NS1 to NS4) and seven structural proteins (VP1 to VP7) [3]. The outler layer consists of two main viral proteins: VP5 and VP2. This one has greater importance, as it determines the virus serotype, in addition to being responsible for binding to the receptor and recruiting specific host immunity [4]. The BTV is commonly transmitted by Culicoides biting midges [5], but also vertically [6], through colostrum [7], semen [8] and reused needles [9].
In Brazil the first viral isolation involving serotype 4 in cattle exported to the USA in 1980. Latter, outbreaks involving sheep and goats occurred in the Parana State in 2001 [10] and 2002 [11], when the clinical disease was first described in Brazil and South America [11]. Since then, several seroepidemiological studies have been carried out in Brazil, as the climate is conducive to proliferation of the Culicoides spp. In sheep flock the seropositivity for BTV has been variable in studies carried out from 2000 to 2023 (0.16–81.40%) in the states of Rio Grande do Sul (RS), Paraíba (PB), São Paulo (SP), Mato Grosso do Sul (MS), Bahia (BA), Distrito Federal (DF), Ceará (CE), Rio de Janeiro (RJ), Paraná (PR) e Amazonas (AM) [12,13,14,15,16,17,18,19,20,21,22,23].
Argentina and Paraguay are countries that also have suitable climatic conditions for spread of the virus and both border Paraná. In Argentina there are few serological studies, however they have demonstrated seropositive animals [11]. Serotype-4 has already been isolated in a sentinel herd [24], and the circulation of this same serotype has been proven [25]. Paraguay to date there are no reports in the literature on the prevalence or circulating serotypes of BTV.
Despite the detection of anti-BTV antibodies in asymptomatic cattle [26] and sheep [22] which demonstrated the virus´s silent spread Parana State, there have been no serological survey with a representative sample of the Parana State to better characterize the sanitary profile.
Even though bluetongue has been reported in Brazilian states with similar climate and indeed most part of the Brazilian territory is in the endemic zone for bluetongue disease due to favorable climatic conditions for the Culicoides [27], there are no data on climate effects and other risk factors related to BTV infection in sheep flocks.
Therefore, the current study aimed to evaluate the seroepidemiological profile through antibodies occurrence, persistence and risk factors to BTV infection in sheep flocks in the state of Parana, Brazil, during the years 2014 to 2017. The outcomes from this study will inform about the sanitary profile and risk factors for bluetongue virus infection in sheep, allowing for evidence-based surveillance and preventive measures to be implemented.
Methods
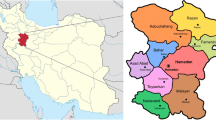
The study was conducted in eight of the ten Parana state mesoregions, Brazil, comprehending 80.65% of the state sheep flock [28], being: Central North, Northwest, Pioneer North, Western Center, West, South Center, Centro Eastern and Metropolitan region of Curitiba. These regions studied are located between latitudes 22°S and 26°S, where the climate is classified according to Koppen as Cfa, from humid subtropical climate with hot summer, where rainfall is well distributed throughout the year and the average temperature is above 22 °C, and as Cfb, from subtropical climate with temperate summer, where the average temperature does not exceed 22 °C [29].
The research was divided into two stages. The first stage aimed to detect BTV antibodies. The number of serum samples was set to 690 animals, according to EpiInfo 7® program [30], considering the prevalence of 50%, confidence level of 95%, and a margin of error of 4% from the total of 650.231 sheep in the state in 2014 [28]. The sampling was carried out randomly from 10% of the animals from 22 flocks of the eight mesoregions above described in March 2014, from asymptomatic sheep of both sexes, older than three months old and of different breeds, never vaccinated against BTV (Table 1).
In the second stage, two farms with low, four with medium and four with high occurrence were reassessed in 2017, according to the epidemiological classification of BTV antibodies detection by Sbizera et al. [22] (2020) (low: 0 to 9.99%; moderate: 10 to 49.99%; high occurrence: 50 to 100%). All those farms were part of the surveillance program accomplished in 2014. The number of samples was set at 270 animals according to the EpiInfo 7® program [30], considering the previously tested population (n = 690) in the first stage, a prevalence of 30%, a confidence level of 95%, and a margin of error of 4%. The sampling was carried out randomly from 10% of the animals from each flock, covering part of the farms collected in 2014 from asymptomatic sheep of both sexes, older than 3 months of age, of different breeds (Table 1).
Blood was collected through jugular venipuncture, sera were separated by centrifugation and stored in sterile microtubes at − 80 °C.
For the detection of BTV antibodies in all the samples from both years, the sera were subjected to a competitive enzyme-linked immunosorbent assay (cELISA), using a commercial Bluetongue Virus Antibody Test Kit and following manufacturer’s instructions (VMRD Inc., Pullman, Washington, USA). Two standard samples were used as positive and negative controls. The plates were read in an ELISA reader with 620 nm filter to obtain the optical density (OD) values. The samples were considered positive if their OD values corresponded to less than 50% of the average OD values of the negative controls, using two standard deviations (two negative and two positive controls).
It was applied an epidemiological questionnaire in all farms of the second stage to verify the main risk factors associated with the persistence or not of BTV infection. The investigated variables were sex (male or female), the animal’s genealogical record in the Brazilian Association of Sheep Breeders (yes or no), the rearing regime (extensive, semi-extensive and intensive), the presence of Culicoides (yes or no), the use of sheep folds (yes or no), the grazing of sheep carried out concomitantly with cattle (yes or no), the presence of a forest (yes or no), the presence of cattle in the neighborhood (yes or no), the presence of dogs at the farm (yes or no) and the breed (Texel, White Dorper, Dorper or Santa Inês).
The statistical analysis were performed in the statistical package R [31]. Descriptive statistical analysis was used to determine the occurrence in two stages. First, chi-square tests were performed to analyze the association between independent variables and the persistence of BTV antibodies, and variables with P < 0.20 in this analysis were included in the multivariate logistic regression analysis. The selection of independent variables was carried out with both side stepwise method according to the Akaike information criterion [32]. Independent variables considered in the complete model were the animal’s genealogical record of the animal in the Brazilian Association of Sheep Breeders, the rearing regime, the presence of Culicoides, the use of sheep folds on the farm, grazing of sheep carried out concomitantly with cattle, the presence of cattle in the neighborhood, the presence of dogs on the farm, forest and breed. After selecting the best model, the p-value, odds ratio and 95% confidence interval were obtained for each independent variable was analyzed considering a significance level of 5%, and only the variables with a significant effect were kept in the model. The fit of the selected model was verified by the Hosmer-Lemeshow test [33].
Results
Studying the occurrence of antibodies against BTV in the animals sampled in 2014, a total of 28.26% of the sheep (195/690) was positive and 95.45% (21/22) of the flocks presented at least one seropositive animal. Even if one flock (4.55%; 1/22) did not have serum-reactive animal, all studied mesoregions had BTV seropositive flocks. The flock without BTV antibody detection in 2014 was located at São José dos Pinhais, Metropolitan Mesoregion of Curitiba, and the flock with the highest occurrence, 80% (20/25), belonged to the municipality of Araruna, Western Center (Table 2).
When evaluating the persistence of BTV antibodies in the animals sampled in 2017, 41.11% of the sheep (111/270) were positive and 90% (9/10) of the flocks had at least one seropositive animal. The highest detection (78.26%; 18/23) occurred in Rosário do Ivaí, Central North Mesoregion. Once again, one flock from the Metropolitan Mesoregion of Curitiba did not have positive serology for BTV (0/27), but one flock from Colombo city did.
In 2017, there was persistence of antibodies to BTV of between mesoregions Central North (62.93%; 68/109), South Center (61.54%; 16/26), Western Center (40%; 12/30), Pioneer North (36.84%; 14/38), Central Eastern (2.5%; 1/40), and there was no persistence in the Metropolitan mesoregion of Curitiba (0/27). The analysis of antibodies persistence in the 10 flocks in 2014 and 2017 can be seen in Table 3, which indicates the antibody detection wavering over the three-year period.
The results obtained for risk factors can be seen in Table 4. It was observed that the risk factors associated with the persistence of BTV infection in sheep (p ≤ 0.05) was the animal’s genealogical record, the rearing regime, the presence of Culicoides, the presence of cattle in the neighborhood, forest and breed.
In the results of the multivariate logistic regression analysis, the significant variables considered as risk factors were Culicoides spp. presence (P < 0.0001; OR = 8.83 and 95% CI 4.28–18.22); genealogical record (P < 0.0001; OR = 0.23 and 95% CI 0.12–0.45) and use of sheep folds (P = 0.0208; OR = 0.36 and 95% CI 0.15-0 0.86).
Discussion
Despite the two outbreaks of BTV infection that occurred in Parana state in 2001 and 2002 [10, 11], this study is the first report that investigates persistence and risk factors associated with BTV seropositivity in sheep flocks covering the majority (8/10) of the Parana state mesoregions. The results highlighted that BTV infection is endemic in this state, since 95.45% of the farms assessed had serum-reactive sheep in 2014, and 90% in 2017, similar to other Brazilian regions, like São Paulo State [14] and the Federal District [18], where 100% of the sheep flocks had anti-BTV antibodies detected.
In the Parana state, BTV-12 was identified in 2001 during the outbreak of bluetongue disease involving sheep and goats [10], and serotypes 3, 14, 18, 19, and 22 were detected from an outbreak involving Bororo deer [34]. Even if our study was restricted to serological testing in asymptomatic sheep flocks instead of the virus molecular detection and characterization, the presence of asymptomatic infection may have occurred due to the possible low pathogenicity of virus species [35]. Moreover, in a year interval similar to our study, in 2013 to 2015, BTV-4 was detected from asymptomatic dairy cattle from the Central North mesoregion of Parana state [26], reinforcing the possible low pathogenicity and the silent spread of this virus species, not only in the Parana state, but also in bordering countries, such as Argentina, where the same serotype has already been detected [24, 25]. Moreover, Flannery et al. [36] reported that viraemia and clinical signs may be reduced for the BTV-8 strain. Notwithstanding, novel putative bluetongue virus serotype 29 was isolated from inapparently infected goat in China [2].
The moderate occurrence (10-49.99%) of seroreactive sheep obtained in 2014 (28.26%) and in 2017 (41.11%) herein reported can be explained by the favorable climate for the Culicoides presence predisposing to BTV infection [14, 15, 21]. The assessment of antibodies positivity results corroborates this climate hypothesis, since in 2014, regions classified as Cfa, with a hot summer, well-distributed rainfall throughout the year and an average temperature above 22 °C, had a higher occurrence, such as the Center Western (80%) and Central North (57.94%), and regions with Cfb climate had a lower occurrence, where the summer is mild and the average temperature does not exceed 22 °C, such as the Central Eastern Mesoregion (5.27%) and Metropolitan Region of Curitiba (1.25%) [29].
Similar results in serological surveys in sheep flocks for detection of BTV antibodies were obtained in Brazilian states with similar climate to those mesoregions of higher seropositivity in the present study, with 65% of seroreactivity in the state of São Paulo [14], 52.37% in the Federal District [15], and 54.1% in Minas Gerais state [21]. All these authors related the results to the climate conditions and the effective presence of the vector, corroborating to the confirmed hypothesis herein exposed that the climate is a risk factor. In South America, even in those countries that not directly border Paraná, such as Peru, detected that hot climate is also a risk factor [37].
On the other hand, Brazilian locations with unfavorable climates for the multiplication of Culicoides had a low occurrence of BTV in sheep flocks, as observed in regions with a semi-arid climate, which is dry and hot, such as the Paraíba state, where Alves et al. [13] and Mota et al. [17] observed 8.4% and 4.30, respectively, and at Bahia state, where Souza et al. [16] demonstrated 0.43% seroreactivity. Oppositely, in Rio Grande do Sul state, where a cooler climate is observed, Costa et al. [12] observed 0.16% of BTV-seropositive sheep, and those are like the Central Oriental (4.30%) and Metropolitan Region of Curitiba (1.25%) mesoregions of the present study, which also present an unfavorable climate for the vector proliferation. Coetzee et al. [38] reported that the BTV distribution is limited to latitudes from 50ºN to 35ºS, which is consistent with the moderate detection of antibodies to BTV in the present work on those farms located between the lowest latitudes (22ºS and 26ºS).
Although some authors claim that there is the transmission of BTV through semen [8], transplacental [6, 39], through the reuse of needles [9], ingestion of infected placenta [40], colostrum [7], and even by direct contact [9], the presence of the vector is still considered fundamental in viral transmission [5]. The presence of Culicoides in the farms was a risk factor highlighted in the present study, and it is important to reemphasis that three of the four farms with the highest seropositivity related the presence of Culicoides. In studies in other regions of the world, such as Bangladesh, the presence of the vector was also considered a risk factor, once again corroborating the results obtained [41].
The epidemiological questionnaire demonstrated that 60% (6/10) of the farms did not carry out the animals genealogical record (55.94% of serum-reactive sheep), confirmed as a risk factor associated with the persistence of BTV-seropositive animals. This can be explained because flocks that do not carry out genealogical control generally do not control zootechnical indices, either, which can hinder the control and prevention of sanitary problems. Batista Filho et al. [41] and Silva et al. [42] also identified the absence of zootechnical control as a risk factor for BTV in cattle, highlighting the importance of sanitary and zootechnical control.
Antibody persistence can be defined as the detection of antibodies in the sera of naturally infected animals by unit of time. Experimental and epidemiological data are consistent with the concept that BTV infections can be prolonged but of finite duration and are not truly persistent [43]. Takamatsu et al. [44], in an in vitro study, suggested that BTV may persist in T cells in the skin of infected sheep for more than 9 weeks after infection. In a study by Batten et al. [45] an inactivated vaccine for BTV serotype 8 was administered in sheep, and one year later a re-vaccination was performed, where they could observe the persistence of antibodies for up to 30 months after the last dose of the vaccine. Based on this information, the present work carried out an interval of three years, to ensure that the 2017 antibodies did not originate from infections remaining in 2014, and it was observed that antibodies to BTV were persistent in greater or lesser occurrence in the studied mesoregions and properties, in 2014 and 2017, corroborating the authors above, that there is the persistence of antibodies to bluetongue disease in sheep.
The increased persistence of the farms mentioned above can also be explained by Gubbins et al. [46] who demonstrated that the optimal mean temperature for the proliferation of vectors ranges from 15 °C to 25 °C, as observed in these municipalities. The authors also reported that higher temperatures (30 °C to 35 °C) and lower temperatures (below 15 °C) are critical for the reproduction and survival of the vectors, which possibly explains the lower seropositivity observed in the farm located in Castro, and the absence of persistence in Colombo, which presents milder temperatures throughout the year.
The risk factors obtained in the present study may also justify the decrease in the persistence of antibodies to BTV. This is because the farm located in Candói had the highest percentage (75%) of the risk factors identified in the study, possibly justifying the largest increase in persistence observed in this farm in 2017. Unlike the farms located in Araruna, Maringá and Rancho Alegre, which showed lower persistence of antibodies to BTV in 2017, even with climatic conditions identical to other properties. Possibly, the lower risk factor indices obtained in Araruna (25%) and Maringá (50%) justify the lower persistence observed in the study. The lower persistence obtained in Rancho Alegre can be explained by the 25% decrease in the flock from 2014 to 2017, due to factors not related to the BTV infection.
The epidemiological merit of the study is evidently genuine and clear when analyzing the results facing the endemicity of BTV in the studied state, since 95.45% of the flocks presented at least one serum-reactive animal in 2014, and 90% (9/10) of the flocks had at least one seropositive animal in 2017.
Conclusion
BTV infection is widespread in the studied mesoregions and persists in those where the climate is favorable to the proliferation of the vector. Therefore, the concept of BTV infection persistence in sheep flocks is linked to the greater or lesser intensity of risk factors associated with the presence of the vector. Despite the variation obtained in the BTV occurrence, the persistence of data against BTV is evident in the Parana state, encouraging the adoption of measures to reduce the risk factors observed in this study, avoiding future problems with the disease. To the best of our knowledge this is the first study on the antibodies occurrence, persistence and risk factors related to BTV infection in sheep flocks covering the majority of the Parana state mesoregions.
Data availability
Not applicable.
References
OIE (2021) Word Organization for Animal Health. Bluetongue Aetiology Epidemiology Diagnosis Prevention and Control References. Retrivied from https://www.oie.int/app/uploads/2021/03/bluetongue.pdf. Acessado em: 10 de março de 2022
Yang H, Gu W, Li Z, Zhang L, Liao D, Song J, Baoxin S, Hasimu J, Li Z, Yang Z, Huachun I (2021) Novel putative Bluetongue virus serotype 29 isolated from inapparently infected goat in Xinjiang of China. Transbound Emerg Dis 1–13. https://doi.org/10.1111/tbed.13927
Roy P (2008) Functional mapping bluetongue virus proteins and their interactions with host proteins during virus replications. Cell Biochemistru Biophyics 50:143–157. https://doi.org/10.1007/s12013-008-9009-4
Cecco BS, Santos IR, Molossi FA, Canal CW, Barros CSL, Driemeier D, Sonne L, Pavarini SP (2023) Viral disease of sheep in Brazil: a review and current status. Cienc Rural 53:8e20220218. https://doi.org/10.1590/0103-8478cr20220218
Morikawa VM, Pellizzaro M, Paploski IAD, Kikuti M, Lara MCCSH, Okuda LH, Biondo AW, Barros Filho IR (2018) Serosurvey of bluetongue, caprine arthritis-encephalitis (CAE) and Maedi-Visna in Barbary sheep (Ammotragus lervia) of a southern Brazilian zoo. Pesq Vet Bras 38:1203–1206. https://doi.org/10.1590/1678-5150-PVB-4590
Chauhan HC, Biswas SK, Chand K, Rehman W, Das B, Dadawala AI, Mondal B (2014) Isolation of bluetongue virus serotype 1 from aborted goat fetuses. Rev Sci Tech 33:803–812. https://doi.org/10.20506/rst.33.3.2319
Backx A, Heutink R, Van Rooij E, Van Rijn P (2009) Transplacental and oral transmission of wild-type bluetongue virus serotype 8 in cattle after experimental infection. Vet Microbiol 138:235–243. https://doi.org/10.1016/j.vetmic.2009.04.003
Kirschvink N, Raes M, Saegerman C (2009) Impact of a natural bluetongue serotype 8 infection on semen quality of Belgian rams in 2007. J Vet 182:244–251. https://doi.org/10.1016/j.tvjl.2008.06.008
Darpel KE, Barber J, Hope A, Wilson AJ, Gubbins S, Henstock M, Frost L, Batten C, Veronesi E, Moffat K, Carpenter S, Oura C, Mellor PS, Mertens PPC (2016) Using shared needles for subcutaneous inoculation can transmit bluetongue virus mechanically between ruminant hosts. Sci Rep 6:20627. https://doi.org/10.1038/srep20627
Clavijo A, Sepulveda L, Riva J, Pessoa-Silva M, Tailor-Ruthes A, Lopez JW (2002) Isolation of bluetongue virus serotype 12 from na outbreak of the disease in South America. Vet Record 7:301–302. https://doi.org/10.1136/vr.151.10.301
Lager IA (2004) Bluetongue virus in South America: overview of viruses, vectors, survillance and unique features. Vet Ital 40:89–93
Costa JRR, Lobato ZIP, Herrmann GP, Leite RC, Haddad JPA (2006) Prevalência De Anticorpos Contra o vírus Da língua azul em bovinos e ovinos do sudoeste e sudeste do Rio Grande do sul. (Bluetongue virus antibodies in cattle and sheep in Southwest and Southeast regions of Rio Grande do sul, Brazil). Arq Bras Med Vet Zootec 58:273–275. https://doi.org/10.1590/S0102-09352006000200017(Portuguese)
Alves FAL, Alves CJ, Azevedo SS, Silva WW, Silva MLCR, Lobato ZIP, Clementino IJ (2009) Soroprevalência E fatores de risco para a língua azul em carneiros das mesorregiões do Sertão E Da Borborema, semi-árido do Estado Da Paraíba, Brasil. (seroprevalence and risk factors for Bluetongue in rams of the Sertão and Borborema mesoregions, semi-arid of Paraíba state, Northeastern Brazil). Ciênc Anim 39:484–489. https://doi.org/10.1590/S0103-84782008005000066(Portuguese)
Nogueira AHC, Pituco EM, Stefano E, Curci VCLM, Cardosa TC (2009) Detecção De Anticorpos Contra o vírus Da língua azul em ovinos na região de Araçatuba, São Paulo, Brasil. Cienc Anim Bras 10:1271–1276 Detection of antibodies against bluetongue virus in sheep in the region of Araçatuba, São Paulo, Brazil
Tomich RGP, Nogueira MF, Lacerda ACR, Campos FS, Tomas WM, Herrera HM, Lima-Borges PA, Pellegrin AO, Lobato ZIP, Silva RAMS, Pellegrin LA, Barbosa-Stancioli EF (2009) Sorologia para o virus Da língua azul em bovinos de corte, ovinos e veados campeiros no Pantanal Sul-matogrossense. Arq Bras Med Vet Zootec 61:1222–1226. https://doi.org/10.1590/S0102-09352009000500028(Portuguese)
Souza TS, Costa JN, Martinez PM, Costa Neto AO, Pinheiro RR (2010) Anticorpos contra o vírus Da língua azul em rebanhos ovinos da microrregião de Juazeiro, Bahia. Arq Inst Biol 77:419–427. https://doi.org/10.1590/1808-1657v77p4192010
Mota IO, Castro RS, Alencar SP, Lobato ZIP, Lima Filho CDF, Araújo Silva TL, Dutra ACT, Nascimento AS (2011) Anticorpos contra o vírus do grupo da língua azul em caprinos e ovinos do sertão de Pernambuco E inferência sobre sua epidemiologia em regiões semiáridas. (antibodies against bluetongue-virus group in goats and sheep from Pernambuco state and inferences on bluetongue epidemiology under tropical conditions. Arq Bras Med Vet Zootec 63:1595–1598. https://doi.org/10.1590/S0102-09352011000600045(Portuguese)
Dorneles EMS, Morcatti FC, Guimarães AS, Lobato ZIP, Lage AP, Gonçalves VSP, Heinemann MB (2012) Prevalence of bluetongue virus antibodies in sheep from Distrito Federal, Brazil. Semin Cienc Agrar 33:1521–1524. https://doi.org/10.5433/1679-0359.2012v33n4p1521
Pinheiro RR, Souza TS, Feitosa ALVL, Aragão MAC, Lima CCV, Costa JN, Andrioli A, Teixeira MFS, Brito RLL (2013) Frequência De Anticorpos Contra o vírus Da língua azul em ovinos do estado do Ceará, Brasil. Arq Inst Biol 77:419–427
Balaro MFA, Lima MS, Fava CD, Oliveira GR, Pituco EM, Brandão FZ (2014) Outbreak of bluetongue vírus serotype 4 in dairy sheep in Rio De Janeiro, Brazil. J Vet Diagn Invest 26:567–570. https://doi.org/10.1177/1040638714538020
Biihrer DA, Albuquerque AS, Romaldini AHCN, Pituco EM, Matos ACD, Lobato ZIP, Varaschin MS, Raymundo DL (2020) Serological survey of bluetongue virus in sheep from Minas Gerais. Pesq Vet Bras 40:261–265. https://doi.org/10.1590/1678-5150-PVB-6318
Sbizera MCR, Cunha Filho LFC, Lunardi M, Pertile SFN, Patelli THC, Barreto JVP, Pituco EM (2020) Detection of bluetongue virus antibodies in sheep from Parana, Brazil. Semin Cienc Agrar 41:879–886. https://doi.org/10.5433/1679-0359.2020v41n3p879
Ferreira Neto JV, Silva APM, Mesquita DC, Araujo JAS, Silva JWP, Pessoa FAC (2022) First record of antibodies to the bluetongue vírus in ewe (Ovis aries) in the state of Amazonas, Brazil. R Bras Ci Vet 2:81–84. https://doi.org/10.4322/rbcv.2022.015
Gorsch CVA, Duffy S, Miquet J, Pacheco J, Bolondi A, Draghi G, Cetra B, Soni C, Ronderos M, Russo S, Ramirez V, Lager I (2002) Bluetongue isolation and characterization of the vírus and identification of vectors in northeastern Argentina. Ver Argent Microbiol 34:150–156
Legisa D, Gonzalez F, De Stefano G, Pereda A, Dus Santos MJ (2012) Phylogenetic analysis of bluetongue vírus serotype 4 field isolates from Argentina. J Gen Virol 94:652–662. https://doi.org/10.1099/vir.0.046896-0
Negri Filho LC, Nogueira AHC, Stefano E, Katto S, Okuda LH, Silva LC, Pituco EM, Okano W (2016) Detecção de anticorpos contra o sorotipo 4 da língua azul (btv-4) em bovinos leiteiros da mesorregião norte central do Parana, Brasil. (Detection of antibodies against bluetongue serotype 4 (btv-4) in dairy cattle from the north central mesoregion of Parana, Brazil). Rev Ed Cont Med Vet Zootec:14
Lobato ZIP, Guedes MIMC, Matos ACD (2015) Bluetongue and others orbiviruses in South America: gaps and challenges. Vet Ital 51:253–262. https://doi.org/10.12834/VetIt.600.2892.1
SEAB (2015) Secretaria De Estado Da Agricultura E do Abastecimento. Dispõe sobre dados da pecuária paranaense. Governo do estado do Parana. Departamento De Economia Rural. (Secretary of State for Agriculture and Supply. Provides data on livestock in Parana. Parana State Government. Department of Rural Economy. Retrivied from www.agricultura.gov.br. (Portuguese).
IAPAR (2021) Agronomic Institute of Parana. Parana Climate Charts. Retrivied from http://www.iapar.br/modules/conteudo/conteudo.php?conteudo=863
CDC Epi Info https://www.cdc.gov/epiinfo/index.html. Accessed 25 July 2022
Core Team R (2021) R: A Language and Environment for Statistical Computing
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Caski F. (Eds.), Proceedings of the Second International Symposium on Information Theory. Budapest., Akademiai Kiado, pp. 267–281
Hosmer DW, Lemeshow S (2000) Applied Logistic Regression, 2nd edn. Wiley, New York
OIE (2016) World Organization for Animal Health. Report archive. Retrivied from http://www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/reportarchive
Purse BV, Carpenter S, Venter GJ, Bellis G, Mullens BA (2015) Binomics of temperate ant tropical Culicoides midges: knowledge gaps and consequences for transmission of Culicoides-borne viruses. R Entomol 60:373–392. https://doi.org/10.1146/annurev-ento-010814-020614
Flannery J, Sanz-Bernardo B, Ashby M, Brown H, Carpenter S, Cooke L, Batten C (2019) Evidence of reduced viremia, pathogenicity and vector competence in a re-emerging European strain of bluetongue virus serotype 8 in sheep. Transb Emerg Dis 66:1177–1185. https://doi.org/10.1111/tbed.13131
Navarro-Mamani DA, Pucllas JJ, Vargas-Calla A, Dextre KP, Quispe YZ, Murga-Moreno CA, Ibelice PC, Ruben VA, Miguel AG, Incil E, Pedro OO, Geronimo HR (2023) National seroprevalence and risk factors of bluetongue vírus in domestic ruminants in Peru. https://doi.org/10.1101/2023.07.06.548015
Coetzee P, Stokstad M, Venter EH, Myrmel M, Van Vuuren M (2012) Bluetongue: a historical and epidemiological perspective with the emphasis on South Africa. Virol J 13:198. https://doi.org/10.1186/1743-422X-9-198
Antoniassi NAB, Pavarini SP, Ribeiro LAO, Silva MS, Flores EF, Driemeier D (2010) Alterações clínicas e patológicas em ovinos infectados naturalmente pelo vírus da língua azul no Rio Grande do sul. (clinical and pathological changes in sheep naturally infected with bluetongue virus in Rio Grande do sul, Brazil). Pesq Vet Bras 30:1010–1016. https://doi.org/10.1590/S0100-736X2010001200002(Portuguese)
Menzies FD, McCullough SJ, McKeown IM, Forster JL, Jess S, Batten C, Oura CA (2008) Evidence for transplacental and contact transmission of bluetongue virus in cattle. Vet Rec 163:203–209. https://doi.org/10.1136/vr.163.7.203
Batista Filho AFB, Oliveira JMB, Silva GM, Oliveira PRF, Borges JM, Brandespim DF, Pinheiro Júnior JW (2018) Ocorrência E fatores de risco da infecção pelo vírus da língua azul em bovinos no Estado do Pernambuco. (occurrence and risk factors of the Bluetongue Virus infeccion in cattle in Pernambuco state, Brazil). Pesq Vet Bras 38:250–255. https://doi.org/10.1590/1678-5150-PVB-4379
Silva TG, Lima MS, Spedicato M, Carmine I, Teodori L, Leone A, Martins MSN, Buchala FG, Hellwig KS, Romaldini AHCN, De Stefano E, Savini G, Pituco EM (2018) Prevalence and risk factors for bluetongue in the state of São Paulo, Brazil. Vet Med Scie 4:280–287. https://doi.org/10.1002/vms3.113
Gasparini M, Laguardia-Nascimento M, Sales EB, Oliveira AGG, Lobato ZIP, Camargos MF, Fonseca Júnior AA (2021) Study of molecular diagnosis and viremia of bluetongue virus in sheep and cattle. Braz J Microbiol 52. https://doi.org/10.1007/s42770-021-00518-y
Takamatsu H, Mellor PS, Mertens PPC, Kirkham PA, Burroughs JN, Parkhouse RME (2003) A possible overwintering mechanism for bluetongue virus in the absence of the insect vector. J Gen Virol 84:227–235. https://doi.org/10.1099/vir.0.18705-0
Batten CA, Edwards L, Oura CAL (2013) Evaluation of the humoral immune responses in adult cattle and sheep, 4- and 2.5-years post-vaccination with a bluetongue serotype 8 inactivated vaccine. Vaccine 31:3783–3785. https://doi.org/10.1016/j.vaccine.2013.06.033
Gubbins S, Carpenter S, Baylis M, Wood JLN, Mellor PS (2008) Assessing the risk of bluetongue to UK livestock: uncertainly and sensitivity analyses of a temperature-dependent model for basic reproduction number. J R Soc 5:363–371. https://doi.org/10.1098/rsif.2007.1110
Funding
This research was funded by the following Brazilian institutes: National Foundation for the Development of Private Higher Education (FUNADESP), and the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design.vMaterial preparation, data collection and analysis were performed by Maria Carolina Ricciardi Sbizera, José Victor Pronievicz Barreto, Simone Fernanda Nedel Pertile, Fabíola Cristine de Almeida Rego, Julio Augusto Nylor Lisboa and Luiz Fernando Coelho da Cunha Filho. The first draft of the manuscript was written by Maria Carolina Ricciardi Sbizera and José Victor Pronievicz Barreto and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval and consent to participate
The project underwent ethical review and was given approval by the institutional animal care and use committee from Ethics Committee on the use of animals (CEUA 006/16), following all the national/international guidance of animal experimentation.
Additional information
Responsible Editor: Luis Augusto Nero.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sbizera, M.C.R., Barreto, J.V.P., Pertile, S.F.N. et al. Longitudinal seroepidemiological survey and risk factors for bluetongue virus infection in sheep in the state of Parana, Brazil, from 2014 to 2017. Braz J Microbiol (2024). https://doi.org/10.1007/s42770-024-01486-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42770-024-01486-9