Abstract
The present study evaluated the antibiofilm and antimicrobial effects of temporary restorative materials on root canals after an intra-oral challenge. Seventy roots were endodontically treated and divided into 5 groups: high-viscosity glass ionomer (HV-GIC), light-activated glass ionomer (RM-GIC), zinc-oxide cement without eugenol (ZO), zinc-oxide cement with eugenol (ZOE), and unsealed roots (negative control). For 28 days, 14 participants used intra-oral devices with five roots, and drops of sucrose were applied onto them. The amount of biofilm and the bacterial counts were analyzed by Kruskal–Wallis and Dunn, and by two-way ANOVA and Tukey (α = 0.05). HV-GIC and RM-GIC better inhibit biofilm, followed by ZO and ZOE. Unsealed roots had the largest biofilm accumulation (p = 0.002) and higher bacterial penetration than restored roots (p = 0.023). A low amount of Streptococcus was found in RM-GIC and ZOE-restored roots without difference from HV-GIC (p = 0.021). The low amount of Enterococcus (p = 0.003) was found in the ZOE-restored roots, without difference from GICs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevention of bacterial penetration into the root canal system is essential for maintaining disinfection [1, 2]. Proper sealing with a temporary restorative material protects the root canal filling and ensures periapical health until the final coronal rehabilitation is completed [3,4,5].
Previous in vitro studies have assessed the sealing characteristics of temporary materials by analyzing their resistance to abrasion and compression [6], dimensional stability [6, 7], marginal leakage [2, 8, 9], and antimicrobial activity [1, 5, 10]. However, few clinical trials [4, 11] have evaluated the bacterial penetration of teeth restored with temporary materials, and the analyses were limited to the crown, without the use of a control group (unsealed teeth) because of ethical issues.
The in situ oral biofilm model can simulate the in vivo environment by using intraoral appliances with dental fragments, which enable further laboratory analysis [12,13,14]. These appliances allow contact between saliva and the substrate, permitting natural plaque development without mechanical disturbances [15]. Such models have played an essential role in cariology by testing the effects of new caries prevention methods.
Few studies have used in situ models to assess the performance of materials inside root canals when exposed to oral fluids [12, 16, 17]. In a previous investigation [12], none of the tested canal sealers avoided degradation of the adhesive interface and caries formation on intracanal dentine after a 14-day simulated challenge.
Considering that temporary materials are crucial for providing an adequate seal during endodontic visits, it is important to assess the antimicrobial activity of these materials after a simulated acid challenge in the human oral microbiome. The study analyzed: (1) the amount of biofilm (mg) formed on the materials/roots; (2) the total counting of microorganisms, and the specific amount of S. mutans and E. faecalis in the root canal system sealed with different temporary materials.
Materials and methods
Ethical aspects and sample selection
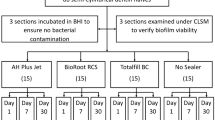
This in situ study has been written according to Preferred Reporting Items for Randomized Trials in Endodontics (PRIRATE) 2020 guidelines. The CONSORT flow diagram is shown in Fig. 1. The research was approved by the research ethics committee (70730217/16) and informed consent was obtained from all participants.
Seventy caries-free human mandibular incisors from a local human biobank were selected. Teeth were extracted within 6 months, stored in thymol solution at 4°C, and washed for 24 h to eliminate residues. The teeth were checked for any fracture lines under a stereomicroscope (Nikon, Tokyo, Japan) and then radiographed to select specimens with fully formed roots and a single canal without calcifications or accentuated curvatures. Teeth with root fillings or restorative procedures were excluded.
Selection of participants and intraoral appliances preparation
The sample size calculation was based on previous in situ studies using cariogenic acid challenge [12, 18], considering the maximal errors of 5% (α) and 20% (β), obtaining a sample size of 11 participants. After adding 25% to the sample due to possible loss, a sample of 15 participants was established.
The inclusion criteria were good general and oral health with no caries or periodontal disease, ability to follow the experimental protocol, nonsmoking, not being pregnant, and no orthodontic appliance or antibiotic use in the last 6 months [12, 13, 19].
Impressions of superior and inferior arches were taken in all participants, and acrylic palatal devices were fabricated for each participant with five spaces (12-mm height × 6-mm width × 5-mm depth) accommodating the roots facing the oral environment [12].
Root canal preparation
The crowns of the incisors were removed (Isomet 1000; Buehler, Lake Buff, IL, USA), and the roots were standardized at 10 mm using a digital caliper (Mitutoyo America, Suzano, SP, Brazil). Apical patency was confirmed by inserting a 10 K-file (Dentsply-Sirona, Ballaigues, VD, Switzerland) through the apical foramen. The working length was established considering a 1-mm step back from the length of the file tip visible at the foramen. Root canals were prepared with an R40 instrument (Reciproc; VDW GmbH, Munich, BY, Germany) with reciprocating movements (VDW GmbH). Root canal preparation was performed according to the manufacturer’s instructions. Irrigation was performed with 10 mL of 2.5% NaOCl.
The roots were sterilized in an autoclave (Dabi Atlante, Ribeirão Preto, SP, Brazil) at 121°C for 15 min using polypropylene tubes (Eppendorf AG, Hamburg, HH, Germany) with distilled water [20]. They were then examined in detail under ×10 stereoscopic glass (Nikon, Tokyo, Japan), and those with cracks were discarded.
The working length of each root canal was verified using a size 40-k file (Dentsply-Sirona). Final irrigation was performed with 5 mL of 2.5% NaOCl and 5 mL of 17% EDTA for 5 min. The canals were irrigated with 5 mL of distilled water, dried with paper points, and filled with a single gutta-percha cone (Reciproc) and AH Plus sealer (Dentsply DeTrey, Konstanz, BW, Germany). The gutta-percha was cut 2 mm from the entrance of the root canal with heated Paiva’s condensers (Golgran Millennium, São Caetano do Sul, Brazil) to accommodate the restorative material and help with the retention of restorations.
Temporary coronal filling and intra-oral phase
Seventy roots were randomly allocated into five groups of 14 roots each. The occlusal surfaces of the 56 roots were sealed with one of the following materials: high-viscosity glass ionomer (HV-GIC) (Ketac Molar EasyMix; 3M ESPE, Saint Paul, MN, USA), resin-modified glass ionomer (RM-GIC) (Riva Light Cure; SDI Limited, Bayswater, WA, Australia), zinc oxide–based cement without eugenol (ZO) (Coltosol, Coltene Whaledent, Cuyahoga Falls, OH, USA), or zinc oxide–based cement with eugenol (ZOE) (IRM; Dentsply-Sirona). The remaining 14 unsealed roots were used as controls. The 2-mm thickness of the filling material was checked with a periodontal probe. The apical foramina and external root surface sealing were done using cyanoacrylate to ensure that microbial penetration only occurred in the coronal portion of the root.
The specimens were stored at 37 °C and relative humidity for 48 h to allow complete setting of the materials. The roots were fixed in a random position on the palatal devices using melted wax, except in the most cervical region. To allow biofilm accumulation, the roots were covered with nylon mesh, leaving a 1-mm space from the cervical portion of the root [12, 13].
No restrictions were made concerning the participants’ diet, but they were instructed to remove the appliances during meals and before consuming liquids. Throughout the experiment, the participants used a dentifrice containing 1450 ppm F (Oral B, Manaus, AM, Brazil), and they were instructed to brush their teeth and the palatal portion of the device three times a day.
Over a 7-day lead-in period, participants were instructed to use only the toothpaste and toothbrush (Oral B) provided by the researchers. The purpose of this lead-in period was to standardize intraoral conditions, and the acid challenge started after the lead-in phase. For 28 days, a 20% sucrose solution was applied perpendicularly onto the roots six times a day simulating a high-acidic challenge [12, 13, 19]. The solutions were prepared every 3 days and delivered to the participants. After 5 min, the device was re-inserted into the mouth without washing the solution.
Analysis of the biofilm formed onto temporary restorative material
On the morning of 28th day, the nylon mesh was removed, and the biofilm from each root slab over the temporary material was collected using a plastic spatula, under a stereomicroscope (Nikon, Tokyo, Japan), and individually placed in previously weighed Eppendorf tubes. Biofilm accumulation was weighed on a 5-digit analytical balance (Ohaus Analytical Plus; Ohaus Corp., Parsippany, NJ, USA) [13]. After weighing, 300 μL of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.6) was placed in each Eppendorf tube and stored at –20 °C.
Microbiological analysis
The roots were sectioned into three thirds. The dentine/filling material chips were removed from the canals with sterile diamond conical burs (KG Sorensen, São Paulo, SP, Brazil) at low speed (Dentsply Sirona, São Paulo, Brazil), using a custom-made metallic apparatus to fix the root sections and ensure the perpendicular insertion of the bur. The last bur was used only in the cervical third [21]. The samples obtained with each bur and in each third were immediately collected into separate test tubes containing reduced transport fluid (RTF). To set the specimen mass, the RTF tubes were weighed before and after collection.
After vortexing for 2 min, bacterial suspensions of each sample were diluted in RTF using a 10-fold serial dilution to 107 before plating on culture plates. Altogether, 50 μL of each dilution was spread onto MM10 SB agar, SB20, and m-Enterococcus agar (Difco, Detroit, MI, USA) using a Drigalsky loop to count total viable bacteria, S. mutans and E. faecalis, respectively. All MM10 SB agar and SB20 plates were incubated under anaerobic conditions, and all m-Enterococcus agar plates were incubated under aerobic conditions for 48 h at 37 °C.
The number of colony-forming units (CFUs) per milligram was determined on two replicate plates per sample and statistically analyzed. The purity of the positive cultures was confirmed by Gram staining, catalase production, and colony morphology on BHI blood agar, and by using a biomechanical identification kit (API 20Strep; BioMériux SA, Marcy-l' Etoile, France). Microbiological assays were carried out under aseptic conditions in a laminar flow chamber (Quimis, Diadema, SP, Brazil). Figure 2 shows the illustration of the methodological sequence.
Illustration of the methodological design: A root sterilization; B root canal preparation; C root canal filling; D temporary filling materials; E intra-oral devices; F selection of participants; G biofilm weighing; H collection of the dentine/filling material chips; I sample weighing; J, K, and L inserting the bacterial suspensions on culture plates to the microbiological analysis; M, N, and O bacterial counts
Data analyses
Statistical analysis was performed using IBM SPSS Statistics (version 25.0; IBM Corp., Armonk, NY, USA). The biofilm data were assessed by nonparametric Kruskal–Wallis and Dunn tests. For microbiological data, parametric data were analyzed using two-way ANOVA (material × root thirds) and post hoc Tukey test. The significance limit was set at 5% for all the analyses.
Results
A total of 14 participants (6 males and 8 females with a mean age of 24.7 years ± 8.41 years) completed the experiment. One of the participants had the roots dislodged from the acrylic palatal device and the ZO restoration lost the adherence to the root surface. These samples were excluded from the statistical analysis.
Biofilm formed onto temporary restorative material
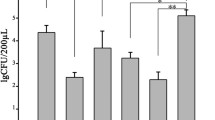
Biofilm accumulation on the roots was significantly affected by the root sealing materials (p = 0.002). HV-GIC (22 mg a) and RM-GIC (20 mg a) restorations were better able to inhibit biofilm accumulation, followed by ZO (41 mg b) and ZOE (48 mg b) restorations. The unsealed roots (90 mg c) had the largest accumulation of biofilm (p > 0.05). Figure 3 shows the medians of biofilm weight (in mg) accumulated on the temporary filling materials after the acid challenge in the oral environment.
Microbial penetration on canal-treated roots
In the total counts, the two-way ANOVA and Tukey test showed significant differences in the microbial penetration among the root thirds (p < 0.001), with the smallest bacterial penetration in the apical third, followed by middle and cervical. The unsealed roots had the highest bacterial penetration than the temporary filling materials, which did not differ statistically among themselves (p = 0.023). No difference was found in the interaction of factors (p = 0.586). The means and standard deviations of the total count of microorganisms (MM10 SB agar) are listed in Table 1.
The S. mutans counts were significantly affected by the temporary restorative material (p = 0.021) and radicular thirds (p = 0.012). The bacterial penetration was higher in the cervical third, followed by middle and apical. A low amount of Streptococcus was found in the ZOE and RM-GIC-restored roots, without difference from HV-GIC. The unsealed roots (control) and ZOE-restored roots had higher bacterial counts, followed by and ZO-restored roots. No significant differences were found between the interactions (p = 0.079). The means and standard deviations of S. mutans counts (SB20) are shown in Table 2.
Regarding the E. faecalis counts, ANOVA showed a significant difference in the temporary restorative material (p = 0.003) and root thirds (p < 0.001). The lowest bacterial penetration was found in the apical, followed by middle and cervical thirds. A lower amount of E. faecalis was observed in the ZOE-sealed roots, without differences from GICs (p > 0.05). The control had the highest bacterial counts, and ZO-sealed roots also had no differences from GICs. No significant differences were found in the interactions (p = 0.083). The means and standard deviations of E. faecalis counts (m-Enterococcus agar) are shown in Table 3.
Discussion
The sealing ability of the temporary materials tested in this study has previously been evaluated in vitro [8, 9]. However, the simulated conditions do not parallel the in vivo environment, which contains microbial communities in their natural habitat [15]. The mechanisms underlying natural oral microbial communities, including their composition, arrangement, and metabolism, remain elusive [15, 22, 23; Choi et al., 2018]. To the best of our knowledge, this is the first study to compare the ability of different temporary materials to prevent biofilm accumulation and bacterial penetration in root canals using an in situ intraoral challenge.
The in situ model was designed to allow accurate biofilm contact with the roots and materials, facilitating the control and standardization of experimental variables [12]. This model is superior to the preceding plaque collection methods in that it does not disturb the three-dimensional architecture of biofilms [15]. In the oral cavity, exposed clean restorations are immediately coated by a salivary pellicle, which dictates how oral microbes adhere to the surface [21].
Although in situ studies can be performed with a small group of participants, some methodological limitations were identified, including a difficulty in selecting participants due to the strictness of the experimental schedule and possible discomfort from using the acrylic palatal device. Therefore, we were always available and in daily contact with the participants for any necessary adjustment in the acrylic device.
The CFU counts method allowed us to quantify the interest bacterial species in each root third separately and identify the viable counts of microorganisms. The present study aimed to evaluate the bacterial contamination in the adhesive interface and inside the root dentin and assessed the filling material and sealer contamination. The dentine/filling material chips were removed from the canals and spread onto the agar plates.
The fermentation of carbohydrates by bacteria creates acids liable for degradation of the mineralized tissue and dentin collagen matrix, which not only degrades the material, but also causes bacterial contamination of the restorative interface [24, 25]. Therefore, we measured the weight of the biofilms formed on the roots. In this study, the biofilm weight revealed inhibition of biofilm formation for all materials compared to unsealed roots. Roots sealed with HV-GIC and RM-GIC were better able to inhibit biofilm formation. GICs have desirable properties, such as the capacity to induce dentin remineralization, biocompatibility, and antibacterial activity per release of fluoride [10, 26]. While frequently used in dentistry because of their high radiopacity, flexural strength, and chemical adhesion to the tooth [26, 27], GICs are sensitive to temperature and moisture changes that interfere with material dissolution [28].
The fluoride-releasing and neutralizing ability of GIC is affected by the nature of the fluoride incorporated, particularly its pH during setting [25, 27]. In the oral environment, the liquid attacks the solid structure of the glass particles, releasing ions of calcium or strontium, aluminum, and fluorine into the aqueous solution by buffering the saliva [25]. Fluoride, aluminum, and strontium have been associated with reduced acidogenicity of S. mutans biofilms [28]. Fluoride can also affect acid production and the formation of extracellular polymeric substances (EPS) in dental plaques [27]. Taken together, these findings explain the low amount of biofilm on the GIC-sealed roots.
Unpolished material surfaces can accumulate more dental biofilm than polished ones because the contact area increases, and depressions can protect bacteria against shear forces [21, 25, 27]. Therefore, in our research, the tested materials were unpolished (as in clinical situations with temporary restorations), which could influence biofilm accumulation.
To improve the mechanical properties of HV-GIC, monomers and initiators that trigger polymerization when light-cured were added to the material composition [26, 28]. However, the RM-GIC set by dual mechanisms has the disadvantages of increased water sorption and contraction, less stability in the physical properties, and a lower release of fluoride when compared to HV-GIC [28, 29]. However, RM-GIC is showed comparable activity to HV-GIC.
During the microbiological analysis, the total microorganism counts showed no significant differences among the tested materials. However, in the S. mutans and E. faecalis counts, ZOE had the greatest sealing ability, which could be explained by eugenol hydrolysis and diffusion capacity [4, 6]. The antibacterial mechanism of eugenol involves a direct interaction between ZnO nanoparticles and cell surfaces, which affects cell membrane permeability, and induces oxidative stress in bacterial cells, resulting in the inhibition of cell growth and eventually cell death [1].
The antibacterial mechanisms of restorative materials justify the lack of correlation between surface biofilm accumulation and bacterial penetration results. The need for the bacteria contact with the restorative material for the antibacterial effect of ZOE can explain the lowest bacterial penetration observed for this material since bacteria have to pass through the material to infect the root canal system. ZOE inhibits biofilm worse probably because the ZnO nanoparticles cannot penetrate and act in biofilm accumulation which differs from the antibacterial activity per release of fluoride of GIC.
Although Enterococcus faecalis is frequently associated with persistent infections [23, 30], this bacterial strain was used due to its ability to live in poor nutrient environments (resistant to antimicrobial agents) and have deep tubular penetration, while Streptococcus mutans was considered for its ability to synthesize extracellular polysaccharides and for being one of the most abundant species of dental biofilm [22].
As the GIC’s temporary materials, ZOE requires mixing the powder and liquid before use, which could influence their abilities [31]. On the other hand, premixed ZO is widely used for its ease of handling [4, 9]. However, in our study, the premixed ZO restorations exhibited the lowest sealing ability. ZO has a high linear expansion coefficient resulting from water sorption, which is important to its sealing ability [4]. Our study used roots with no remaining dental walls, so ZO’s weak adhesion to teeth was expected.
The findings among GIC materials show that, although the acid conditions of biofilms stimulate fluoride release to inhibit biofilm formation, the microbial environment changes the morphology of these materials and accelerates material aging [25, 27]. The colonizing organisms adhere to the degraded material, inducing deterioration [25] and possibly reducing marginal sealing over time. The surface deterioration of GIC-sealed roots could explain the intermediate results in the microbiological analysis and the disparity of these results with the analysis of the biofilm formed onto temporary restorative material.
After 28 days of acid exposure, we found bacterial penetration in all experimental groups, and the amount of microorganisms decreased from the cervical to apical root thirds. Bacteria can pass through root filling within 5–73 days from the coronal to the apical end [31]. A previous study [2] has reported reduced sealing ability over time using temporary materials.
Although this in situ model can reproduce the clinical bacteriological situation, it cannot simulate the mechanical forces of teeth during function and their influence on the performance of the materials. Overall, our investigation demonstrated that glass ionomer and zinc oxide–based cements with eugenol could be used as temporary restorative materials between and after root canal treatment, preventing biofilm formation and root canal contamination. Further clinical studies should correlate the characteristics of the filling materials and bacterial activity of temporary materials after exposure to the oral environment.
Conclusions
None of the temporary filling materials tested prevented biofilm formation or root canal contamination after the highly acidic challenge. However, HV-GIC and RM-GIC had a superior ability to inhibit biofilm accumulation, whereas ZOE reduced bacterial penetration into the root canals.
References
Peralta SL, Leles SB, Dutra AL, Guimarães VBDS, Piva E, Lund RG (2018) Evaluation of physical-mechanical properties, antibacterial effect, and cytotoxicity of temporary restorative materials. J Appl Oral Sci 26:e20170562. https://doi.org/10.1590/1678-7757-2017-0562
Srivastava PK, Nagpal A, Setya G, Kumar S, Chaudhary A, Dhanker K (2017) Assessment of coronal leakage of temporary restorations in root canal-treated teeth: an in vitro study. J Contemp Dent Pract 18:126–130. https://doi.org/10.5005/jp-journals-10024-2002
Louzada LM, Arruda-Vasconcelos R, Duque TM, Casarin RCV, Feres M, Gomes BPFA (2020) Clinical investigation of microbial profile and levels of endotoxins and lipoteichoic acid at different phases of the endodontic treatment in teeth with vital pulp and associated periodontal disease. J Endod 46:736–747. https://doi.org/10.1016/j.joen.2020.02.005
Shanmugam S, PradeepKumar AR, Abbott PV, Periasamy R, Velayutham G, Krishnamoorthy S, Mahalakshmi K (2020) Coronal bacterial penetration after 7 days in class II endodontic access cavities restored with two temporary restorations: a randomised clinical trial. Aust Endod J 46:358–364. https://doi.org/10.1111/aej.12415
Porenczuk A, Grzeczkowicz A, Maciejewska I, Gołaś M, Piskorska K, Kolenda A, Gozdowski D, Kopeć-Swoboda E, Granicka L, Olczak-Kowalczyk D (2019) An initial evaluation of cytotoxicity, genotoxicity and antibacterial effectiveness of a disinfection liquid containing silver nanoparticles alone and combined with a glass-ionomer cement and dentin bonding systems. Adv Clin Exp Med 28:75–83. https://doi.org/10.17219/acem/76160
Mushashe AM, Gonzaga CC, Tomazinho PH, da Cunha LF, Leonardi DP, Pissaia JF, Correr GM (2015) Antibacterial effect and physical-mechanical properties of temporary restorative material containing antibacterial agents. Int Sch Res Notices 2015:697197. https://doi.org/10.1155/2015/697197
Djouiai B, Wolf TG (2021) Tooth and temporary filling material fractures caused by Cavit, Cavit W and Coltosol F: an in vitro study. BMC Oral Health 21:74. https://doi.org/10.1186/s12903-021-01431-4
Markose A, Krishnan R, Ramesh M, Singh S (2016) A comparison of the sealing ability of various temporary restorative materials to seal the access cavity: an in vitro study. J Pharm Bioallied Sci 8(Suppl 1):S42–S44. https://doi.org/10.4103/0975-7406.191965
Naseri M, Ahangari Z, Shahbazi Moghadam M, Mohammadian M (2012) Coronal sealing ability of three temporary filling materials. Iran Endod J 7:20–24
Hasegawa T, Takenaka S, Ohsumi T, Ida T, Ohshima H, Terao Y, Naksagoon T, Maeda T, Noiri Y (2020) Effect of a novel glass ionomer cement containing fluoro-zinc-silicate fillers on biofilm formation and dentin ion incorporation. Clin Oral Investig 24:963–970. https://doi.org/10.1007/s00784-019-02991-0
Beach CW, Calhoun JC, Bramwell JD, Hutter JW, Miller GA (1996) Clinical evaluation of bacterial leakage of endodontic temporary filling materials. J Endod 22:459–462. https://doi.org/10.1016/S0099-2399(96)80077-X
Silva-Neto RD, Sousa-Neto MD, Pécora JD, Palma-Dibb RG, Souza-Gabriel AE (2018) Wear profile of canal wall surfaces and bond strength of endodontic sealers after in situ acid challenge. Int Endod J 51:364–374. https://doi.org/10.1111/iej.12858
Souza-Gabriel AE, Turssi CP, Colucci V, Tenuta LM, Serra MC, Corona AS (2015) In situ study of the anticariogenic potential of fluoride varnish combined with CO2 laser on enamel. Arch Oral Biol 60:804–810. https://doi.org/10.1016/j.archoralbio.2015.01.016
Arweiler NB, Netuschil L, Beier D, Grunert S, Heumann C, Altenburger MJ, Sculean A, Nagy K, Al-Ahmad A, Auschill TM (2014) Action of food preservatives on 14-days dental biofilm formation, biofilm vitality and biofilm-derived enamel demineralisation in situ. Clin Oral Investig 18:829–838. https://doi.org/10.1007/s00784-013-1053-9
Abdullah N, Al-Marzooq F, Mohamad S, Abd Rahman N, Chi Ngo H, Perera Samaranayake L (2019) Intraoral appliances for in situ oral biofilm growth: a systematic review. J Oral Microbiol 11:1647757. https://doi.org/10.1080/20002297.2019.1647757
Virtej A, MacKenzie CR, Raab WH, Pfeffer K, Barthel CR (2007) Determination of the performance of various root canal disinfection methods after in situ carriage. J Endod 33:926–929. https://doi.org/10.1016/j.joen.2006.11.025
Barthel CR, Zimmer S, Zilliges S, Schiller R, Göbel UB, Roulet JF (2002) In situ antimicrobial effectiveness of chlorhexidine and calcium hydroxide: gel and paste versus gutta-percha points. J Endod 28:427–430. https://doi.org/10.1097/00004770-200206000-00002
Hass V, de Paula AM, Parreiras S, Gutiérrez MF, Luque-Martinez I, de Paris MT, Bandeca MC, Loguercio AD, Yao X, Wang Y, Reis A (2016) Degradation of dentin-bonded interfaces treated with collagen cross-linking agents in a cariogenic oral environment: an in situ study. J Dent 49:60–67. https://doi.org/10.1016/j.jdent.2016.02.009
Li Y, Carrera C, Chen R, Li J, Lenton P, Rudney JD, Jones RS, Aparicio C (2014) Fok A (2014) Degradation in the dentin-composite interface subjected to multi-species biofilm challenges. Acta Biomater 10:375–383. https://doi.org/10.1016/j.actbio.2013.08.034
Pelozo LL, Silva-Neto RD, de Oliveira LPB, Salvador SL, Corona SAM, Souza-Gabriel AE (2022) Comparison of the methods of disinfection/sterilization of extracted human roots for research purposes. Dent Med Probl 59:381–387. https://doi.org/10.17219/dmp/144762.4
Hu H, Burrow MF, Leung WK (2022) Evaluation of 12-hour in situ bacterial colonization on smooth restorative material surfaces. J Dent 119:104071. https://doi.org/10.1016/j.jdent.2022.104071
Vaknin M, Steinberg D, Featherstone JD, Feuerstein O (2020) Exposure of Streptococcus mutans and Streptococcus sanguinis to blue light in an oral biofilm model. Lasers Med Sci 35:709–718. https://doi.org/10.1007/s10103-019-02903-4
Choi YS, Kim C, Moon JH, Lee JY (2018) Removal and killing of multispecies endodontic biofilms by N-acetylcysteine. Braz J Microbiol 49:184–188. https://doi.org/10.1016/j.bjm.2017.04.003
Contreras-Guerrero P, Ortiz-Magdaleno M, Urcuyo-Alvarado MS, Cepeda-Bravo JA, Leyva-Del Rio D, Pérez-López JE, Romo-Ramírez GF, Sánchez-Vargas LO (2020) Effect of dental restorative materials surface roughness on the in vitro biofilm formation of Streptococcus mutans biofilm. Am J Dent 33:59–63
Padovani G, Fúcio S, Ambrosano G, Sinhoreti M, Puppin-Rontani R (2014) In situ surface biodegradation of restorative materials. Oper Dent 39:349–360. https://doi.org/10.2341/13-089-C
Dias AGA, Magno MB, Delbem ACB, Cunha RF, Maia LC, Pessan JP (2018) Clinical performance of glass ionomer cement and composite resin in class II restorations in primary teeth: a systematic review and meta-analysis. J Dent 73:1–13. https://doi.org/10.1016/j.jdent.2018.04.004
Hao Y, Huang X, Zhou X, Li M, Ren B, Peng X, Cheng L (2018) Influence of dental prosthesis and restorative materials interface on oral biofilms. Int J Mol Sci 19:3157. https://doi.org/10.3390/ijms19103157
Tüzüner T, Dimkov A, Nicholson JW (2019) The effect of antimicrobial additives on the properties of dental glass-ionomer cements: a review. Acta Biomater Odontol Scand 5:9–21. https://doi.org/10.1080/23337931.2018.1539623
Moberg M, Brewster J, Nicholson J, Roberts H (2019) Physical property investigation of contemporary glass ionomer and resin-modified glass ionomer restorative materials. Clin Oral Investig 23:1295–1308. https://doi.org/10.1007/s00784-018-2554-3
Duan M, Sun Q, Fan W, Fan B (2022) Enhanced antibacterial effect against Enterococcus faecalis by silver ions plus Triton X-100 with low concentrations and cytotoxicity. Braz J Microbiol 53:161–169. https://doi.org/10.1007/s42770-021-00643-8
Balkaya H, Topçuoğlu HS, Demirbuga S (2019) The effect of different cavity designs and temporary filling materials on the fracture resistance of upper premolars. J Endod 45:628–633. https://doi.org/10.1016/j.joen.2019.01.010
Acknowledgements
The authors are grateful to all the volunteers for participating in the study. The authors deny any conflicts of interest related to this study.
Funding
This study was supported by CAPES (Coordination for the Improvement of Higher Education Personnel – Brazil).
Author information
Authors and Affiliations
Contributions
Silva-Neto R.D.: conceptualization, methodology, investigation, and data curation. Pelozo L.L.: methodology and writing - original draft. Corona S.A.M.: software, validation, and formal analysis. Salvador S.L.: writing - review and editing and project administration. Sousa-Neto M.D.: visualization and resources. Souza-Gabriel A.E.: supervision, resources, and writing - review and editing. All the authors have contributed significantly and are in agreement with the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Tânia A. Tardelli Gomes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Clinical implications
None of the temporary filling materials can prevent biofilm formation and bacterial penetration in the canals throughout 28 days under acidic challenge. However, GICs have a superior ability to inhibit biofilm accumulation, whereas ZOE reduced bacterial penetration.
Supplementary information
ESM 1
(PDF 216 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva-Neto, R.D., Pelozo, L.L., Corona, S.A. et al. Antibiofilm and antimicrobial activity of temporary filling materials on root canals: an in situ acid challenge. Braz J Microbiol 54, 2781–2789 (2023). https://doi.org/10.1007/s42770-023-01103-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01103-1