Abstract
Slow-growing breeds are more resistant to Salmonella infection compared to fast-growing broilers. However, it is unclear whether that is associated with innate resistance or rather rely on differences in Salmonella-induced gut responses. We investigated the microbial composition and gene expression of nutrient transporters, mucin, and interleukin in the gut of a fast-growing (Cobb500) and a slow-growing naked neck (NN) chicken breeds challenged with Salmonella Enteritidis. Hatchlings were inoculated at two days of age using sterile broth (sham) or Salmonella Enteritidis (SE) and distributed according to a completely randomized design into four treatments: Cobb-sham; Cobb-SE; NN-sham; and NN-SE. Cecal SE counting and microbial composition by 16 S rRNA sequencing were determined at 24-, 96-, and 168-hours post-inoculation (hpi). Gene expression of amino acid (Asct1) and peptide transporters (PepT1), glucose transporters (Sglt1, Glut2 and Glut5) and mucin (Muc2) in the jejunum and expression of interleukins (IL1 beta, IL8, IL17 and IL22) in the cecum was assessed by qPCR at 24 and 168 hpi. NN birds were colonized by SE just as Cobb birds but showed innate upregulation of Muc2, IL8 and IL17 in comparison to Cobb. While nutrient transporter mRNA expression was impaired in SE-challenged Cobb birds, the opposite was observed in NN. There were no differences in microbial diversity at different sampling times for Cobb-SE, whereas the other groups had higher diversity and lower dominance at 24 hpi compared with 96 hpi and 168 hpi. NN birds apparently develop earlier gut microbial stability, have higher basal level of mucin gene expression as well as differential nutrient transporter and interleukin gene expression in the presence of SE which might mitigate the effects of SE infection compared to Cobb birds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gut health is crucial to support animal performance because of greater ability to utilize and metabolize dietary nutrients. The uptake of protein and carbohydrate digestion products in the intestine relies on transporters present on enterocytes. Apical transporters involved in the absorption of proteolysis products include Pept1 (peptide transporter, SLC15A1) and Asct1 (alanine, serine, cysteine, threonine transporter, SLC1A4), and there are many transporters located in the basolateral membrane for specific amino acids [1,2,3,4]. Glucose and fructose transporters are Sglt1 (sodium-dependent glucose transporter; SLC5A1) and Glut5 (fructose transporter, SLC2A5) in the apical membrane, and Glut2 (glucose transporter, SLC2A2) in the basolateral membrane [3, 5].
Besides competing for nutrients, enteric pathogens may damage the epithelium, negatively affecting digestion and absorption [6] and consequently, compromising performance. Salmonella enterica is one of the most important zoonotic agents associated with the consumption of poultry products worldwide [7], and Enteritidis (S. Enteritidis, SE) is one of the leading serovars involved in foodborne salmonellosis outbreaks in humans [8]. Salmonella infection in the birds is age dependent [7], probably related to the development of the immune system and lower leukocyte population in the gut lamina propria [9].
Birds selected for fast growth and high production may become more susceptible not only to respiratory and cardiovascular diseases [10, 11], but also to infectious diseases, whereas slow-growing birds have genetic elements associated with improved resistance against pathogens [12]. Susceptibility might be associated with differences in immunological mechanisms between fast- and slow-growing birds. While the activation of macrophages and T cells favoring the oxidative process is observed in meat-type chickens both in homeostasis and after sanitary challenges [13], slow-growing birds have a rapid pro-inflammatory response, with greater heterophil numbers, as observed in Fayoumis birds, a rustic African breed showing resistance against S. Enteritidis infection [14]. Fast-growing birds inoculated with S. Enteritidis showed higher bacterial counts in the liver and more evident weight loss compared with slow-growing birds [15]. In addition to genetic background, different response patterns are affected by age, the organ or intestinal segment, native intestinal microbiota, diet, and type of pathogen [16, 17]. Therefore, comparative physiological, immunological and microbiological investigations considering slow- and fast-growing birds could shed light on important drivers affecting gut health in modern poultry production.
Previous studies in our laboratory have evidenced a less compromised performance of naked neck birds challenged with SE than Cobb birds, even though SE colonization was similar in both breeds (non-published results). It is hypothesized that these results are not associated with innate resistance of naked neck birds against SE but rather rely on putative differences in Salmonella-induced gut responses between slow and fast-growing birds. Therefore, this study assessed the Salmonella counts, microbial composition and expression of interleukin genes in the cecum of Cobb and naked neck (NN) two-day-old chicks inoculated with SE or nutrient broth (sham), as well as mucin and nutrient transporter gene expression in the jejunum.
Materials and methods
Animals and management practices
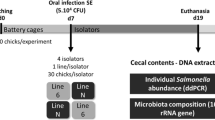
Cobb500 and naked neck fertile eggs (n = 120/each) were incubated at 37.7oC and relative humidity of 60%. Eggs were automatically turned every two hours and candling was performed at 11 days to discard dead embryos and infertile eggs. After hatch, individual hatchling weight was recorded, and the negative Salmonella status was confirmed using cloacal swabs from 20 birds per incubator. Hatchlings (n = 50/breed) with similar average weight were then distributed in a completely randomized design according to 2 × 2 factorial with two breeds (Cobb and naked neck, NN) and two inoculation conditions (Salmonella Enteritidis culture - SE; or sterile nutrient broth - sham). In this study, each bird was considered the experimental unit, since animals from a treatment were submitted to the same environment and conditions. Therefore, there were four treatments: Cobb-sham; Cobb-SE; NN-sham; and NN-SE.
Birds were individually identified with leg bands and kept in boxes with a minimum area of at least 0.05 m2 per bird (1.25 m x 1 m). Boxes were covered with nylon to avoid Salmonella cross-contamination between boxes by flies and other vectors. Water and food were provided ad libitum, and environment and management conditions were similar for both breeds. Room temperature and relative humidity were monitored with thermo-hygrometers (Oregon Scientific, Portland, USA). Corn and soybean meal-based diets were formulated with 22.20% CP, 2,950 kcal/kg ME, 1.31% digestible lysine, 0.94% digestible methionine + cystine and 0.852% digestible threonine in the initial phase (1 to 10 d) according to Rostagno et al. [18].
All management practices, as well as slaughter and sampling procedures were approved by the Ethical Committee for the Use of Animals from Universidade Federal da Paraiba (protocol 186/15) in compliance with the National Council for Animal Experimentation Control – CONCEA (Federal Law nº 11.794/08) as established in Art. 225 of the Brazilian National Constitution on the guidance for the use of animals for scientific purposes.
Inoculation and bacterial counts
Inoculation was performed according to Moreira Filho et al. [19]. All birds in each box were inoculated at 2 days of age (d) into the crop using either 0.5 mL sterile nutrient broth or 0.5mL of nalidixic acid-resistant Salmonella (1.6 × 109 CFU/mL). Cecal contents were sampled individually from five birds per treatment for bacterial counts when birds were 3d (24 hpi), 6d (96 hpi) and 9d of age (168 hpi). Samples were weighed and serially diluted with PBS pH 7.4 and twenty-microliter aliquots from each dilution were streaked onto brilliant green agar with nalidixic acid (100 µg/mL), followed by incubation at 37ºC for 24 h. Colonies were counted and values were expressed as colony forming units per gram of cecal content (CFU/g). The 72 h-interval between samplings was chosen based on the epithelial turnover rate in chickens [20].
Microbial composition analyses
Total DNA was extracted from cecal contents sampled individually from three birds at 3d (24 hpi), 6d (96 hpi) and 9d of age (168 hpi) using a commercial kit (PowerSoil DNA Isolation, Qiagen, Germany) following the provided protocol. The microbial 16S rRNA gene (V3-V4 region) was amplified using the primers 341F and 785R (5’TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3’ and 5’GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3’). Polymerase chain reaction (PCR) conditions were 95ºC for 3 min, followed by 25 cycles at 95ºC for 30 s, 55ºC for 30 s, and 72ºC for 30 s and a final extension to 72ºC for 5 min. Library preparation was performed as per the standard Illumina 16 S rRNA gene protocol. Amplification products were evaluated in 1.5% agarose gel, purified using AMPure beads (Beckman Coulter, USA) and quantified by fluorometry (Qubit2.0, Life Invitrogen, USA). Quality was assessed using a capillary electrophoresis system (Fragment Analyzer, Agilent, USA) before sequencing in the Illumina MiSeq with Illumina V2 kit (2 × 250 cycles).
Demultiplexed paired-end reads in fastq format were processed using QIIME2 [21]. Sequences were joined, selected by size (> 240 bp), quality filtered (minimum Phred score > 20) and dereplicated using VSEARCH. Chimeras were removed using UCHIME. De novo clusterization with 99% of similarity was performed to obtain the amplicon sequence variants (ASVs). The sample with lowest number of sequences was used to standardize the number of sequences per sample. Taxonomic classification was attributed using the Naïve Bayes method with SILVA database (https://www.arb-silva.de/) with 99% for region V3-V4. Alpha and beta diversity were assessed with phyloseq and DESEq2 packages in R [22]. Multiple groups were analyzed with STAMP (https://beikolab.cs.dal.ca/software/STAMP), using the Farthest Neighbor and ANOVA with significance level of 5%.
Gene expression analyses
Jejunal and cecal mucosa was individually sampled at 24 and 168 hpi from five birds per treatment, snap-frozen and kept at -80oC. Total RNA was isolated from individual jejunum samples using the RNeasy Mini kit (Qiagen, Germany). Concentration and purity were determined at 260/280 and 260/230 using a microvolume spectrophotometer (Colibri, Titertek-Berthold, Germany). Reverse transcription was performed with AffinityScript QPCR cDNA Synthesis Kit (Agilent, USA) and relative gene expression was determined by real time PCR using Brilliant III Ultra-Fast SYBR QPCR Master Mix (Agilent) according to provided guidelines. Cycling was carried out in a Stratagene Mx3005P thermocycler (Agilent). Primer sequences targeting sodium-dependent glucose transporter (Sglt1), glucose transporter (Glut2), fructose transporter (Glut5), peptide transporter (Pept1), alanine, serine, cysteine, threonine transporter (Asct1), interleukin 1 beta (IL1-beta); interleukin 8 (IL8); interleukin 17 (IL17); interleukin 22 (IL22) and reference genes (glyceraldehyde-3-phosphte dehydrogenase/Gapdh, and hydroxymethylbilane synthase/Hmbs) are shown in Online Resource 1.
Statistical analyses
Bacterial counts (CFU/g) were transformed in Log10 to be analyzed in a completely randomized design, comparing the groups of SE-inoculated birds of the two genotypes (n = 5 per breed per sampling point). Relative gene expression was calculated using the method 2−ΔΔCt [23]; Ct values of each sample were standardized for the reference gene Gapdh. Gene expression data was analyzed in each post-inoculation sampling, considering four treatments in a 2 × 2 factorial (Cobb or NN; SE or sham), and each bird as repetition (n = 5 per tissue per treatment). A significant interaction indicates that changes in gene expression caused by SE-inoculation are different between breeds and/or indicate that, within a single breed, SE-inoculation will affect gene expression. Thus, when there was interaction, the four treatments were compared by Tukey’s test at 5% of probability. When there was no interaction, means were compared either between breeds (independent of inoculation) or between inoculation treatments (independent of breed), using Tukey’s test at 5% probability.
Results
Salmonella cecal counts
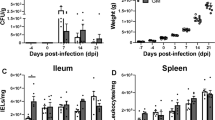
Salmonella counts were not different between breeds at 24 and 96 hpi (p > 0.05). At 168 hpi, counts were lower in NN birds (p < 0.05; Fig. 1). All sham-inoculated birds showed negative results for SE in all sampling periods.
Gene expression
The statistically significant results of gene expression are shown in Table 1 and complete gene expression data is shown in Online Resource 2. Data with significant interaction (p < 0.05) were further analyzed to show differences in gene expression caused by SE-inoculation within a single breed and/or differences between breeds within each inoculation group (Table 2).
There was no interaction between factors on the expression of cytokines in the cecum and transporters in the jejunum at 24 hpi (Table 1), therefore, inoculation and breeds are discussed separately. IL1 beta (p = 0.042) expression was upregulated when the birds were inoculated with Salmonella, but there was no effect of genotype (p = 0.323). These effects were not seen at 168 hpi. Neither breed nor inoculation affected IL8, IL17 and IL22 at 24 hpi, or IL1 beta and IL22 at 168 hpi.
There was interaction between breed and inoculation for Muc2 (p = 0.0014) at 24 hpi and for Glut2 (p = 0.0002), IL8 (p = 0.011) and IL17 (p = 0.074) at 168 hpi (Table 2). The analysis within each factor evidenced that Muc2 expression in Cobb-sham birds was lower than NN-sham birds (0.277 vs. 1.362) and was upregulated when Cobb birds were inoculated with SE (1.886). In naked neck birds, Muc2 expression was similar between sham- and SE-inoculated animals (1.362 vs. 1.128). Similarly, at 168 hpi, IL8 and IL22 were upregulated in Cobb-SE compared with Cobb-sham birds (3X higher and 2X higher, respectively), whereas expression was not changed between sham- and NN-SE birds at 168 hpi. IL8 and IL22 expression in Cobb-sham was also lower when compared with NN-sham birds (3X and 2X lower, respectively) (Table 2). Glut2 expression was higher in Cobb than in naked neck when birds were sham-inoculated (2.109 vs. 1.735). Muc2 expression significantly decreased in Cobb-SE (0.866, 2X lower) and significantly increased in NN-SE birds (3.438, 2X higher) when compared to the sham-inoculated birds within the same breed (Table 2).
Microbial composition analyses
Ten different phyla and 192 genera were identified considering all groups. These data are presented in Online Resources 3 and 4.
Alpha diversity analyses evidenced that Cobb-sham, NN-sham and NN-SE had lower (p < 0.05) observed ASVs and Shannon indexes and higher (p < 0.05) dominance index at 24 hpi when compared to 96 hpi and 168 hpi. There were no differences in ASVs, Shannon or dominance indexes at different sampling times for Cobb-SE (Table 3).
Differential abundance analyses were carried out for genera with significant statistical difference (p < 0.05) between sampling times within each treatment (Figs. 2 and 3, Online Resources 5–8). In Cobb-sham chicks, abundance of Dickeya and unclassified Enterobacterales abundance was higher at 24 hpi when compared with 96 and 168 hpi. The abundance of Erysipelatoclostridium, Flavonifractor, Lactobacillus, Sellimonas, Streptococcus and Tepidibacter abundance was higher at 96 hpi. Finally, at 168 hpi, higher abundance was seen for [Ruminococcus]_torques_group, Anaerostignum, Anaerostipes, Anaerotruncus, Butyricicoccus, Candidatus_Arthromitus, Lachnoclostridium, Lachnospiraceae_unclassified, Negativibacillus, Oscillibacter, Oscillospiraceae_unclassified, Oscillospiraceae_uncultured, Ruminococcaceae_Incertae_Sedis and Ruminococcaceae_unclassified.
Cobb-SE presented higher abundances of the genera Clostridium_sensu_stricto_1, Enterobacterales_unclassified, Epulopiscium and Escherichia-Shigella at 24 hpi. [Clostridium]_methylpentosum_group, Clostridioides and Sellimonas abundances were higher at 96 hpi. Clostridia_vadinBB60_group, Ruminococcaceae_Incertae_Sedis and Tyzzerella abundances were higher at 168 hpi.
At 24 hpi, NN-sham birds had higher abundances of the genera Acinetobacter, Dickeya and Enterobacterales_unclassified, while at 96 hpi, higher abundances of Lachnospiraceae_CHKCI001, Lactobacillus, Sellimonas and Tepidibacter were observed. Genera [Ruminococcus]_gauvreauii_group, Anaerostignum, Colidextribacter, Lachnospiraceae_UCG-010, Negativibacillus, Oscillibacter and Ruminococcaceae_Incertae_Sedis showed higher abundances at 168 hpi.
Only Enterobacterales_unclassified had higher abundance at 24 hpi compared with 96 and 168 hpi in NN-SE chicks. Abundance at 96 hpi was higher for Anaerostipes, Clostridioides, Lactobacillus and Oscillospirales_unclassified. At 168 hpi, abundance was higher for Anaerostignum, Bifidobacterium, Butyricicoccaceae_UCG-009, Candidatus_Soleaferrea, Colidextribacter, Ethanoligenenaceae_uncultured, Intestinimonas, Lachnospiraceae_GCA-900,066,575, Oscillospiraceae_UCG-005, Oscillospiraceae_uncultured, Ruminococcaceae_DTU089, Ruminococcaceae_Incertae_Sedis and Tyzzerella.
Discussion
Salmonella counts in the cecum and gene expression
Differences in gastrointestinal ontogeny between fast- and slow-growing birds have been reported earlier and correlated to lower colonization and faster clearance of Salmonella in the latter [16]. Mucin is one of the major components of the mucosal barrier and has a fundamental role in the prevention of pathogen invasion [24]; it is a simple and effective measure to prevent Salmonella adhesion and provide clearance by peristalsis [7]. The higher expression of Muc2 in the jejunum of NN-sham birds indicate an innate ability for clearance of bacteria and may be responsible for the lower counts in SE-inoculated birds at 168 hpi. This assumption is corroborated by the fact that, in Cobb breed, increased expression of Muc2 was observed only in SE-challenged birds. The lower SE counts (Fig. 1) are in line with the Muc2 expression data and indicate that the more rustic slow-growing breed might use interrelated mechanisms that improve the response to the pathogen such as mucin production.
Further evidence of the importance of mucin for gut protection is that mucin production is started as early as 20–21 days of incubation with an increased density of goblet cells [25]. A steady number of goblet cells was reported in the duodenum of Cobb birds at hatch, whereas the density of goblet cells increased until 4 days of age, suggesting that the mucus layer must be well-formed earlier to provide a protective barrier in the newly hatched chick against oral infection by pathogens [26].
Forder et al. [27] have reported lower Muc2 expression in Cobb birds challenged with Eimeria spp. and Clostridium perfringens, suggesting an impairment of the mucosal activity with time due to the deterioration of the intestinal mucosa, resulting in less replenishment of the mucus layer and greater susceptibility to other bacterial infections. The expression of Muc2 was more prominent in the naked neck birds in the present study and was not changed in this breed in the presence of SE; on the other hand, Muc2 expression increased approximately five times in Cobb-SE birds. These apparently contradictory responses might be explained by different inoculation ages, intestinal segments or by different effects of the bacterial agents on the mucus layer. C. perfringens and Eimeira spp. cause a more severe enteritis and greater desquamation of epithelial and goblet cells, decreasing Muc2 expression and consequently increasing the probability of new infections [27, 28].
Prominent morphophysiological changes occur in the end of incubation and first days after hatch, enabling birds to properly digest and absorb exogenous food. Studies on the gene expression of intestinal transporters in slow-growing birds compared to fast-growing breeds are scarce. On the other hand, the expression of intestinal transporters is vastly reported in Cobb birds considering factors such as diet supplementation, feed restriction and age [29,30,31]; however, no changes were reported in Glut2 expression in these previous studies. In the results presented herein, the downregulation in Glut2 expression in Cobb-SE compared to Cobb-sham birds might reflect greater mucosal damage, whereas upregulation was seen in naked neck birds. According to Gilbert et al. [5], the upregulation of transporter genes is probably related to a greater ability of absorption of its substrate. Thus, glucose and peptide absorption might be improved in naked neck chicks in the presence of SE as part of the response against the pathogen. Downregulation of Asct1 and Eaat3 mRNA levels has been reported in Clostridium perfringens-challenged birds, suggesting reduced uptake of amino acids and glutamate, the latter an energy source, which might respond for changes in morphology and growth performance [32]. The authors also reported downregulation of Glut2 in Eimeria-challenged birds, similar to our findings.
Diet utilization depends not only on these complex physiological changes, but also on the establishment of a commensal microbiota that is essential for gastrointestinal homeostasis. In this sense, the gut immune system must be able to differentiate between putatively deleterious antigens and inoffensive antigens, such as those from commensal bacteria and dietary proteins, and gut health depends on the balance between response and tolerance [33, 34]. The inflammatory response is minimized to reduce negative effects on the host health [35], and disease tolerance in the gut evolves concomitantly with microbiota development, thus, proinflammatory cytokine levels are seen as a specific defense mechanism of the host.
The cecum of newly hatched birds shows greater abundance of heterophils in the lamina propria, followed by macrophages and T lymphocytes. Macrophage and heterophils play a key role on the response to Salmonella and are directly related to pro-inflammatory cytokine expression (IL1 beta and IL8) [7]. These pro-inflammatory mediators activate the recruitment of leukocytes in the developing gut [36], a process that lasts one to two weeks during the development of the immune system. Inoculation was performed at 2d-old chicks in our study and IL1 beta expression was higher in SE-inoculated birds at 24 hpi, although there were no differences between breeds (Table 1). IL1 beta production is related to the rapid inflammatory response as an attempt to fight the bacterial invasion [37]. On the other hand, IL8 expression increased in Cobb-SE chicks at 168 hpi, whereas levels were higher in NN birds, including sham-inoculated birds. These findings show that the higher IL8 expression levels in NN is not necessarily associated with SE infection. It can be inferred that basal activation is more prominent in naked neck birds or that macrophage response is activated in Cobb birds only in the presence of SE. Indeed, Rychlik et al. [7] also reported higher expression of IL1 and IL8 in slow-growing birds.
IL17 and IL22 are frequently expressed belatedly, but in specific conditions. Although in the present study only IL17 expression was affected by SE-infection in Cobb breed (Table 2), Chranova et al. [38] reported increased expression of both cytokines 24 h after Salmonella-infection. Van Hemert et al. [16] also reported greater expression of interleukins that are produced by Th17 cells after Salmonella inoculation, including IL17 and IL22. IL17 stimulates heterophils against microbial invasion and promotes increased number of regulatory T cells in the cecum of Salmonella-infected birds, which is associated with greater expression of anti-inflammatory cytokines and lower expression of pro-inflammatory cytokines.
IL17 receptors were seen in dendritic cells, macrophages and T lymphocytes, indicating its potential to regulate the immune response [39]. On the other hand, IL22 receptor was observed only in non-immune cells, stimulating these cells to produce antimicrobial peptides, and also stimulating the growth and regeneration during infection. Normal microbiota, therefore, stimulates the pro-inflammatory response that, due to the absence of positive regulation of IL22, is not deleterious to tissues [40].
Once Salmonella adheres to gut mucosa cells, a response is mounted to restrict Salmonella dissemination to other tissues [7]. Heterophil numbers do not change significantly, but macrophage infiltration is fast followed by a decrease in macrophage numbers at 6 days post-inoculation (dpi), whereas T-lymphocytes will decrease only at 10dpi in chicks from a commercial fast-growing breed [10]. The upregulation of cytokines in sham-inoculated birds at 168 hpi observed in our study indicate that leukocyte recruitment might be affected by commensal microbiota establishment, or it may be different from Cobb birds.
Microbial composition analyses
Colonization of the gastrointestinal tract in hatchlings occurs rapidly and the commensal microbiota complexity and diversity evolves, until stabilization [41]. The commensal microbiota is important for the gut morphofunctional development and is crucial for protection against pathogens, competing for colonization sites. It also affects the development of Peyer patches and immunoglubulin A production [42].
According to our results, Salmonella inoculation did not induce dramatic shifts in microbial composition of birds, corroborating Videnska et al. [43]. Nevertheless, we observed a significant reduction in microbial dominance and increased diversify at 96 and 168 hpi in NN-birds (Table 3), indicating a faster recovery of the microbial stability, which is also supported by the faster clearance of Salmonella Enteritidis in the gut of NN-birds at 196 hpi compared with the Cobb breed (Fig. 1). Interestingly, the SE challenge caused greater shifts in the expression of intestinal transporters and cytokines in the gut of Cobb birds compared with NN-birds (Tables 1 and 2). Therefore, these findings indicate that NN chicks had a better response against Salmonella infection.
Diversity indexes in sham-inoculated chicks showed a common pattern independent of the breed, i.e., Shannon index was lower at 24 hpi and increased at 96 and 168 hpi in both NN-sham and Cobb-sham. On the contrary, dominance index was higher at 24 hpi and decreased at 96 and 168 hpi. Interestingly, Salmonella inoculation did not change this pattern in naked neck birds, whereas in Cobb birds the alpha diversity was similar between 24 hpi, 96 hpi and 168 hpi. These results suggest that Salmonella inoculation affected the development, succession, and balance/stability of the microbiota of Cobb birds, but not in NN birds. This can be also supported by the marked increased abundance of Tyzzerella in SE-inoculated Cobb birds at 168 hpi compared with 24 and 96 hpi (Online Resources 8). Increased Tyzzerella abundance has been reported in heat-stressed broilers showing damaged intestinal villus-crypt structures, and microbial gut disbiosis [44].
The similar pattern of microbiota development shared by NN-SE and sham-inoculated birds is further supported by the genera that are more abundant in each time post-inoculation. These three groups of birds showed dominance of Dickeya and unclassified Enterobacterales at 24 hpi, followed by an increase in Lactobacillus abundance at 96 hpi and greater diversity at 168 hpi. On the other hand, Cobb-SE birds showed no dominance of taxonomic groups at 24 hpi. Furthermore, this is the only group showing a greater abundance of Clostridium, Escherichia and Epulopiscium at 24 hpi when compared with 96 e 168 hpi. Differently from the other groups, Cobb-SE did not show differential abundance of Lactobacillus at 96 hpi, and only three genera were highly abundant at 168 hpi. The lower microbial diversity in Cobb-SE birds at 168 hpi can favor Salmonella persistence in the gut, as Salmonella abundance in chickens appears to be decreased with higher diversity of the microbial population [45]. Equilibrium during microbial succession is important for the development and immune response of the host. The change in the succession patterns and in the cecal microbiota establishment in Cobb birds associated with Salmonella-challenge may be related to a lower ability to respond to bacterial infections, probably due to a later maturation of the immune system, which is corroborated by cytokine expression patterns.
In summary, naked neck chicks do not seem to be more resistant to Salmonella Enteritidis infection, i.e., they are colonized just as Cobb birds, as evidenced by similar Salmonella counts in the cecum of both breeds until 96 hpi. On the other hand, innate upregulation of Muc2 (mucin production), and of the cytokines IL8 and IL17 in comparison to Cobb birds might play an important role on pathogen clearance at 168 hpi. Furthermore, this can be also affected by significant differences in the expression of nutrient transporters between the two breeds. While glucose uptake could be impaired in Cobb-SE birds, the opposite is observed in NN-SE birds. Nonetheless, peptide uptake may also be improved in this breed due to the higher Pept1 gene expression. Lastly, the greater microbiota stability observed in naked neck compared with Cobb chicks possibly contribute to a better response against Salmonella infection.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available but can be made available from the corresponding author on reasonable request.
References
Bröer S (2008) Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88:249–286. https://doi.org/10.1152/physrev.00018.2006
Barri A, Honaker CF, Sottosanti JR, Hulet RM, McElroy AP (2011) Effect of incubation temperature on nutrient transporters and small intestine morphology of broiler chickens. Poult Sci 90:118–125. https://doi.org/10.3382/ps.2010-00908
Miska KB, Fetterer RH (2019) Expression of amino acid and sugar transporters, aminopeptidase, and the di- and tri-peptide transporter PepT1; differences between modern fast growing broilers and broilers not selected for rapid growth. Poult Sci 98:2272–2280. https://doi.org/10.3382/ps/pey583
Zhang H, Li D, Liu L, Xu L, Zhu M, He X, Liu Y (2019) Cellular composition and differentiation signaling in chicken small intestinal epithelium. Anim (Basel) 11:870. https://doi.org/10.3390/ani9110870
Gilbert ER, Li H, Emmerson DA, Webb KE Jr, Wong EA (2007) Developmental regulation of nutrient transporter and enzyme mRNA abundance in the small intestine of broilers. Poult Sci 86:1739–1753. https://doi.org/10.1093/ps/86.8.1739
Gunal M, Yayli G, Kaya O, Karahan N, Sulak O (2006) The effect of antibiotic growth promoter, probiotic or organic acid supplementation on performance, intestinal microflora and tissue of broilers. Int J Poult Sci 5:149–155. https://doi.org/10.3923/ijps.2006.149.155
Rychlik I, Elsheimer-Matulova M, Kyrova K (2014) Gene expression in the chicken caecum in response to infections with non-typhoid Salmonella. Vet Res 45:119. https://doi.org/10.1186/s13567-014-0119-2
ECDC EFSA (2017) Multicountry outbreak of Salmonella Enteritidis infections linked to Polish eggs. European Centre for Disease Prevention and Control and European Food Safety Authority: Stockholm and Parma. http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1353/pdf. Accessed Apr 2020
Van Immerseel F, De Buck J, De Smet I, Mast J, Haesebrouck F, Ducatelle R (2002) The effect of vaccination with a Salmonella Enteritidis aroA mutant on early cellular responses in caecal lamina propria of newly-hatched chickens. Vaccine 20:3034–3041. https://doi.org/10.1016/s0264-410x(02)00227-x
Bayyari GR, Huff WE, Rath NC, Balog JM, Newberry LA, Villines JD, Skeeles JK, Anthony NB, Nestor KE (1997) Effect of the genetic selection of turkeys for increased body weight and egg production on immune and physiological responses. Poult Sci 76:289–296. https://doi.org/10.1093/ps/76.2.289
Crossley DA II, Altimiras J (2012) Effect of selection for commercially productive traits on the plasticity of cardiovascular regulation in chicken breeds during embryonic development. Poult Sci 91:2628–2636. https://doi.org/10.3382/ps.2012-02344
Wigley P (2004) Genetic resistance to Salmonella infection in domestic animals. Res Vet Sci 76:165–169. https://doi.org/10.1016/S0034-5288(03)00117-6
Coble DJ, Redmond SB, Hale B, Lamont SJ (2011) Distinct lines of chickens express different splenic cytokine profiles in response to Salmonella Enteritidis challenge. Poult Sci 90:1659–1663. https://doi.org/10.3382/ps.2010-01279
Redmond SB, Chuammitri P, Andreasen CB, Palić D, Lamont SJ (2009) Chicken heterophils from commercially selected and non-selected genetic lines express cytokines differently after in vitro exposure to Salmonella enteritidis. Vet Immunol Immunopathol 132:129–134. https://doi.org/10.1016/j.vetimm.2009.05.010
Van Hemert S, Hoekman AJ, Smits MA, Rebel JM (2006) Gene expression responses to a Salmonella infection in the chicken intestine differ between lines. Vet Immunol Immunopathol 114:247–258. https://doi.org/10.1016/j.vetimm.2006.08.007
Cheng HW, Eicher SD, Chen Y, Singleton P, Muirt WM (2001) Effect of genetic selection for group productivity and longevity on immunological and hematological parameters of chickens. Poult Sci 80:1079–1086. https://doi.org/10.1093/ps/80.8.1079
Davison F, Kaspers B, Schat KA (2008) Avian immunology. Elsevier, Amsterdam
Rostagno HS, Albino LFT, Donzele JL, Gomes PC, Oliveira RF, Lopes DC, Ferreira AS, Barreto SLT, Euclides RF (2011) Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais, 3rd edn. Universidade Federal de Viçosa, Viçosa
Moreira Filho ALB, Oliveira CJB, Freitas Neto OC, de Leon C, Saraiva M, Andrade M, White B, Givisiez PEN (2018) Intra-amnionic threonine administered to chicken embryos reduces Salmonella enteritidis cecal counts and improves posthatch intestinal development. J Immunol Res. https://doi.org/10.1155/2018/9795829
Uni Z, Geyra A, Ben-Hur H, Sklan D (2000) Small intestinal development in the young chick: crypt formation and enterocyte proliferation and migration. Br Poult Sci 41:544–551. https://doi.org/10.1080/00071660020009054
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Caporaso JG (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. https://doi.org/10.1038/s41587-019-0209-9
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/. Accessed Apr 2020
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San DiegoCalif) 25:402–408. https://doi.org/10.1006/meth.2001.1262
Chang CH, Teng PY, Lee TT, Yu B (2020) Effects of multi-strain probiotic supplementation on intestinal microbiota, tight junctions, and inflammation in young broiler chickens challenged with Salmonella enterica subsp. Enterica. Asian-Australas J Anim Sci 33:1797–1808. https://doi.org/10.5713/ajas.19.0427
Ozaydin T, Celik I (2012) Histological, histochemical and immunohistochemical investigations on the developing small intestines of broilers embryos. J Anim Vet Adv 11:2936–2944. https://doi.org/10.3923/javaa.2012.2936.2944
Reynolds KL, Cloft SE, Wong EA (2020) Changes with age in density of goblet cells in the small intestine of broiler chicks. Poult Sci 99:2342–2348. https://doi.org/10.1016/j.psj.2019.12.052
Forder RE, Nattrass GS, Geier MS, Hughes RJ, Hynd PI (2012) Quantitative analyses of genes associated with mucin synthesis of broiler chickens with induced necrotic enteritis. Poult Sci 91:1335–1341. https://doi.org/10.3382/ps.2011-02062
Golder HM, Geier MS, Forder RE, Hynd PI, Hughes RJ (2011) Effects of necrotic enteritis challenge on intestinal micro-architecture and mucin profile. Br Poult Sci 52:500–506. https://doi.org/10.1080/00071668.2011.587183
Duarte CR, Vicentini-Paulino ML, Buratini J Jr, Castilho AC, Pinheiro DF (2011) Messenger ribonucleic acid abundance of intestinal enzymes and transporters in feed-restricted and refed chickens at different ages. Poult Sci 90:863–868. https://doi.org/10.3382/ps.2010-01015
Ebrahimi R, Faseleh Jahromi M, Liang JB, Soleimani Farjam A, Shokryazdan P, Idrus Z (2015) Effect of dietary lead on intestinal nutrient transporters mRNA expression in broiler chickens. Biomed Res Int. https://doi.org/10.1155/2015/149745
Ruhnke I, Röhe I, Goodarzi Boroojeni F, Knorr F, Mader A, Hafeez A, Zentek J (2015) Feed supplemented with organic acids does not affect starch digestibility, nor intestinal absorptive or secretory function in broiler chickens. J Anim Physiol Anim Nutr (Berl) 99(Suppl S1):29–35. https://doi.org/10.1111/jpn.12313
Criado-Mesas L, Abdelli N, Noce A, Farré M, Pérez JF, Solà-Oriol D, Martin-Venegas R, Forouzandeh A, González-Solé F, Folch JM (2021) Transversal gene expression panel to evaluate intestinal health in broiler chickens in different challenging conditions. Sci Rep 11:6315. https://doi.org/10.1038/s41598-021-85872-5
Mowat AM (2003) Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol 3:331–341. https://doi.org/10.1038/nri1057
Beal RK, Powers C, Davison TF, Smith AL (2006) Immunological development of the avian gut. In: Perry GC (ed) Avian gut function in Health and Disease. CAB International, Wallingford, pp 85–103
Kogut MH, Swaggerty CL, Byrd JA, Selvaraj R, Arsenault RJ (2016) Chicken-specific kinome array reveals that Salmonella enterica serovar Enteritidis modulates host immune signaling pathways in the cecum to establish a persistence infection. Int J Mol Sci 17:1207. https://doi.org/10.3390/ijms17081207
Bar-Shira E, Friedman A (2006) Development and adaptations of innate immunity in the gastrointestinal tract of the newly hatched chick. Dev Comp Immunol 30:930–941. https://doi.org/10.1016/j.dci.2005.12.002
Kaiser P, Rothwell L, Galyov EE, Barrow PA, Burnside J, Wigley P (2000) Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology 146:3217–3226. https://doi.org/10.1099/00221287-146-12-3217
Crhanova M, Hradecka H, Faldynova M, Matulova M, Havlickova H, Sisak F, Rychlik I (2011) Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect Immun 79:2755–2763. https://doi.org/10.1128/IAI.01375-10
Reynolds JM, Angkasekwinai P, Dong C (2010) IL-17 family member cytokines: regulation, and function in innate immunity. Cytokine Growth Factor Rev 21:413–423. https://doi.org/10.1016/j.cytogfr.2010.10.002
Rutz S, Eidenschenk C, Ouyang W (2013) IL-22, not simply a Th17 cytokine. Immunol Rev 252:116–132. https://doi.org/10.1111/imr.12027
Pan D, Yu Z (2014) Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 5:108–119. https://doi.org/10.4161/gmic.26945
Kosiewicz MM, Zirnheld AL, Alard P (2011) Gut microbiota, immunity, and disease: a complex relationship. Front Microbiol 2:180. https://doi.org/10.3389/fmicb.2011.00180
Videnska P, Sisak F, Havlickova H, Faldynova M, Rychlik I (2013) Influence of Salmonella enterica Serovar Enteritidis infection on the composition of chicken cecal microbiota. BMC Vet Res 9:1–8. https://doi.org/10.1186/1746-6148-9-140
Liu G, Zhu H, Ma T, Yan Z, Zhang Y, Geng Y, Zhu Y, Shi Y (2020) Effect of chronic cyclic heat stress on the intestinal morphology, oxidative status and cecal bacterial communities in broilers. J Therm Biol 91:102619. https://doi.org/10.1016/j.jtherbio.2020.102619
Pedroso AA, Lee MD, Maurer JJ (2021) Strength lies in diversity: how community diversity limits salmonella abundance in the chicken intestine. Front Microbiol 12:694215. https://doi.org/10.3389/fmicb.2021.694215
Acknowledgements
The authors thank to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Finance code 001) for scholarships granted to MRBS, ALBMF, MFSA, NVMS, GFCS and MLPL; Conselho Nacional de Pesquisa e Desenvolvimento (CNPq) for scholarship (311860/2016-8) and research funding granted to PENG (458340/2014-6) and Financiadora de Estudos e Projetos (FINEP) for equipment supply in the scope of MCT-Infra institutional projects.
Author information
Authors and Affiliations
Contributions
ALBMF, PENG, MFSA and CJBO conceived and designed research. MRBS, MFSA, NMVS, MLPL, GFCS conducted experiment, laboratorial and bioinformatic analyses. CJBO and OCFN contributed with reagents. MRBS, PENG, ALBMF, OCFN, MFSA, NMVS, GS, MLPL and CJBO analyzed data. MRBS, PENG, CJBO and GFCS wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All management practices, as well as slaughter and sampling procedures were approved ty the Ethical Committee for the use of Animals from Universidade Federal da Paraiba (protocol 186/15) in compliance with the National Council for Animal Experimentation Control – CONCEA [Conselho Nacional de Controle de Experimentação Animal - CONCEA] (Federal Law nº 11.794/08, Lei Arouca) as established in art. 225 of the Brazilian National Constitution on the guidance for the use of animals for scientific purposes.
Competing interests
The authors state that there is no conflict of interest.
Additional information
Responsible Editor: David Germano Gonçalves Schwarz
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1.26 MB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santos, M.R.B., Moreira Filho, A.L., Freitas Neto, O.C. et al. Shifts in microbiota and gene expression of nutrient transporters, mucin and interleukins in the gut of fast-growing and slow-growing chickens infected by Salmonella Enteritidis. Braz J Microbiol 55, 1987–1996 (2024). https://doi.org/10.1007/s42770-024-01297-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-024-01297-y