Abstract
Candida spp., causes invasive fungal infections, especially in immune-compromised patients and the propensity of antifungal resistance against azole-based drugs need to be addressed. This study is thus aimed to characterize the anticandidal effect of the cinnamic acid extracted from the barks of Cinnamomum cassia. Five species of Fluconazole-resistant Candida sp. were retrieved from the department repertoire. The extraction of CA was performed by three different methods followed by silica gel column chromatography. Eluant was subjected to FTIR and XRD analysis for confirmation. The anticandidal activity of the CA was checked by the agar disc diffusion method and the MIC and MFC were determined. The anti-biofilm effect of CA was assessed using the CLSM technique followed by the biocompatibility check using MTT assay in normal HGF cell lines. CA was best extracted with the hot maceration method using ethanol with a maximum yield of 6.73 mg. Purification by column chromatography was achieved using benzene, acetic acid, and water (6:7:3) mobile phase. CA was confirmed by FTIR with absorption peaks and by XDR based on strong intensity. CA was found to possess promising anticandidal activity at 8 µg/mL with MIC and MFC values determined as 0.8 µg/mL and 0.08 µg/mL respectively. Antibiofilm activity by CLSM analysis revealed biofilm inhibition and was biocompatible at 8.5 µg/ml concentrations in HGF cell lines until 24 h. The study findings conclude that CA is the best alternative to treat candidal infection warranting further experimental preclinical studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungal infections contribute to an increased mortality rate worldwide and the most frequent reasons for invasive mycotic infection are caused by Candida species, particularly Candida albicans [1]. Reports document that more than 50,000 deaths occur annually with 250,000 people being infected due to invasive Candidal infections and the incidence rate is reported as 2–14 cases/ 10,000 [2, 3]. Oral candidiasis (oral thrush), is a superficial infection of the mucous membranes typically manifested by the yeast C. albicans [4]. In immune-compromised patients, particularly those with AIDS, patients under cancer therapy, or undergoing organ transplantation Candida species is known for their pathogenesis and further disease progression. Being commensals, conditions of dysbiosis often prevail in these patients leading to critical systemic infections [5, 6]. Oral candidiasis is caused by a wide variety of Candida species, among them, C. albicans being associated with 80% of the oral lesions [7]. Other documented species are Candida glabrata, Candida tropicalis, Candida kruesi, and Candida parapsilosis, with a lower prevalence rate but often involved in both superficial and systemic infections [8, 9]. These Candidal types are also responsible for around 90% of oral candidemia [10].
Candida species colonize the host epithelial tissue initiating the infection process [11] through the adhesins, which are surface-attaching proteins found in the Candidal cells, playing a key role in the disease pathogenesis [12]. Production of hydrolases, the yeast-to-hypha transition, touch sensing, and thigmotropism, as well as adherence to and invasion into host cells further enhances the disease [13]. Treatment for candidal infections often involves the topical and systemic administration of antifungal agents and recently more reports on antifungal resistance have sparked a renewed interest and threat globally. According to the CDC 2019 report, Candida species show resistance to the polyenes, azoles, and echinocandins class of antibiotics. At present there are currently only a few antifungals effective against potentially fatal fungal diseases and most of the other antifungals are not effective due to the resistance exhibited by the candidal strains [1] with no new antifungals entered for clinical trials in recent decades. The eukaryotic nature of the fungal cell wall is the major obstacle to synthesize newer classes of antifungal drugs. Least penetration effects of the antifungal drugs in to the fungal cell wall and cell membrane, challenges the researchers and mycologists to find a suitable alternative strategy to treat resistant fungal species [14,15,16]. Antifungal resistance being a global threat to the medical community [17], majority of the medically important fungi exhibit resistance to polyenes, echinocandins, and azoles. The mechanisms behind the resistance are (i) drug target alteration, (ii) drug target overexpression, and (iii) overexpression of efflux pumps. Along with these underlying mechanisms a fluctuating clinical environment, lowered drug effectiveness, and the host immune system also contributes to the progression of the fungal disease [18].
In this context, the World Health Organisation estimates that 80% of the world's population implements plant extracts or their active ingredients in traditional medicines [19]. Innovative solutions to curb antifungal resistance using active compounds from medicinal plants would be a boon to fungal treatment [20, 21]. Crude extracts of various plant parts have historically been employed as therapeutic medicines against fungal diseases [22]. Many plant extracts are widely used to treat infectious diseases, predominantly targeting drug-resistant pathogens focusing on the vital phytoconstituents of the plant for their broad-spectrum properties [23]. Among many phytoconstituents, one of the large classes of phenolic acids derived from plants is cinnamic acid (CA), which is a type of organic acid that occurs naturally in plants with a wide range of biological functions [24]. Cinnamic acid derivatives are significant and promising substances with a high potential for development into medications in the hunt for new pharmacologically active molecules. The key compound present in Cinnamomum cassia is aromatic carboxylic acid which is also normally present in honey, whole grains, vegetables, and fruits [25]. Lima et al. [26] has proved nitro-cinnamate with maximum fungicidal activity against three types of C. albicans. Another study documents the effect of cinnamaldehyde extract against C. albicans and C. glabrata species [27]. However, there are no further experimentally evidenced based studies with cinnamic acid against different species of Candida especially against the resistant strains. The present study is thus aimed to investigate the anticandidal activity of Cinnamomum cassia-derived cinnamic acid against different types of candidal species with further assessments on its bioactive properties.
Materials and methods
Chemicals
Cinnamon bark was obtained from Government Herbal Farm, Yelagiri, Tamilnadu and the taxonomical nomenclature was confirmed as C.cassia by a Botanist, University of Madras. Solvents for the extraction such as methanol, ethyl acetate, and ethanol were purchased from SLC Chemicals (Delhi, India). Sabouraud Dextrose Agar (SDA), and Sabouraud Dextrose Broth (SDB) were purchased from Himedia (Mumbai, India). The LIVE/DEADTM Cell Imaging Kit (488/570) was purchased from Thermo Fisher Scientific, (USA). Deionized water was obtained from Indion Lab-Q Water Maker for the aqueous extraction procedure.
Test organisms and antifungal profiling
Five different fluconazole-resistant species of C. albicans, C. glabrata, C. tropicalis, C. kruesi, and C. parapsilosis (clinical isolates) were retrieved from the repertoire of the Department of Microbiology and were used for the study. The cultures of C. albicans, C. glabrata, C. tropicalis, C. krusei, and C. parapsilosis were inoculated initially into 30 mL of sterilized Sabouraud Dextrose Broth. The susceptibility assays for clinical isolates of Candida species were evaluated using the microbroth dilution method in sterile flat-bottom 96-well microplates, following the procedures outlined in the NCCLS guidelines, M27-A3 document [28]. Amphotericin B was used as a positive control.
Extraction methods
Hot maceration
A Soxhlet solvent extraction apparatus was used for the hot maceration process using 100 g of dried cinnamon bark powder. With the help of the isomantle, the solvent is heated to 50˚C, at which point it starts to evaporate and passes through the apparatus and into the condenser and the extraction was performed for sixteen hours. Following the extraction process, the products were carefully collected and purified at a constant temperature of 50 °C using a rotary evaporator. Using rotavapor, the extracted samples were placed into a fume hood for an hour to evaporate the remaining ethanol. The extract was placed in a porcelain bowl until all the leftover ethanol had evaporated. The yield was weighed using a digital weighing balance in mg/g and was refrigerated until further use.
Cold maceration
In this method, 100 g of C. cassia powder was soaked in 500 ml of 100% cold ethanol in a screw cap bottle. Using a glass rod, the mixture was thoroughly swirled and was maintained in a shaker for 72 h with a low RPM. After this, the extracted compounds were filtered using the Whatman No. 1 filter paper. Further, the obtained yield was kept in a rotary evaporator at 40 °C and then stored in a refrigerator.
Aqueous extract
The 100 g of C. cassia bark powder were immersed in 500 ml distilled water and then mixed with a stainless-steel hand blender. The mixture was kept in a magnetic stirrer for 20 min and was filtered through the Whatman No 1 filter paper followed by concentration using a rotary evaporator at 50 °C. Using a digital weighing balance, the amount of balance material was weighed in mg/g and was refrigerated until further bioassay evaluation.
Purification by column chromatography
20 × 20 cm silica gel F60 packaged column chromatography was used for the purification process using a 74-micron particle-size silica gel as the stationary phase. The extraction process was carried out using the protocol as described earlier [29]. The mobile phase used was benzene: acetic acid: and water (6:7:3) as described in an earlier study and the samples were collected from the column every 5 min [30]. Identification of phenolic acid compound was assessed by combining 1 ml of purified solvent with 1 ml of Na2CO3 and 0.5 ml of Folin-Denis (FD) reagent and the result was recorded.
Characterization of cinnamic acid
Fourier transform infrared (FT–IR) spectroscopic analysis
The FT-IR spectrum of the compound was recorded in Bruker Alpha II 66 V spectrometer in the range of 4000–400 cm−1. The spectral resolution is ± 2 cm−1. The spectra were recorded in the range of 4000–100 cm−1 with a scanning speed of 30 cm−1 min−1 of spectral width 2 cm−1. The frequencies of all sharp bands were accurate to ± 1 cm−1.
X-ray diffraction (XRD) analysis
X-ray diffractometer (D8 Advance, Bruker, Germany) was used to perform XRD analysis. using CuKα radiation (λ = 1.5406 Å), 40 kV- 40 mA, 2θ/θ scanning mode. The advanced diffractometer with an area detector was operated at Cu Kα radiation of k = 0.154 nm. The analysis was performed at room temperature with a voltage of 40 kV and a current of 40 mA. The scanning range of 2θ was set from 5° to 60º with a scanning speed of 2º/min.
Assessment of antifungal activity
By using the agar-disk diffusion method, the antimicrobial properties of the extracted CA were evaluated against the clinical isolates of C. albicans, C. glabrata, C. tropicalis, C. krusei, and C. parapsilosis. In brief, an inoculum comprising 108 cfu/mL of each 10 µL of fungal solution was made as lawn culture on the surface of the SDA plates. The plates were then inoculated with an 8 µL concentration of CA and allowed to diffuse at room temperature for two hours. The culture plates were then incubated at 37 °C for 72 h (a clear zone around the disk was considered as an antifungal activity). HiAntibiotic ZoneScale™ (Himedia, India) was used to measure the fungal growth inhibition zone diameters (mm), and the results were expressed as the mean value of a triplicate experiment.
Determination of minimum inhibitory concentration (MIC) value
Following the M27-A3 guidelines of the Clinical and Laboratory Standards Institute, the antifungal activities of CA was assessed with Amphotericin B as a positive control against 100 μL of 2 × 103(CFU)/mL C. albicans, C. glabrata, C. tropicalis, C. kruesi, and C. parapsilosis using the microdilution method in a 96-well flat-bottomed microtiter plate. 8 μL CA was added and was serially diluted. A negative control group (drug-free) SDB was carried out, and the plate was incubated at 35˚C for 72 h. MIC was identified as the lowest drug concentration that resulted in 50% growth inhibition. Using a microplate reader (Readwell TOUCHTM, Automatic ELISA Plate Analyser, ROBONIK, INDIA) optical density was measured at 600 nm. The percentage of inhibition (I) of the growth was recorded using the standard formula [Growth (%) = (Sample OD value)/ (Control OD value)] × 100.
Determination of minimum fungicidal concentration (MFC)
In-vitro minimal fungicidal activity (MFCs) was calculated for each strain with a slight modification in the method as documented earlier [31]. Following a 72-h incubation period, 20 μL was sub cultured onto the sterile SDA plates. The plates were incubated at 37 °C for 72 h and the MFC value was measured as 99–99.5% inhibition by CA and no growth or < 3 colonies in an SDA plate [32].
Assay for biocompatibility
The MTT assay was used to assess the cell viability of CA. In brief, Human Gingival Fibroblast (HGF) cells were seeded onto 12-well plates at a density of 3 × 105 cells/well, cultivated overnight, and then exposed to 8 µg of CA up to 24 and 48 h. The culture medium was periodically changed after incubation using 5 mg/ml of PBS. The cells were allowed to lyse to release the formazan by incubating them in a 1 ml HCl/ 0.05 N-isopropanol solution for 15 min and the OD value was measured based on the absorbance at 550 nm in an ELISA reader (Readwell TOUCHTM, Automatic ELISA Plate Analyser, ROBONIK, INDIA). The percentage of the viable proliferating cells was calculated using the formula: % of cell viability = [(OD550 nm (treated cell) − OD (blank)/ (OD (control cell) − OD (blank)] × 100.
Evaluation of time period for maximum biofilm formation
The time taken to form the maximum biofilms by the Candida species was conducted based on an earlier study with slight modifications [33]. This assay was aimed to determine the precise day of biofilm formation by the mixed Candida species for it to be considered for further CLSM analysis. Standard crystal violet staining was used to assess the impact of various culture times on the development of the biofilms formed by C. albicans, C. glabrata, C. tropicalis, C. kruesi, and C. parapsilosis. The yeast cells were cultured in 96-well plates containing SDB and biofilm was formed from 1—5 days. The growth pattern of combined Candida species biofilms was assessed over an extended culture period. Biofilm formation and reduction were quantified by measuring OD values at 600 nm using an ELISA reader.
Analysis for the live cells using CLSM
Tooth models were coated with artificial saliva to resemble the oral cavity and were placed in a 24-well culture plate under aseptic conditions. To create the salivary pellicle, the plate was incubated aerobically for one hour at 35 °C on an orbital shaker. Following the transfer of saliva-coated teeth to a second 24-well culture plate, each well was filled with five different species of candidal suspension. These sets underwent an adhesion phase of 90 min of aerobic incubation in an orbital shaker at 35 °C. The tooth was then slowly rinsed with PBS and placed onto fresh 24-well culture plates that contained SDB culture medium and were enriched with sucrose. The tooth models were left for 3 days (as evaluated in the above method) to form the multi-species biofilm. Following 3-day intervals, the teeth in the treatment group were cleaned with PBS and then given a 6-h treatment with 8 µg of CA. The plates were kept in an incubator at 35 °C for 72 h. After treatment, the teeth were slightly washed with sterile distilled water. The control and treated tooth models were labelled with 2.5-lM SYTO-9 and Propidium iodide (PI) fluorescent stain (Invitrogen Molecular Probes, USA) with further incubation for 20 min at 35 °C in the dark. Using a Leica microscope (CMS GmbH—DMI8 Germany) equipped with a 63 9, 0.8 numerical aperture oil-immersion objective lens, the structural organization of the biofilm was evaluated. An argon laser tuned at 488 nm and a helium–neon laser tuned at 543 nm wavelength were used in CLSM to measure SYTO-9 (green; 480/500) and PI (red; 555/580). The presence of live and dead candida cells was labelled as red and green, respectively.
Results
Antifungal profiling for the resistant strains
Resistance against fluconazole was observed as of guidelines and fluconazole resistant strains from each species were subjected for the antifungal bioassay. The determined MIC breakpoints and selection was given in Table 1.
Yield of CA in various extraction methods
Three different methods of extraction such as soxhlet, cold, and aqueous extraction procedures were used to extract CA from the cinnamon bark. The extraction yield from each method was given in Table 2 with the maximum extract of 6.73 mg using the hot maceration method. Based on the process time, solvent use, and energy expenditure hot maceration method was evaluated as the best method to extract CA.
CA purification by column chromatography
From the C. cassia extract, benzene, acetic acid, and water (6:7:3) as mobile phase was successful in obtaining the eluants. Presence of phenolic acid was confirmed using a negative reaction upon adding ferric chloride. The absence of the formation of violet color indicated the pure form of CA.
FT–IR analysis of CA
The FTIR spectra of CA is displayed in Fig. 1. The absorption peaks that are lower than 3000 cm−1 (2825 cm−1) and higher than 3000 cm−1 (3062 and 30264 cm−1) are ascribed to the saturated and unsaturated C–H vibrations of cinnamic acid, respectively. The carboxyl groups are responsible for the significant absorption at 1668 cm−1, whereas the aromatic ring's C = C group is responsible for the absorption bands at 1623 cm−1, 1492 cm−1, and 1446 cm−1.
X-ray diffraction (XRD) analysis of CA
The XRD pattern of the synthesized CA is shown in Fig. 2 and it is clear that the XRD patterns represent CA's strong intensity and crystalline structure. The peaks at 2-Theta CA revealed medium-to-strong reflections that were sharp and well-defined peaks at 2-Theta CA showed sharp and well-defined reflections with medium-to-strong intensities. The main 2 h reflections were found at 9.69º, 15.13º, 18.69º, 19.75º, 20.13º, 21.79º, 23.00º, 23.83º, 25.34º, 27.23 º, 29.66º, 30.71º and 40.62º, revealing the crystalline form of CA. The practical size of the prepared CA was calculated using Scherrer's formula. D = Kλ/β1/2Cosθ, where D is the particle size, λ is the X-ray wavelength (Cu ka, 1.54060 Å), θ is the diffraction angle, and β is the fall width at half maximum (FWHM in radians), and K = 0.89 is the Scherer constant associated with the form and index of the crystals.
Antifungal activity and MIC/MFC value of CA
CA obtained through hot maceration demonstrated substantial inhibition against all five resistant yeast species tested through the agar well diffusion method and it ranged from 20 ± 0.5 to 25 ± 1.0 (Table 3, Fig. 3). Amphotericin B was used as a control which exhibited an MIC value at 32 µg/mL against all the resistant strains. The pathogens C. albicans and C. parapsilosis exhibit 50% inhibition at a concentration of 0.8 µg/mL. Conversely, a concentration of 0.08 µg/mL demonstrates 50% inhibition against other species such as C. glabrata, C. tropicalis, and C. krusei. The minimum inhibitory concentration (MIC) of cinnamic acid was established at 0.8 µg/mL, effectively inhibiting the visible growth of Candida species in microwell culture plates following a 24-h incubation period (Table 4). In the Minimum fungicidal concentration (MFC) analysis, fungal colonies diminished progressively with increasing concentrations of CA. Concentrations of 0.8 µg/mL for C. albicans and C. parapsilosis and 0.08 µg/mL for other species are identified as the MFC and exhibited 99–99.5% killing activity (Fig. 4).
Biocompatibility of CA
CA did not affect the viability of normal human gingival fibroblast (HGF) cells at the evaluated concentrations (7.5 to 9 µg/ml) after 5 to 24 h (Fig. 5). After CA treatment for 24 h, there was no significant differences in the number of cells as compared to controls. C. cassia derivative, CA, did not affect the cell viability up to 8.5 µg/ml concentration.
Antibiofilm effect of CA against Candida species
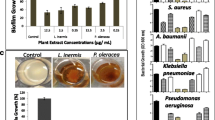
Figure 6 illustrates the biofilm formation of the tested Candida strains, with elevated OD600 values observed on the initial two days, indicating cell proliferation. By the third day, a notably higher OD value suggested the highest biofilm formation. However, on the fourth and fifth day, the OD values decreased, indicating the deterioration of the biofilm strength. Based on this the third-day biofilm was selected for the confocal microscopic studies after treatment with CA.
CLSM results
Using a Leica DMi8 microscope, the biofilm mass of both control and CA-treated biofilms was examined using live/dead (SYTO 9/PI) staining. The live yeast cells emit green fluorescence in the first column, red fluorescence in the second column, and a combination in the third column (Fig. 7). The results of the control and treatment group combined pictures clearly show that SYTO 9 fluorescence was reduced and PI fluorescence was increased in the treatment group 80% dead and 20% of viable cells.
Discussion
Invasive fungal infections are becoming a common medical issue, leading to a global health burden. Invasive candidiasis, which includes Candida bloodstream infections and deep-seated candidiasis, presents a persistent health challenge. These infections, stemming from various Candida species, carry significant morbidity and mortality rates [34]. In immunocompromised patients, systemic fungal infections cause more morbidity and mortality [35]. The propensity of drug resistance among the candidal strains affects the treatment strategies with the existing arsenal of antifungals, such as azoles, polyenes, and echinocandins, that are employed in the treatment of invasive infections. Thus, there is an urgent need for novel antifungals, yet the pursuit of drug discovery is challenged by the phylogenetic preservation of targetable proteins and pathways in both fungi and humans [36].
This has led to the need for newer antifungal agents that may be synthesized or produced from plant-based products. Plant-based therapeutic application to treat systemic fungal infections is evident from many studies [37]. In this context, amidst many reported potent phytoconstituents, the pharmacognostic studies on the cinnamon plant have revealed vital chemical constituents with valuable biological functions. The phytoconstituents as reported from the C. cassia encompass majorly, of eugenol, cinnamaldehyde, and cinnamic acid [38]. However, not many studies have been evident to prove its antifungal role against drug-resistant candidal strains. Thus, in the present investigation, we intend to screen for the vital bioactivity of the extracted CA against the drug-resistant strains of Candidal species.
Solvent extraction systems play a vital role in releasing the important phytoconstituents from the plant source and its application varies with different solvent extraction methods. In the present study, we applied three types of extraction methods, and we compared the yields in different methods. The findings revealed that there is a significant correlation between the solvent extraction methods applied and the extraction yield. Compared to the cold and aqueous extraction methods, the ethanol extraction through hot maceration was the best based on the obtained yield. This is because ethanol may penetrate the cinnamon bark matrix increasing the swelling process as reported in earlier studies and is shown as the best method to extract CA [39, 40]. The yield in the present study was 6.73 mg/mL from 100 g of C. cassia bark correlating with an earlier study where 150 g of cinnamon bark yielded 10.5 mg/mL of yield substantiating the higher yield in the hot maceration method [41].
FT-IR spectra and characterization of CA revealed two different bands at 3024 cm − 1 and 3063 cm − 1, attributing to the stretching of the C-H alkene bond, revealing two other bands C = O and C = C bonds that have correlated with an earlier study [42]. The major compound's aromatic ring presence is confirmed by the presence of C = C bands. The C-H bonds observed in the present study also were similar and correlated with the earlier report [43]. XRD pattern at 2 h reflections were also observed at different ranges with the reflections as sharp and well-defined, intensity varying from medium to strong. These reflections closely resemble those observed in the crystalline structure of CA, as documented in an earlier study [44]. These observations confirmed the presence and the structure of the CA extracted from the bark of the C. cassia.
It is a known fact that the extraction method using various solvents holds promise for the antimicrobial bioassay. In this line, we performed the antimicrobial test using the ethanol extract of CA and various previous documents have applied the same for the anticandidal activity of cinnamic extract [45]. The underlying antimicrobial effect of CA has been associated with its ability to penetrate the cytoplasmic membrane, altering the membrane structure and leading to increased cellular permeability and leakage resulting in cell death [46]. In the present study, the antimicrobial activity was observed at 8 µg/mL concentration correlating with an earlier study documenting the same at 10 µg/ml [47]. The antimicrobial activity against the drug-resistant strains at this lower concentration is a promising finding in the present investigation.
Similarly, MIC value was determined as 0.8 µg/ml for C. albicans, C. parapsilosis, and 0.08 µg/ml for C. glabrata, C. tropicalis, and C. krusei. Prior research has documented the antifungal efficacy of cinnamic acid and related compounds against resistant C. albicans with an MIC value of 128 µg/ml in contrast with the present study [26]. In terms of MFC, 99% of colony reduction was observed at different dilutions of CA (0.8 µg/ml and 0.08 µg/ml correlating with an earlier study [48].
Another promising finding of the present study was the biocompatible nature of the CA checked in the HGF cells at three distinct concentrations 7.5 µg to 9 µg until 24 h. This observation substantiates its oral application as a drug of choice which needs further experimental-based research. Earlier such a study has reported the biocompatibility of CA-mediated gold nanoparticles at a concentration of 0, 0.25, 0.5, 0.75, and 1 mg/mL at 24 h in human fibroblast cells [49]. However, the biocompatibility checks at the lowest concentration of 8.5 µg/mL in the present study for longer hours are to be noted for the selection of the compound to design a novel drug in the future.
In our findings, we observed the biofilm formation progressing from initial adhesion to maturation. Notably, the OD600 of the multispecies biofilm reached its peak on the third day and thus the initial two days were considered as the initial adhesion phase, while on the fourth and fifth days, the biofilm was disrupted likely due to nutrient depletion. This study identifies the third day as the critical period for multispecies biofilm formation among Candida species. Interestingly, previous research on Staphylococcus aureus and Escherichia coli biofilms indicated the second day as the best biofilm formation [33]. Regarding the antibiofilm property, cinnamic acid demonstrated significant antifungal activity against Candida species. Previous such studies have highlighted the antibiofilm effects of Hydroxypropyl chitosan-cinnamic acid (HPCS-CA) derivatives, inhibiting biofilm formation by Staphylococcus aureus and Escherichia coli [33]. Our findings indicate that biofilms treated with CA for 6 h exhibited a substantial reduction in live cells, further supporting their anti-biofilm activity.
The limitation of the present study involves the lack of the clinical application of CA through in-vivo studies and this study is basically on the observation that primarily stems from in-vitro research. It is a known fact that natural compounds like CA demonstrating anticandidal effects in laboratory settings may not necessarily translate to similar outcomes inside the host. The future prospects of the study are thus set to delve deeper into understanding its mechanisms of action and exploring its efficacy against various Candida species and strains. Moreover, there's potential for optimization of extraction methods to enhance the yield and purity of cinnamic acid from C. cassia. Future studies may also be focused on evaluating its synergistic effects with other antimicrobial agents or exploring novel delivery mechanisms to improve its bioavailability and efficacy. Additionally, investigations into the safety profile and potential adverse effects of cinnamic acid extract will be crucial for its clinical translation. Overall, continued research efforts in this direction hold significant promise for harnessing the Candidal infections using CA.
Conclusion
CA was successfully extracted and characterized in the present study from the barks of C. cassia. The anticandidal effect of CA was promising against five different species of Candida that exhibited resistance against the routine antifungal fluconazole. CA may be considered as the best alternative drug candidate to treat drug-resistant traits of Candida species. The findings of the present study document the antimicrobial, antibiofilm, and biocompatible nature of CA. The study further warrants the need for experimental evidence-based research works to design and synthesize CA as a novel drug to treat candidal infections in health care settings.
References
Lee Y, Puumala E, Robbins N, Cowen LE (2021) Antifungal drug resistance: molecular mechanisms in Candida albicans and beyond. Chem Rev 121:3390–3411. https://doi.org/10.1021/acs.chemrev.0c00199
Arendrup MC (2010) Epidemiology of invasive candidiasis. Curr Opin Crit Care 16:445–452. https://doi.org/10.1097/MCC.0b013e32833e84d2
Kullberg BJ, Arendrup MC (2015) Invasive candidiasis. N Engl J Med 373:1445–1456. https://doi.org/10.1056/NEJMra1315399
Vazquez JA, Sobel JD (2002) Mucosal candidiasis. Infect Dis Clin North Am 16:793–820. https://doi.org/10.1016/s0891-5520(02)00042-9
Cornely OA, Lass-Flörl C, Lagrou K, Arsic-Arsenijevic V, Hoenigl M (2017) Improving outcome of fungal diseases - guiding experts and patients towards excellence. Mycoses 60:420–425. https://doi.org/10.1111/myc.12628
Ellis D (2002) Amphotericin B: spectrum and resistance. J Antimicrob Chemother 1:7. https://doi.org/10.1093/jac/49.suppl_1.7
Millsop JW, Fazel N (2016) Oral candidiasis. Clin Dermatol 34:487–494. https://doi.org/10.1016/j.clindermatol.2016.02.022
Hellstein JW, Marek CL (2019) Candidiasis: red and white manifestations in the oral cavity. Head Neck Pathol 13:25–32. https://doi.org/10.1007/s12105-019-01004-6
Hachem R, Hanna H, Kontoyiannis D, Jiang Y, Raad I (2008) The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer 112:2493–2499. https://doi.org/10.1002/cncr.23466
Turner SA, Butler G (2014) The Candida pathogenic species complex. Cold Spring Harb Perspect Med 4:119–778. https://doi.org/10.1101/cshperspect.a019778
Yu SY, Zhang L, Chen S, Kong F, Xiao M, Wang H, Hou X, Zhou ML, Zhang G, Zhang JJ, Duan SM, Kang W, Xu YC (2019) Candida isolates causing refractory or recurrent oropharyngeal candidiasis in 11 hospitals in China. Infect Drug Resist 12:865–875. https://doi.org/10.2147/IDR.S199359
Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J (2012) Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev 36:288–305. https://doi.org/10.1111/j.1574-6976.2011.00278.x
Mayer FL, Wilson D, Hube B (2013) Candida albicans pathogenicity mechanisms. Virulence 4:119–128. https://doi.org/10.4161/viru.22913
Roemer T, Krysan DJ (2014) Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med 4:119–703. https://doi.org/10.1101/cshperspect.a019703
Revie NM, Iyer KR, Robbins N, Cowen LE (2018) Antifungal drug resistance: evolution, mechanisms and impact. Curr Opin Microbiol 45:70–76. https://doi.org/10.1016/j.mib.2018.02.005
Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A (2017) The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis 17:383–392. https://doi.org/10.1016/S1473-3099(17)30316-X
Verweij PE, Gangneux JP, Bassetti M, Brüggemann RJM, Cornely OA, Koehler P, Lass-Flörl C, van de Veerdonk FL, Chakrabarti A, Hoenigl M (2020) European confederation of medical mycology; international society for human and animal mycology; European society for clinical microbiology and infectious diseases fungal infection study group; ESCMID study group for infections in critically Ill patients. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe 1:53–55. https://doi.org/10.1016/S2666-5247(20)30027-6
White TC, Holleman S, Dy F, Mirels LF, Stevens DA (2002) Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother 46:1704–1713. https://doi.org/10.1128/AAC.46.6.1704-1713.2002
World Health Organization (WHO) (2008) Traditional medicines. Available online: http://www.who.int/mediacentre/factsheets/fs134/en/
Fisher MC, Alastruey-Izquierdo A, Berman J, Bicanic T, Bignell EM, Bowyer P, Bromley M, Brüggemann R, Garber G, Cornely OA, Gurr SJ, Harrison TS, Kuijper E, Rhodes J, Sheppard DC, Warris A, White PL, Xu J, Zwaan B, Verweij PE (2022) Tackling the emerging threat of antifungal resistance to human health. Nat Rev Microbiol 20:557–571. https://doi.org/10.1038/s41579-022-00720-1
Petrovska BB (2012) Historical review of medicinal plants’ usage. Pharmacogn Rev 6:1–5. https://doi.org/10.4103/0973-7847.95849
Alviano DS, Alviano CS (2009) Plant extracts: search for new alternatives to treat microbial diseases. Curr Pharm Biotechnol 10:106–121. https://doi.org/10.2174/138920109787048607
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12:564–582. https://doi.org/10.1128/CMR.12.4.564
Lafay S, Gil-Izquierdo A (2008) Bioavailability of phenolic acids. Phytochem Rev 7:301–311. https://doi.org/10.1007/s11101-007-9077-x
Chandra S, Roy A, Jana M, Pahan K (2019) Cinnamic acid activates PPARα to stimulate Lysosomal biogenesis and lower Amyloid plaque pathology in an Alzheimer’s disease mouse model. Neurobiol Dis 124:379–395. https://doi.org/10.1016/j.nbd.2018.12.007
Lima TC, Ferreira AR, Silva DF, Lima EO, de Sousa DP (2018) Antifungal activity of cinnamic acid and benzoic acid esters against Candida albicans strains. Nat Prod Res 32:572–575. https://doi.org/10.1080/14786419.2017.1317776
Bakhtiari S, Jafari S, Taheri JB, Kashi TSJ, Namazi Z, Iman M, Poorberafeyi M (2019) The effects of cinnamaldehyde (cinnamon derivatives) and nystatin on Candida Albicans and Candida Glabrata. Open Access Maced J Med Sci 7:1067–1070. https://doi.org/10.3889/oamjms.2019.245
Pfaller MA, Espinel-Ingroff A, Jones RN (2004) Clinical evaluation of the sensititre yeast one colorimetric antifungal plate for antifungal susceptibility testing of the new triazoles voriconazole, posaconazole, and ravuconazole. J Clin Microbiol 42:4577–4580. https://doi.org/10.1128/JCM.42.10.4577-4580.2004
Wang H, Yang L, Zu Y, Zhao X (2014) Microwave-assisted simultaneous extraction of luteolin and apigenin from tree peony pod and evaluation of its antioxidant activity. Sci World J 1:506–971. https://doi.org/10.1155/2014/506971
O’Neil MJ (2006) The Merck index: an encyclopedia of chemicals, drugs, and biologicals, 13th edn. Merck, Whitehouse Station
Espinel-Ingroff A, Fothergill A, Peter J, Rinaldi MG, Walsh TJ (2002) Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp.: NCCLS collaborative study. J Clin Microbiol 40:3204–3208. https://doi.org/10.1128/JCM.40.9.3204-3208.2002
de Aquino LJ, Costa CR, de Araújo CR, Souza LK, Silva Mdo R (2009) Susceptibility testing of Candida albicans isolated from oropharyngeal mucosa of HIV (+) patients to fluconazole, amphotericin B and Caspofungin. killing kinetics of caspofungin and amphotericin B against fluconazole resistant and susceptible isolates. Braz J Microbiol 40:163–169. https://doi.org/10.1590/S1517-838220090001000028
Yue L, Wang M, Khan IM, Xu J, Peng C, Wang Z (2021) Preparation, characterization, and antibiofilm activity of cinnamic acid conjugated hydroxypropyl chitosan derivatives. Int J Biol Macromol 189:657–667. https://doi.org/10.1016/j.ijbiomac.2021.08.164
Riera FO, Caeiro JP, Angiolini SC, Vigezzi C, Rodriguez E, Icely PA, Sotomayor CE (2022) Invasive Candidiasis: update and current challenges in the management of this mycosis in South America. Antibiotics (Basel) 11:877–889. https://doi.org/10.3390/antibiotics11070877
Rayens E, Norris KA (2018) Prevalence and healthcare burden of fungal infections in the United States, 2018. Open Forum Infect Dis 9:593–602. https://doi.org/10.1093/ofid/ofab593
Alabi PE, Gautier C, Murphy TP, Gu X, Lepas M, Aimanianda V, Sello JK, Ene IV (2023) Small molecules restore azole activity against drug-tolerant and drug-resistant Candida isolates. mBio 14:479–523. https://doi.org/10.1128/mbio.00479-23
Kokoska L, Kloucek P, Leuner O, Novy P (2019) Plant-derived products as antibacterial and antifungal agents in human health care. Curr Med Chem 26:5501–5541. https://doi.org/10.2174/0929867325666180831144344
Vallverdú-Queralt A, Regueiro J, Martínez-Huélamo M, Rinaldi Alvarenga JF, Leal LN, Lamuela-Raventos RM (2014) A comprehensive study on the phenolic profile of widely used culinary herbs and spices: rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem 154:299–307. https://doi.org/10.1016/j.foodchem.2013.12.106
Xiao W, Han L, Shi B (2008) Microwave-assisted extraction of flavonoids from Radix Astragali. Sep Purif Technol 62:614–618. https://doi.org/10.1016/j.seppur.2008.03.025
Lee HG, Jo Y, Ameer K, Kwon JH (2018) Optimization of green extraction methods for cinnamic acid and cinnamaldehyde from Cinnamon (Cinnamomum cassia) by response surface methodology. Food Sci Biotechnol 27:1607–1617. https://doi.org/10.1007/s10068-018-0441-y
Mousa NK, Abow AJ, Abdul-Rahman SA, Ali IA, Ahmed AM (2013) Thin layer chromatography, high performance liquid chromatography and melting point for extraction and purification of cinnamic acid from cinnamon bark (Cinnamon aromaticum). J Environ Stud 11:11–18. https://doi.org/10.21608/JESJ.2013.192102
Beyki M, Zhaveh S, Khalili ST, Rahmani-Cherati T, Abollahi A, Bayat M, Tabatabaei M, Mohsenifar A (2014) Encapsulation of Mentha piperita essential oils in chitosan–cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind Crops Prod 54:310–319. https://doi.org/10.1016/j.indcrop.2014.01.033
Kalinowska M, Świsłocka R, Lewandowski W (2007) The spectroscopic (FT-IR, FT-Raman and 1H, 13C NMR) and theoretical studies of cinnamic acid and alkali metal cinnamates. J Mol Struct 834:572–580. https://doi.org/10.1016/j.molstruc.2006.11.043
Shayanfar A, Asadpour-Zeynali K, Jouyban A (2013) Solubility and dissolution rate of a carbamazepine–cinnamic acid cocrystal. J Mol Liq 187:171–176. https://doi.org/10.1016/j.molliq.2013.06.015
Bhalodia NR, Shukla VJ (2011) Antibacterial and antifungal activities from leaf extracts of Cassia fistula l.: an ethnomedicinal plant. J Adv Pharm Technol Res 2:104–109. https://doi.org/10.4103/2231-4040.82956
Wu VC, Qiu X, de los Reyes BG, Lin CS, Pan Y (2009) Application of cranberry concentrate (Vaccinium macrocarpon) to control Escherichia coli O157:H7 in ground beef and its antimicrobial mechanism related to the downregulated slp, hdeA and cfa. Food Microbiol 26:32–8. https://doi.org/10.1016/j.fm.2008.07.014
Mojtabavi S, Khoshayand MR, Fazeli MR, Faramarzi MA, Samadi N (2021) Development of an enzyme-enhancer system to improve laccase biological activities. Int J Biol Macromol 173:99–108. https://doi.org/10.1016/j.ijbiomac.2021.01.068
Latti P, Ramanarayanan S, Prashant GM (2019) Antifungal efficacy of spice extracts against Candida albicans: an in vitro study. Indian J Community Med 44:77–80. https://doi.org/10.4103/ijcm.IJCM_140_19
Chanda N, Shukla R, Zambre A, Mekapothula S, Kulkarni RR, Katti K, Bhattacharyya K, Fent GM, Casteel SW, Boote EJ, Viator JA, Upendran A, Kannan R, Katti KV (2011) An effective strategy for the synthesis of biocompatible gold nanoparticles using cinnamon phytochemicals for phantom CT imaging and photoacoustic detection of cancerous cells. Pharm Res 28:279–291. https://doi.org/10.1007/s11095-010-0276-6
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that is no conflict of interest in association with the research study.
Additional information
Responsible Editor: Rosana Puccia
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kannan, K.P., AS, S.G. Anticandidal effect of cinnamic acid characterized from Cinnamomum cassia bark against the fluconazole resistant strains of Candida. Braz J Microbiol (2024). https://doi.org/10.1007/s42770-024-01469-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42770-024-01469-w