Abstract
Candida species resistant to fluconazole have raised concern in the scientific medical community due to high mortality in patients with invasive disease. In developing countries, such as Brazil, fluconazole is the most commonly used antifungal, and alternative treatments are expensive or not readily available. Furthermore, the occurrence of biofilms is common, coupled with their inherent resistance to antifungal therapies and the host’s immune system, these microbial communities have contributed to making infections caused by these yeasts an enormous clinical challenge. Therefore, there is an urgent need to develop alternative medicines, which surpass the effectiveness of already used therapies, but which are also effective against biofilms. Therefore, the present study aimed to describe for the first time the antifungal and antibiofilm action of the derivative 2-amino-5,6,7,8-tetrahydro-4 H-cyclohepta[b]thiophene-3-isopropyl carboxylate (2AT) against clinical strains of Candida spp. resistant to fluconazole (FLZ). When determining the minimum inhibitory concentrations (MIC), it was found that the compound has antifungal action at concentrations of 100 to 200 µg/mL, resulting in 100% inhibition of yeast cells. Its synergistic effect with the drug FLZ was also observed. The antibiofilm action of the compound in subinhibitory concentrations was detected, alone and in association with FLZ. Moreover, using scanning electron microscopy, it was observed that the compound 2AT in isolation was capable of causing significant ultrastructural changes in Candida. Additionally, it was also demonstrated that the compound 2AT acts by inducing characteristics compatible with apoptosis in these yeasts, such as chromatin condensation, when visualized by transmission electron microscopy, indicating the possible mechanism of action of this molecule. Furthermore, the compound did not exhibit toxicity in J774 macrophage cells up to a concentration of 4000 µg/mL. In this study, we identify the 2AT derivative as a future alternative for invasive candidiasis therapy, in addition, we highlighted the promise of a strategy combined with fluconazole in combating Candida infections, especially in cases of resistant isolates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biofilms are defined as structured microbial communities surrounded by a protective extracellular matrix, attached to a biotic (host tissues) or abiotic (medical devices) surface. These communities can initiate or prolong infections by providing a safe, treatment-resistant environment from which cells can invade local tissue and simultaneously establish new foci of infection [1, 2].
Among the most frequent pathogens that occur forming biofilms, yeasts of the genus Candida stand out. Infections caused by these microorganisms can occur in superficial or invasive forms. These, when disseminated, reach high mortality rates, which increase even further in the context of host immunosuppression and infection by drug-resistant Candida species [3, 4]. In this scenario, practically all Candida species associated with candidiasis are capable of establishing resistant biofilms on different types of surfaces, which represents a significant threat to the emergence of infection and subsequent success of the invasive disease [5].
Currently, there is a limitation in the classes of antifungal medications available to treat patients who have these infections. When in biofilms, Candida spp. can withstand very high concentrations of antifungal drugs compared to infections caused by planktonic cells, thus making these infections difficult to treat [6, 7].
Currently, there is an alarming increase in cases of resistance in Candida species, making it a clinical challenge in medical centers around the world. Faced with the need for new antifungal agents, thiophenic derivatives have stood out due to their promising biological applications and important pharmacological potential [8, 9]. These derivatives are part of a group of sulfur-based heterocyclic compounds with therapeutic activities against various diseases. And, they exhibit a wide range of biological activities, such as antifungal, antiviral, antibacterial, anti-inflammatory and cytotoxic [10].
It is indispensable to highlight that there are no established therapies that target Candida biofilms. However, combination medications have been reported as a possible treatment alternative. This may involve combinations of antifungal agents belonging to different classes as potential therapeutic options. Combination therapy promotes a broader spectrum of action, reduced dosages and less antifungal tolerance [11, 12].
Therefore, the present study aimed to evaluate the antifungal and antibiofilm activity of the 2AT derivative alone and in association with the antifungal FLZ against resistant Candida strains. Moreover, we seek to understand the basis of the molecule’s mechanism of action, as well as its toxicity.
Materials and methods
Strains
Eleven clinical isolates from the Candida were obtained from blood samples and identified by MALDI TOF from the URM Culture Collection Micoteca of the Federal University of Pernambuco.
Compound
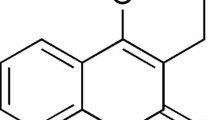
The 2AT, represented in Fig. 1, was synthesized by the Molecule Synthesis and Vectorization Laboratory of the State University of Paraíba, according to a procedure previously described by [13]. Briefly, and using the classic Gewald reaction [14], equimolar amounts of cycloheptane, elemental sulfur and isopropylcyano acetate were reacted in a basic medium, through a one pot procedure. After completion of the reaction, the product was purified by successive recrystallization in absolute ethanol.
To determine the biological activities described in our study, we used the CLSI M27-S4 guidelines, with some modifications [15]. For the tests, a stock solution was prepared in which we dissolved 10 mg of compound 2 AT in a mixture of ethanol and dimethyl sulfoxide. This solution was then diluted in RPMI 1640 medium, buffered to pH 7.0 with 0.165 M morpholinopropanesulfonic acid (Sigma-Aldrich) to reach concentrations ranging from 2 µg/mL to 1.024 µg/mL.
Biofilm quantitative analysis
Candida spp. isolates were cultured aerobically at 37 °C/18 h on Sabouraud dextrose agar (SAB). Suspensions were prepared in Yeast Nitrogen Base-YNB broth (Difco) supplemented with 50 mM glucose. After 18 h of incubation, the suspensions were centrifuged at 3200 rpm for 4 min then washed twice with PBS (pH 7.2). Cells were adjusted to a final concentration of 107 cells/mL with a spectrophotometer at 530 nm [15–16] 100 µL of the cell suspensions were added to the wells of 96-well flat-bottom microdilution plates (Techno Plastic Products, Switzerland), kept at 37 °C for 1.5 h at 75 rpm (adhesion phase). After biofilm formation, the wells were washed twice with 200 µl of PBS, then 200 µl of PBS and 12 µl of the XTT-menadione solution were added to each well. Subsequently, 100 µl of the reaction solution was transferred to a new microtiter plate and the absorbance was measured with a spectrophotometer plate reader at 530 nm.
Checkerboard
Combinations of the fluconazole and compost thiophen (1:1) were tested in duplicates using the M27-S4 method from the Clinical Laboratory Standards Institute (Wayne, 2012) [17] with readings being performed within 24 h. To evaluate antifungal interactions, the fractional inhibitory concentration (FIC) was calculated for each combination.
The Fractional Inhibitory Concentration index was assessed by the compost thiophen /fluconazole combination using the checkerboard dilution method [18]. The FIC was calculated for each agent by dividing the inhibition concentration of the antifungal combination by its MIC value. The interaction of the combination was defined by the FIC values and synergism was defined by a FIC ≤ 0.5, while additivity was defined as a FIC > 0.5 < 1. Indifference was defined as a FIC > 1 < 4, whereas antagonism was defined as a FIC > 4.
FLZ and 2AT anti-biofilm evaluation
Following the steps for biofilm formation (described above), 100 µL of 2AT (40 − 5 mM) and FLZ (64–0,125 µg/mL) (isolated and combined) were added during the adhesion period (1.5 h) and their anti-biofilm action was evaluated within 48 h. The XTT reduction assay was performed after 48 h and the isolated and synergistic effect of the drugs were evaluated. Controls (positive and negative), as well as the reference strain C. albicans ATCC 14,053, were included. The test was performed in triplicate.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 6 software with a significance level of α = 0.05. The dependent (incubation time and biofilm development) and independent (oxidative activity) variables were statistically analyzed. An ANOVA was used to test the null hypothesis when there were no differences between treatments. Tukey’s HSD test was used following the ANOVA for comparisons.
Scanning electron microscopy
The isolates were subjected to the 2AT derivative, using the concentrations and criteria described in the susceptibility tests. The yeast cells were washed with 1x PBS (3 times) and gently centrifuged to remove the culture medium.
They were subsequently fixed with Karnovsky (2.5% glutaraldehyde; 4.0% formaldehyde and 0.1 M phosphate buffer). Post-fixation was performed with 1% osmium tetroxide (Electron Microscopy Science) followed by increasing dehydration with ethanol (30%, 50%, 70%, 90% and 100%). After dehydration, the material was taken to the critical point for complete drying of the samples and metallized with gold/palladium on the FINE COAT ION SPUTTER JFC-1100, JEOL equipment. Visualization of Candida spp. and image capture were carried out using a scanning electron microscope EVO LS-15, ZEISS.
Transmission electron microscopy
Yeast cells treated and untreated with the compound 2AT were processed for transmission electron microscopy. The cells were fixed for 2 h at 4 °C in a solution of glutaraldehyde and paraformaldehyde (3% and 4% respectively) in 0.1 M cacodylate buffer, pH 7.2. After washing in the same buffer, cells were post-fixed for 1 h with 1% osmium tetroxide in 0.1 M cacodylate buffer at pH 7.2. They were then dehydrated in graded series with acetone and embedded in Epon-82 (Sigma-Aldrich, St Louis, USA) for 72 h at 60 °C. Ultrathin sections were stained with uranyl acetate and lead citrate (5% and 2% respectively) and observed on a FEI Tecnai™ Spirit G 2 BioTWIN (FEI, Oregon, USA).
Cytotoxicity
Macrophage culture
Macrophage (J774) were cultivated in RPMI (Sigma Aldrich, St. Louis, USA) supplemented with 10% fetal bovine serum (FBS) and kept in an incubator stove at 5% CO2, at 37 ºC.
Cytotoxicity analysis of compounds
The cytotoxicity in mammalian cells was assessed using tests with 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium (MTT) bromide. Macrophages were cultured in 96-well cell culture plates at a concentration of 2 × 106 cells per plate and incubated in an atmosphere containing 5% CO2 at 37 °C. After 24 h, the supernatant was removed, and the cells were incubated with various concentrations of the 2AT (ranging from 4000 to 250 µg/mL) for 72 h. Absorbance readings of the solubilized formazan crystals were performed using an ELISA Benchmark Plus spectrophotometer (Bio-Rad, California, USA) with a wavelength of 570 nm. The concentration at which a 50% reduction in cellular viability occurred was determined through linear regression analysis. Each experiment was conducted in biological duplicate. Macrophages incubated in culture medium and in the standard drug FLZ were used as negative and positive controls, respectively.
Results and discussion
Antifungal and synergistic action of the 2AT derivative
Our results showed that all Candida strains evaluated showed resistance to the antifungal FLZ, with minimum inhibitory concentrations (MIC) ranging from 8 to > 64 (Table 1). These data demonstrate that despite the usefulness of this drug in the treatment of fungal infections, there has been an increase in the global incidence of Candida isolates resistant to FLZ [19–20].
Candida infections generally present therapeutic failure, contributing to the mortality associated with invasive candidiasis. Despite advances in the field of antifungal therapy, these rates have remained unchanged for more than a decade. Such infections could be treated more efficiently if more effective therapeutic alternatives were available for cases of resistance [21].
The emergence of resistance threatens the effectiveness of azoles, which are the most widely used class of antifungals and the only oral treatment option available for candidemia. A promising strategy to increase the effectiveness of FLZ is to identify synergistic drugs that can increase its antifungal effect, or even reduce the emergence of this resistance, making it fungicidal [22].
Thiophenes derivatives are heterocyclic compounds that have versatile chemical characteristics and many biological activities. This makes these molecules promising for the development of new drugs, and justifies the interest in characterizing their antifungal properties, especially in cases of resistance [23].
In our study, we observed that the thiophene derivative 2AT showed 100% fungal action, with MICs ranging from 100 to 200 µg/ml when evaluated alone, and when associated with FLZ it showed a synergistic effect (FIC < 4) against all strains resistant to this drug (Table 1). Among the strains of Candida spp. analyzed, we observed that in the isolates C. albicans (HG 04, 07, 11e ATCC 14053) and C. parapsilosis (HG 01 and 05), the 2AT derivative showed fungicidal action at concentrations of 100 µg/ml, the lowest MIC value detected, as seen in Table 1.
This antifungal potential of 2-aminothiophene compounds has already been observed in other studies. Lua et al. [24] observed that compounds derived from 2-aminothiophenes showed promising in vitro antifungal activity against dermatophyte fungi and C. parapsilosis. Neves et al. [25] also detected activity of this class of thiophenes against C. albicans and Cryptococcus neofarmans.
Regarding the synergistic potential of thiophene compounds, Mohammed et al. [26] observed that pyrazole derivatives containing the thiophene ring demonstrated synergistic effects when combined with Ciprofloxacin and Ketoconazole, reducing MICs in resistant bacterial pathogens. In fungal pathogens, Ajdacic et al. [27] who observed that thiophene-based compounds exhibited excellent activity against voriconazole-resistant C. albicans. In another study, Yin et al. [28] observed that 5-phenylthiophene derivatives showed fungicidal activity and inhibited the growth of C. albicans resistant to FLZ.
As for enhancing FLZ activity, there are targets that are known to increase its effectiveness. In cases of resistance, FLZ can be synergized if a compound helps it cause damage by inhibiting functions that confer that resistance. Such as the transcription factors Ndt80, which is known to play an important role in the expression of enzymes related to ergosterol biosynthesis, morphological transition and is also related to FLZ resistance [11, 29, 30].
Furthermore, Neto et al. [31] observed that N-substituted 2-(5-nitro-thiophene)-thiosemicarbazone derivatives are potential antifungal agents with activity associated with the inhibition of enzymes related to ergosterol biosynthesis. Therefore, the possible inhibition of enzymes or genes related to ergosterol synthesis could be a potential synergistic target of fluconazole when associated with the compound 2AT.
Biofilm production of Candida strains and antibiofilm potential of 2AT and FLZ
The formation of biofilms is of extreme clinical relevance, as there is a link between antifungal resistance and virulence. In our research, it was observed that all isolates used are capable of forming an active biofilm in vitro within 48 h (Fig. 2). These data corroborate Boher et al. [32] who confirmed in their results that there is a positive correlation between increased MICs of azoles and more pronounced biofilm formation.
Biofilm formation by clinical isolates of Candida spp. Strains HG 01 (C. parapsilosis), HG 05 (C. parapsilosis), HG 08 (C. glabrata) and HG 11 (C. albicans) were the largest biofilm formers in vitro. In contrast, strain HG10 (C. tropicalis) was the least biofilm-forming strain. Data represent means and standard deviation in triplicate. For analysis, Tukey’s multiple comparison test was performed for all means obtained at a significance level of 5%. Symbols “****” indicate significant differences between the percentage of biofilm production (p ≤ 0.0001)
Regarding the antibiofilm effect, it was found that the 2AT compound decreased fungal proliferation, adhesion and biofilm formation of fungal cells. Furthermore, when combined with FLZ it resulted in a strong antibiofilm effect, which suppressed the local growth of Candida cells that didn’t adhere to the surface (Fig. 3).
Antibiofilm effect of strains in the absence of FLZ/2AT (control), treated with FLZ (fluconazole), treated with (compound 2AT for 24 h), treated with the combination of (FLZ and 2AT for 24 h), treated with (FLZ and 2AT for 48 h). For analysis, Tukey’s multiple comparison test was performed for all means obtained at a significance level of 5%. The symbols “****” indicate significant differences between the percentage between isolates treated for 24 h and 48 h alone and in combination with FLZ and 2AT (p ≤ 0.0001)
It is important to highlight that isolate from the C. parapsilosis complex (HG 01 and HG 05), together with a strain of C. albicans (HG 11) had a higher production compared to other strains, as seen in Fig. 2. Despite this, we observed a significant reduction in its formation when these strains were exposed to FLZ and 2AT in combination.
C. glabrata (HG 06, 08 and 09), demonstrated the lowest biofilm production capacity, with the exception of isolate HG 08. C. glabrata is also one of the main species responsible for candidiasis and has high antifungal tolerance, in addition to important adhesion characteristics [33]. Martínez-Herrera et al. [34] emphasized in their study that the high mortality rate reported in cases of invasive infections by C. glabrata has been related to both low intrinsic susceptibility and true resistance to fluconazole. Furthermore, certain isolates can acquire cross-resistance with other antifungals [33].
In our study, C. tropicalis strains did not demonstrate high biofilm production, contrary to the study by Konečná et al. [35] who described that the species could be categorized as a strong biofilm producer. C. albicans strains (HG 07, 11 and ATCC 14053) showed a greater capacity to form biofilms than C. tropicalis (HG 02, 03 and 12), although studies by Atiencia-Carrera et al. [36] show that C. tropicalis is the most prevalent species among biofilm-forming organisms, even more than C. albicans. Of the C. albicans isolates (HG 04, 07, 11 and ATCC 14,053), all of them showed a moderate capacity for biofilm formation, except for isolate HG 11. In addition to this capacity, all of them had their biofilm formation reduced when exposed to the association of FLZ and 2AT.
There are few studies on the antibiofilm potential of thiophene compounds in fungal biofilms. Yin et al. [28] who observed that 5-phenylthiophene derivatives, in addition to their fungicidal activity against fluconazole-resistant C. albicans, also had excellent antibiofilm potential. Neves et al. [25] also found that 2-aminothiophene nanoparticles had antifungal and antibiofilm activity against C. neoformans.
The results observed in our study may have occurred due to the ability of the 2AT derivative to reduce the adhesion of Candida cells. Lu et al. [11] state that an impaired adhesion process is beneficial for potentiating fluconazole. Furthermore, the association may act on genes involved in hyphal growth. Studies have observed that the deletion of Ntd80 causes Candida to have a general defect in its growth. When absent, in C. parapsilosis, it prevents the species from forming biofilms [37].
Structural analysis
The structural analysis of yeast cells treated with the compound thiophene and visualized by scanning electron microscopy showed changes in Candida spp. at subinhibitory concentrations (Fig. 4).
Electron micrographs of Candida albicans ATCC 14053 (ab) in the absence of the compound 2AT and (cd) under the effect of the compound. a Presence of many cells and some hyphae and biomass production (arrowhead). b Fungal cells have intact and smooth walls, with the presence of polar scars (white arrows). c Under the effect of the thiophene compound, there is a significant reduction in cells and biomass. d Cells treated with 2AT, showing rough cell walls (white arrows)
Among the changes observed are the reduction in biomass and roughness of the yeast cell walls. It is worth mentioning that the results obtained were observed at a subinhibitory concentration of 0.78 µg/ml (50% fungal growth) since at a concentration of 100 µg/ml there is a fungicidal action on 100% of Candida cells. Ishida et al. [38] used enzyme inhibitors Δ 24(25) sterolmethyltransferase and squalene synthase in the treatment of C. albicans. These inhibitors act on the main ergosterol biosynthesis pathway, causing changes in the shape and thickness of the cell wall, as well as mitochondrial swelling and abnormalities in the nuclear structure. In our analysis, we observed similar structural patterns, suggesting that the compound may share the same mechanism of action and be related to the synthesis of ergosterol.
These changes are also compatible with the action of amphotericin B on fungal cells. This medication binds to ergosterol and works by extracting sterols from cell membranes, weakening it and causing leakage of cytosolic contents, leading to cell death [39]. Furthermore, Grela et al. [40] observed that amphotericin affects the integrity of the cell wall during the budding of daughter cells, and this occurs due to the consequent decrease in the rigidity of the lipid bilayer.
Transmission microscopy
Transmission electron microscopy of cells treated with the compound thiophene at concentrations of 0.78 µg/ml revealed morphological changes characteristic of apoptosis, such as chromatin condensation and margination (Fig. 5).
Transmission micrographs of Candida albicans ATCC 14053 cells in the absence of 2AT (ab) and treated at subinhibitory concentration (cd). (ab) Untreated C. albicans cells, showing nuclei (n) and mitochondria (white arrowheads). (cd) C. albicans cells treated with the thiophene derivative, showing mitochondrial swelling (m), cell wall thickening (red arrow) and membrane disintegration (black arrows) and chromatin migration (white asteristic), and presence of aggregations of small vacuoles (white arrowheads) present in apoptosis and necrotic processes
De Araújo et al. [31] observed similar findings when determining that thiophene-thiosemicarbazone caused retraction of the plasma membrane, changes in the shape of mitochondria and the nucleus, causing apoptosis in C. albicans cells due to oxidative stress. In another study, Fayed et al. [10] observed that cyclohepta[b]thiophene can induce damage to an important enzyme in many basic biological processes involving the DNA of bacteria and yeast.
In addition to these findings, it is also possible to observe aggregations of small vacuoles (Fig. 5), results also found and compatible with the mechanism of action of amphotericin B. These results corroborate the studies Grela et al. [40] who found that Candida cells presented this response when exposed to amphotericin B. According to the study, these vacuoles provide a means of self-defense against some toxic activity for yeast cells.
In our study, thiophene may have induced Candida membrane damage, and when in combination with FLZ may also have caused direct DNA damage [11]. The enhancement of the effect of FLZ when combined with thiophene, exhibited changes at the nuclear level in Candida cells, corroborating the data from Lu et al. [11]. In the study, the authors demonstrated that disturbances in the DNA damage response and cell cycle functions of these yeasts can improve the effectiveness of fluconazole.
Furthermore, thiophene can also act on mitochondria, since they are decisive for cell death, as observed by De Araújo Neto et al. [30]. The action of thiophene together with FLZ can cause membrane potential disturbances that lead to a decrease in metabolic energy production and a reduction in the transcription and translation of mitochondrial genes, leading to apoptosis and/or necrosis [30].
Assessment of cytotoxicity
Regarding the cytotoxicity of the thiophene derivative 2AT, we evaluated the cell viability of J774 macrophages in the presence of the compound at concentrations ranging from 250 to 4000 µg/mL. The data revealed that the evaluated cell line was not affected when incubated with the thiophene derivative up to a concentration of 250 µg/mL, presenting an IC50 of 10,152.52 and cell viability of 72% at a concentration of 4000 µg/mL and cell inhibition of 27.74% (Fig. 6). Our results indicate that there is selectivity of the compound with fungal cells. These data corroborate those of Sousa et al. [39] evaluated the cytotoxicity of another 2-amino-thiophene derivative SB-200 against Zophobas morio larvae and observed 100% survival.
Conclusions
The present study describes a promising drug candidate for the treatment of Candida fungal infections. The 2AT derivative exhibited antifungal activity against C. albicans, C. glabrata, C. tropicalis and C. parapsilosis, species responsible for the majority of invasive fungal infections. Furthermore, it has demonstrated significant efficacy in reducing biofilm formation.
It is known that biofilm formation by these yeasts has impacted susceptibility to antifungals, leading to resistance, which demonstrates the importance of research aimed at the prevention and control of these clinical microbial communities.
The compound showed synergism with fluconazole, suggesting that its effect in association with this drug can enhance its antifungal and antibiofilm effects. This may be a promising approach to increasing the efficacy of fluconazole, especially in cases of resistance. Furthermore, the compound showed no in vitro toxicity in J774 macrophages, showing only minimal toxicity at very high concentrations.
Furthermore, we detected structural changes that indicate loss of cell wall integrity, as well as changes in the membrane, mitochondria and nucleus in Candida spp. From these results, we emphasize the antifungal and antibiofilm potential of the 2AT derivative in resistant Candida isolates, but in-depth studies must be carried out to understand the complete mechanism of action of this molecule and in vivo research is strongly encouraged.
References
Ramage G, Borghi E, Rodrigues CF, Kean R, Williams C, Lopez-Ribot J (2023) Our current clinical understanding of Candida biofilms: where are we two decades on? APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 131:636–653. https://doi.org/10.1111/apm.13310
Eix EF, Nett JE (2020) How Biofilm Growth affects Candida-host interactions. Front Microbiol 11:1437. https://doi.org/10.3389/fmicb.2020.01437
Ajetunmobi OH, Badali H, Romo J, Ramage G, Lopez-Ribot JL (2023) Antifungal therapy of Candida biofilms: past, present and future. Biofilm 5:100126. https://doi.org/10.1016/j.bioflm.2023.100126
Murphy SE, Bicanic T (2021) Drug Resistance and Novel Therapeutic approaches in Invasive Candidiasis. Front Cell Infect Microbiol 11:759408. https://doi.org/10.3389/fcimb.2021.759408
Kaur J, Nobile CJ (2023) Antifungal drug-resistance mechanisms in Candida biofilms. Curr Opin Microbiol 71:102237. https://doi.org/10.1016/j.mib.2022.102237
Massey J, Zarnowski R, Andes D (2023) Role of the extracellular matrix in Candida biofilm antifungal resistance. FEMS Microbiol Rev 47:0168–6445. https://doi.org/10.1093/femsre/fuad059
Wall G, Lopez-Ribot JL (2020) Current antimycotics, new prospects, and future approaches to antifungal therapy. Antibiotics 9:445. https://doi.org/10.3390/antibiotics9080445
Archna, Pathania S, Chawla PA (2020) Thiophene-based derivatives as anticancer agents: an overview on decade’s work. Bioorg Chem 101:1–37. https://doi.org/10.1016/j.bioorg.2020.104026
De Araújo Neto LN, de Lima MDCA, de Oliveira JF, De Souza ER, Feitosa SEM, De Souza Lima GM, Buonafina MDS, Brayner FA, Alves LC, Sandes JM, De Castro MVS, Neves MCAB, Mendonça-Junior RP FJB (2020) Thiophene-thiosemicarbazone derivative (L10) exerts antifungal activity mediated by oxidative stress and apoptosis in C. Albicans. Chem Biol Interact Apr 320:109028. https://doi.org/10.1016/j.cbi.2020.109028
Fayed EA, Mohsen M, El-Gilil SMA, Aboul-Magd DS, Ragab A (2022) Novel cyclohepta[b]thiophene derivative incorporating pyrimidine, pyridine, and chromene moiety as potential antimicrobial agents targeting DNA gyrase. J Mol Struct 1262:133028. https://doi.org/10.1016/j.molstruc.2022.133028
Lu H, Shrivastava M, Whiteway M, Jiang W (2021) Candida albicans targets that potentially synergize with fluconazole, Critical Reviews in Microbiology, 47: 323–337. https:https://doi.org/10.1080/1040841X.2021.1884641
De Barros PP, Rossoni RD, de Souza CM, Scorzoni L, Fenley JC, Junqueira JC (2020) Candida Biofilms: an update on Developmental mechanisms and Therapeutic challenges. Mycopathologia 185:415–424. https://doi.org/10.1007/s11046-020-00445-w
Da Cruz RMD, Zelli R, Benshain S, Siqueira-Júnior JP, Décout JL, Mingeot-Leclercq MP, Mendonça-Junior FJB (2020) Synthesis and Evaluation of 2-Aminothiophene Derivatives as Staphylococcus aureus Efflux Pump Inhibitors. MedChem, 15, 716–725. https://doi.org/10.1002/cmdc.201900688
Gewald K (1965) Heterocyclen aus CH-aciden Nitrilen, VII. 2-Amino-thiophene aus α-Oxo-mercaptanen und methylenaktiven Nitrilen. Chem Ber 98:3571–3577. https://doi.org/10.1002/cber.19650981120
Silva WJ, Seneviratne J, Parahitiyawa N, Rosa EAR, Samaranayake LP, Del Bel Cury AA (2008) Improvement of XTT assay performance for studies involving Candida albicans biofilms. Braz Dent J 19:364–369. https://doi.org/10.1590/S0103-64402008000400014
Clinical and Laboratory Standards Institute (2012) Reference method for broth dilution antifungal susceptibility testing of yeasts; fourth informational supplement. Clinical and Laboratory Standards Institute, Wayne. (Document M27-A4)
Clinical and Laboratory Standards Institute (2008) Reference method for broth dilution antifungal susceptibility testing of yeasts. 3ed. Wayne: Clinical and Laboratory Standards Institute. (Approved standard. M27-A3)
Lewis RE, Diekema DJ, Messer MA, Pfaller, Klepser ME (2002) Comparison of Etest, chequerboard dilution and time–kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J Antimicrob Chemother 49:345–351. https://doi.org/10.1093/jac/49.2.345
Daneshnia F, Júnior JNA, Ilkit M, Lombardi L, Perry AM, Gao M, Nobile CJ, Egger M, Perlin DS, Zhai B, Hohl TM, Gabaldón T, Colombo AL, Hoenigl M, Arastehfar M (2023) Worldwide emergence of fluconazole-resistant Candida parapsilosis: current framework and future research roadmap. Lancet Microbe 4:e470–e480. https://doi.org/10.1016/S2666-5247(23)00067-8
Costa-de-Oliveira S, Rodrigues AG (2020) Candida albicans Antifungal Resistance and Tolerance in Bloodstream infections: the Triad yeast-host-antifungal. Microorganisms 8:1–19. https://doi.org/10.3390/microorganisms8020154
Dawoud AM, Saied SA, Torayah MM, Ramadã AE, Elaskary SA (2024) Antifungal susceptibility and virulence determinants profile of candida species isolated from patients with candidemia. Sci Rep 14:11597. https://doi.org/10.1038/s41598-024-61813-w
Escribano P, Guinea J (2022) Fluconazole-resistant Candida parapsilosis: a new emerging threat in the fungi arena. Front Fungal Biology 3:1010782. https://doi.org/10.3389/ffunb.2022.1010782
Mabkhot YN, Alatibi F, El-Sayed NNE, Kheder NA, Al-Showiman SS (2016) Synthesis and structure-activity relationship of some New Thiophene-based heterocycles as potential Antimicrobial agents. Molecules 21:1036. https://doi.org/10.3390/molecules21081036
Luna IS, Wendell WN, Lima-Neto RG, Albuquerque APB, Pitta MGR, Rêgo MJBM, Neves RP, Scotti MT, Mendonça-Junior FJB (2021) Design, synthesis and antifungal activity of New Schiff bases bearing 2-Aminothiophene derivatives obtained by Molecular Simplification. J Braz Chem Soc 32:5: 1017–1029. https://doi.org/10.21577/0103-5053.20210004
Neves WW, Neves RP, Macêdo DPC, Eleamen GRA, Kretzschmar EAM, Oliveira EE, Mendonça-Júnior FJB, Lima-Neto RG (2020) Incorporation of 2-amino-thiophene derivative in nanoparticles: enhancement of antifungal activity. Braz J Microbiol 51:647–655. https://doi.org/10.1007/s42770-020-00248-7
Mohamed HA, Ammar YA, Elhagali GAM, Eyada HA, Aboul-Magd DS, Ragab A (2022) In Vitro Antimicrobial evaluation, single-point resistance study, and Radiosterilization of Novel Pyrazole incorporating Thiazol-4-one/Thiophene derivatives as dual DNA gyrase and DHFR inhibitors against MDR pathogens. ACS Omega 7:6: 4970–4990. https://doi.org/10.1021/acsomega.1c05801
Ajdačić V, Senerovic L, Vranić M, Pekmezovic M, Arsic-Arsnijevic V, Veselinovic A, Veselinovic J, Šolaja BA, Nikodinovic-Runic J, Opsenica IM (2016) Synthesis and evaluation of thiophene-based guanylhydrazones (iminoguanidines) efficient against panel of voriconazole-resistant fungal isolates. Bioorg Med Chem 24(6):1277–1291. https://doi.org/10.1016/j.bmc.2016.01.058
Yin W, Liu L, Jiang H, Wu T, Cui H, Zhang Y, Gao Z, Sun Y, Qin Q, Zhao L, Su X, Zhao D, Cheng M (2022) Design, synthesis, and evaluation of novel 3-thiophene derivatives as potent fungistatic and fungicidal reagents based on a conformational restriction strategy European. J Med Chem 233:114195. https://doi.org/10.1016/j.ejmech.2022.114195
De Oliveira Santos GC, Vasconcelos CC, Lopes AJO, De Sousa Cartágenes MDS, Filho AKDB, Do Nascimento FRF, Ramos RM, Pires ERRB, De Andrade MS, Rocha FMG, De Monteiro A, C (2018) Candida Infections and therapeutic strategies: mechanisms of action for traditional and alternative agents. Front Microbiol 9:1351. https://doi.org/10.3389/fmicb.2018.01351
De Araújo Neto LN, do Carmo Alves de Lima M, de Oliveira JF, de Souza ER, Buonafina MDS, Vitor Anjos MN, Brayner FA, Alves LC, Neves RP, Mendonça-Junior FJB (2017) Synthesis, cytotoxicity and antifungal activity of 5-nitro-thiophene-thiosemicarbazones derivatives. Chemico-Biol Interact 272:172–181. https://doi.org/10.1016/j.cbi.2017.05.005
Vandeputte P, Pradervand S, Ischer F, Coste AT, Ferrari S, Harshman K, Sanglard D (2012) Identification and functional characterization of Rca1, a transcription factor involved in both antifungal susceptibility and host response in Candida albicans. Eukaryot Cell 11:916–931. https://doi.org/10.1128/EC.00134-12
Bohner F, Papp C, Gácser A (2022) The effect of antifungal resistance development on the virulence of Candida species. FEMS yeast research. 22:foac019. https://doi.org/10.1093/femsyr/foac019
McCarty TP, White CM, Pappas PG (2021) Candidemia and Invasive Candidiasis. Infect Dis Clin North Am 35:389–413. https://doi.org/10.1016/j.idc.2021.03.007
Martínez-Herrera E, Frías-De-León MG, Hernández-Castro R, García-Salazar E, Arenas R, Ocharan-Hernández E, Rodríguez-Cerdeira C (2022) Antifungal Resistance in Clinical isolates of Candida Glabrata in Ibero-America. J Fungi 8:14. https://doi.org/10.3390/jof8010014
Konečná K, Němečková I, Diepoltová A, Vejsová M, Janďourek O (2021) The impact of Cultivation Media on the in vitro Biofilm Biomass production of Candida Spp. Curr Microbiol 78:2104–2111. https://doi.org/10.1007/s00284-021-02452-6
Atiencia-Carrera MB, Cabezas-Mera FS, Vizuete K, Debut A, Tejera E, Machado A (2022) Evaluation of the biofilm life cycle between Candida albicans and Candida tropicalis. Front Cell Infect Microbiol 12:953168. https://doi.org/10.3389/fcimb.2022.953168
Lo WH, Deng FS, Chang CJ, Lin CH (2020) Synergistic Antifungal Activity of Chitosan with Fluconazole against Candida albicans, Candida tropicalis, and Fluconazole-Resistant Strains. Molecules, 21: 5114. https://doi.org/10.3390/molecules25215114
Ishida K, Rodrigues JC, Ribeiro MD, Vila TV, De Souza W, Urbina JA, Nakamura CV, Rozental S (2009) Growth inhibition and ultrastructural alterations induced by Delta24(25)-sterol methyltransferase inhibitors in Candida spp. isolates, including non-albicans organisms. BMC Microbiol 9:74. https://doi.org/10.1186/1471-2180-9-74
Sousa JPA, Sousa IMS, Rodrigues RRL, Nunes TAL, Machado YAA, Araujo AC, Silva IGM, Barros-Cordeiro KB, Báo SN, Alves MMM, Mendonça-Junior FJB, Rodrigues KAF (2023) Antileishmanial activity of 2-amino-thiophene derivative SB-200. Int Immunopharmacol 110750. https://doi.org/10.1016/j.intimp.2023.110750
Grela E, Zdybicka-Barabas A, Pawlikowska-Pawlega B, Cytrynska M, Wlodarczyk M, Grudzinski W, Luchowski R, Gruszecki WI (2019) Modes of the antibiotic activity of amphotericin B against Candida albicans. Sci Rep 9:17029. https://doi.org/10.1038/s41598-019-53517-3
Acknowledgements
The authors would like to thank Jana Messias Sandes, Gabriel Gazzoni and Rafael Padilha from the Electron Microscopy Laboratory of the Keizo Asami Immunopathology Laboratory– LIKA for their contribution to the production of Scanning and Transmission Electron Microscopy images.
Author information
Authors and Affiliations
Contributions
Adryelle Idalina da Silva Alves: Conceptualization, Writing - Original Draft, Investigation, Writing - Review & Editing. Bruna Rodrigues de Sousa: Formal analysis. Janderson Weydson Lopes Menezes da Silva: Investigation. Dyana Leal Veras: Resources, Conceptualization, Methodology. Fábio André Brayner: Resources, Methodology. Luiz Carlos Alves: Resources, Methodology. Francisco Jaime Bezerra Mendonça-Junior: Resources, Writing - Review & Editing. Cicero Pinheiro Inácio: Supervision, Writing - Review & Editing. Rejane Pereira Neves: Conceptualization, Supervision, Writing - Review & Editing, Administerial de projects.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: Rosana Puccia.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva Alves, A.I., de Sousa, B.R., da Silva, J.M. et al. Synergistic antifungal effect of thiophene derivative as an inhibitor of fluconazole-resistant Candida spp. biofilms. Braz J Microbiol (2024). https://doi.org/10.1007/s42770-024-01470-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42770-024-01470-3