Abstract
The microbiota of aquatic animals is heavily influenced by their environment, offering a potential source for biotechnologically relevant microorganisms. In this investigation, bacterial strains from fish and fish products were investigated to determine their antimicrobial effects against fish and food pathogens. Twelve strains, including five Lactococcus, two Enterococcus hirae, two Enterococcus mundtii, and three Latilactobacillus sakei were selected as producing bacteriocin-like substances with antimicrobial properties that were active against a broad spectrum of bacteria, such as Listeria monocytogenes, Staphylococcus aureus, and Pseudomonas aeruginosa. Selected strains were identified via 16S rRNA sequencing. Most strains exhibited sensitivity to eight types of antibiotics (erythromycin, tetracycline, chloramphenicol, vancomycin, fosfomycin, gentamicin, ampicillin, and netilmicin), lacked hemolysin and gelatinase virulence factors, and did not produce histamine. These findings suggest that marine fish may be a promising source of lactic acid bacteria strains with antimicrobial potential for use as biopreservatives in the food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seafood is a significant part of the global economy, with a production of 179 million tonnes in 2018. China is the largest producer of seafood, followed by Indonesia, India, Vietnam, and the United States [1]. The seafood industry has an estimated value of $153 billion in 2018, with a substantial amount of the global catch being processed into products like canned fish, fish meal, and fish oil. Seafood is also a popular food item globally, with an estimated per capita consumption of 20.5 kg in 2018 [2].
Morocco boasts abundant fisheries resources, which is reinforced by the diversity of products available, including pelagic fish like sardines, swordfish, horse mackerel, mackerel, and anchovies, as well as white fish such as hake, pageot, sole, tongue, umbra, and scorpion fish. Cephalopods like octopus, sepia, and cuttlefish, crustaceans including Blackspot seabream and king shrimp, shellfish, seaweed, and echinoderms-urchin are also available [3]. Despite the wealth of fisheries resources in Morocco, there is limited research on the microbiology of fresh or processed seafood, such as semi-preserved anchovies and smoked fish. Similarly, there is a dearth of studies on traditionally processed products, like dried mussels, known as Bouzroug in southern Morocco. These food products, which are rich in water and nutrients, provide an ideal environment for the proliferation of various microorganisms. Fresh foods like raw fish can be vulnerable to contamination by bacterial pathogens, including Staphylococcus aureus, Clostridium perfringens, Bacillus cereus, and Listeria monocytogenes. Additionally, psychrotrophic Pseudomonas spp. may also be present [4].
Lactic acid bacteria (LAB) constitute a group of Gram-positive, catalase-negative, non-spore-forming and non-motile bacteria. These microorganisms have traditionally been recognized as "GRAS: Generally Recognized as Safe" due to their safety profile, and they are known to exhibit antimicrobial activity against various foodborne pathogens and spoilage organisms. However, it is important to note that certain lactic acid bacteria may pose health risks.". This activity stems from the production of several inhibitory compounds, such as organic acids, hydrogen peroxide, diacetyl, and bacteriocins. Several studies have focused on the isolation of LAB from seafood products. For instance, Ishikawa et al. [5] identified strains of the lactic acid genus from seawater and fresh fish, while Matamoros et al. [6] obtained strains from modified atmosphere packaging (MAP) and smoked fish. Balcázar et al. [7] reported the isolation of Lactococcus lactis CLFP 101, Lactobacillus fermentum CLFP 242, and Lactobacillus plantarum CLFP 238 from rainbow trout, while Ahn et al. [8] isolated Weissella cibaria from fermented shrimp.
This study aimed to assess the bacteriocinogenic potential of lactic acid bacteria isolated from fish and fish products and investigate the properties of the selected strains based on criteria for their potential use as food bio-protectives against significant fish related foodborne pathogens.
Materials and methods
Sample collection
Freshwater fish species (n = 50) including Oncorhynchus mykiss (n = 5), Trachinotus ovatus (5), Farfantepenaeus kerathurus (5), Mytilus edulis (5), Gymnocranius griseus (5), Sardina pilchardus (5), Sepia officinalis (5), Merlangius merlangus (5), Mullus (5), and Rastrelliger brachysoma (5) were collected from various locations in Morocco. The fish were carefully placed in clean plastic bags and promptly transported to the laboratory under refrigerated conditions for subsequent analysis. Upon arrival, the fish were stored under appropriate refrigeration conditions and analyzed within 24 h of collection.
Culture conditions
Pathogenic bacteria used in this study were grown on TSB (Biokar Diagnostics, France); Listeria monocytogenes CECT 7467, Listeria monocytogenes CECT 4032, Escherichia coli ATCC 25922, Staphylococcus aureus CECT 976, Pseudomonas aeruginosa CECT 118, Bacillus subtilis DSMZ 6633 (Table 1). Positive control for bacteriocinogenic Enterococcus hirae F420 and Enterococcus faecium F58, were cultured on deMan, Rogosa and Sharpe Agar (MRS) (Biokar Diagnostics, France).
Isolation of lactic acid bacteria and detection of inhibitors
Under aseptic conditions, 10 g of various parts of each fish specimen were removed and suspended in 90 mL of sterile 0.85% saline solution. The suspension was homogenized for 2 min using a Stomacher Equipment (Seward, United Kingdom). Subsequently, 100 µl of serial dilutions were spread on de Man Rogosa and Sharp (MRS) agar and GM17 (Biokar Diagnostics, France) plates, and then anaerobically incubated (using the Oxoid Gas Pack Anaerobic system) at 30°C for 48 h. Following incubation, two sets of agar plates were used to replicate a random sample of several colonies for bacteriocin screening. Six mL of Listeria monocytogenes CECT 7467 overnight cultures were placed on one of the plates, which was used as an indicator strain [9]. The plates were incubated at 37°C, and the presence of zones of inhibition surrounding individual colonies was examined. All strains were kept at 4°C and as frozen stocks in 35% glycerol at -24°C to maintain long-term cultures. The cultures were multiplied twice in their appropriate broth media at 30°C prior to use.
Stability of bacteriocin-like activity in the presence of proteolytic enzymes
The antibacterial activity of cell-free supernatants obtained from an early-stationary-phase culture of isolated strains was tested for sensitivity to enzymes, using Listeria monocytogenes CECT 7467 as the indicator strain. The active supernatants were incubated at 37 °C for 2 h in the presence of 1 mg.ml−1 of proteinase K, dissolved in buffers recommended by the supplier (Qiagen, Germany). Untreated bacteriocin solutions, not treated with enzymes, were used as controls. The remaining activity of the treated and untreated samples was determined using the well-diffusion method [9].
Inhibitory activity spectrum
Two different antagonism assays were conducted following the protocols described by Achemchem et al. [10], namely the spot-on lawn assay and the well diffusion assay. To conduct the primary screening, 5 μl aliquots were extracted from overnight cultures of 720 candidate colonies that had been grown in MRS broth. These aliquots were then spotted onto buffered MRS agar plates and allowed to grow overnight. The plates were then covered with 6 mL of soft agar medium (0.75% agar) that had been pre-seeded with overnight culture indicator strains grown in the proper medium. Bacterial candidates displaying antimicrobial activity were further tested for production in liquid media using the well-diffusion assay. To conduct the cell-free supernatant assay, 100 μl of the supernatant was carefully added to wells (8 mm) containing the appropriate media that had been pre-seeded with 100 μl of the indicator strain. To prevent any potential zone of inhibition arising from acid production, the culture supernatants were adjusted to a pH of 7.0 using 1 N NaOH and subsequently filtered using a 0.45 μm filter. The plates were then incubated at the optimal temperature as specified in the experimental protocol. The diameter of the zone of bacterial growth (5 mm) or the well (8 mm) was measured and used to calculate the inhibition zone size.
Morphological, physiological and biochemical characterization of LAB isolates
The LAB isolates were identified at the generic level using standard biochemical tests. These tests involved Gram-staining, catalase and oxidase assays, as well as an examination of morphological characteristics such as color, texture, and surface growth of the isolates on the plates. Furthermore, additional tests were carried out to assess the gas production from glucose, the ability to grow at different temperatures in MRS broth (10, 40, and 50°C), and in the presence of 6.5% NaCl (Labo Chemie, India), as well as the ability to hydrolyze esculin in the presence of 4% bile (Biokar Diagnostics, France). Additionally, carbohydrate assimilation and fermentation of different compounds were studied using API 50CH and API 20E strips [11].
Molecular identification
In order to identify the LAB isolates that were selected, we conducted further identification using 16S rRNA gene sequencing. The DNA extraction from liquid bacterial cultures is performed using the automated kit "MagPurix Bacterial DNA Extraction Kit" on the platform. The extracted DNA is quantified using Nanodrop 8000. PCR is carried out on the DNA samples to obtain a 1500bp amplicon using universal primers FD1/RP2, following the protocol with MyTaq DNA polymerase kit from Bioline, consisting of 5 × buffer (5 µl), FD1 10µM (1 µl), RP2 10µM (1 µl), Taq DNA polymerase 5U/µl (0.2 µl), DNA (100 ng), and sufficient H2O to make up a total volume of 25 µl. The PCR program is as follows: 95°C for 2 min; (95°C for 30 s, 52°C for 30 s, 72°C for 30 s) for 35 cycles; 72°C for 3 min, using the "Applied Biosystems VERITYTM 2990211635" thermocycler from ABI. The resulting PCR products are then subjected to electrophoresis by migration on a 1% agarose gel with a 1kb molecular weight marker. as described by Kadri et al. [12].The PDR (5'-GTATTACCGCGGCTGCTG-3'), PCF(5'-CTACGGGAGGCAGCAGTGGG-3'), and PEF (5'-CATGGCTGTCGTCAGCTCGT-3') primers were used for sequencing the 16S rRNA gene. After the amplification of the products, purification was performed using the ExoSAP-IT PCR cleanup Kit, manufactured by Applied Biosystems. To carry out the sequence reactions, the Big Dye terminator cycle sequencing kit version 3.1 (Applied Biosystems). After the sequencing reactions, the products were purified using the BigDye® XTerminator™ Purification Kit from Applied Biosystems. The purified products were then loaded onto an ABI 3130xL capillary sequencer, following the manufacturer's instructions. These manipulations are carried out within the Technical Support Units for Scientific Research (UATRS) under the National Center for Scientific and Technical Research (CNRST)-Morocco.
To perform a sequence alignment analysis, we compared the obtained sequences (query) with those in the Gene Bank database using BLAST (Basic Local Alignment Search Tool) available at NCBI website. Phylogenetic analysis was conducted using MEGA v11.0 to display a maximum likelihood phylogenetic tree, using Kimura-2 together in the bootstrap test (500 replicates) [13].
Haemolytic activity
The production capacity of the extracellular protein haemolysin was assessed on BHI agar plates supplemented with 5% sheep's blood. Following the preparation of the inoculum, the isolates were spread onto the surface of BHI-blood agar and incubated for 48 h at 37°C. The activity of the haemolysin protein was confirmed by the presence of different types of halos [14].
Production of histamine
In order to evaluate the production of biogenic amines, we performed the decarboxylase test by spotting LAB isolates onto plates containing Maijala agar medium that had been supplemented with 20 g/l of histidine as a precursor. Positive colonies were identified by the presence of a purple color surrounding them [15].
Proteolytic activity and lipolytic activity
To investigate the potential for hydrolysis of milk casein and lipolytic activity, a small volume of each LAB strain was applied onto specific PCA agar plates (Biokar Diagnostics, France). The PCA agar plates supplemented with 1.5% (v/v) UHT skim milk were used to assess the hydrolysis of milk casein. After 48-h of incubation at 30°C, transparent zones surrounding colonies were observed, indicating the presence of proteolysis [16]. For the evaluation of lipolytic activity, PCA agar plates enriched with 10 g/l Tween 80 were used, and the colonies were observed every day for 24 h for the development of halos, using the methodology described by Elmoslih et al. [17].
Gelatinase activity
To detect the production of gelatinase, gelatine at a concentration of 30 g/l was added to PCA agar plates. The plates were placed at 4°C for five hours after being incubated at 30°C overnight, and any zones of turbidity around the colonies were examined, indicating gelatine hydrolysis [18].
Aggregation assays
The assessment of auto-aggregation capability of certain LAB strains was executed through a reference method, as per Mohd-Zubri et al. [19]. The absorbance of the suspension was measured at 600 nm and the auto-aggregation percentage of each strain was calculated as follow:
where At represents the absorbance at time point (t = 24 h) and A0 represents the absorbance at t = 0.
The coaggregation assay was conducted following the protocol described by Mohd-Zubri et al. [19]. The cell suspensions were incubated at room temperature for 24 h and samples were taken at (t = 24h). The absorbance of the suspension was measured at 600 nm and the percentage of coaggregation was calculated using the following formula:
where Alab + Apat represents the initial absorbance of the mixture of LAB strain and pathogen, while the variable Amix indicates the absorbance of the same mixture at the sampling time.
Cross-antibacterial activity among selected strains
To investigate the potential cross-antibacterial activity among selected strains, a cross-streak method was employed following the protocol of Guo et al. [20]. The isolates were streaked perpendicular and across from one another on MRS agar plates. The plates were then incubated at 37°C under anaerobic conditions for 48 h to observe any antagonistic interactions between the isolates.
Antimicrobial susceptibility assay
The Kirby-Bauer disc diffusion method was employed to determine the antibiotic susceptibility of the tested strains, following the protocol described by Govindaraj et al. [21]. The antibiotics used in this study included streptomycin (25 µg), setilmicin (30 µg), panamycin (30 µg), ciprofloxacin (5 µg), penicillin (10 units), erythromycin (15 µg), tetracycline (30 µg), chloramphenicol (30 µg), vancomycin (30 µg), fosfomycin (200 µg), gentamicin (10 µg), and ampicillin (20µg). The isolate of interest was swabbed onto Mueller–Hinton agar, and antibiotic discs were placed on the agar before incubation at 37°C for 24 h. The diameter of the inhibition zone surrounding each disc was measured and susceptibility was classified in accordance with The European Committee on Antimicrobial Susceptibility Testing guidelines.
Statistical analysis
All experiments were realized in duplicate. The mean ± standard deviations (SD) were used to present the data. One-way analysis of variance (ANOVA) was performed using IBM SPSS Statistics 26 software (IBM, Chicago, IL, USA). Differences were assessed by Tukey's test at p < 0.05.
Results
Isolation of marine lactic acid bacteria and screening for antibacterial activity
Marine fish are known to harbor diverse bacterial populations that can vary significantly depending on the fish species, geographic location, and environmental conditions. In this study, we quantified the abundance of marine bacteria in fish and assessed their potential as a source of antimicrobial agents against pathogenic bacteria.
The results showed that the average count of marine bacteria in fish was 5,75 log CFU/g, with a wide range of variability observed between samples (ranging from 4.14 to 7.86 log CFU/g) due to differences in the fish's collection sites and origins (Table 2). To investigate the antimicrobial potential of these marine bacteria, we isolated a total of 720 strains from 50 fish samples and screened them for antagonistic activity against two common pathogenic bacteria; L. monocytogenes CECT 7467 and S. aureus CECT 976, using the double-layer agar method. Of the tested strains, 34 exhibited inhibitory effects against at least one of these bacterial pathogens.
Preliminary identification of isolates
The bacterial isolates were subjected to oxidase, catalase, and Gram staining tests, revealing that 29 of the isolates belonged to the LAB group, as evidenced by their Gram-positive, catalase-negative, and oxidase-negative properties. Morphological analysis further revealed that 6 of the isolates exhibited bacillary form, while the remaining isolates were coccoid in shape. These results are presented in Table 3.
Screening for antimicrobial activity on liquid medium
Antimicrobial activity of the bacterial isolates was evaluated in liquid medium against pathogenic microorganisms, including L. monocytogenes, E. coli, and S. aureus. The findings revealed that among the tested isolates, only 9 coccoid-form isolates exhibited a positive effect against at least one of the pathogenic microorganisms, while a three bacillary-form isolate exhibited a positive effect against S. aureus. Notably, in tests against the identified indicator bacteria, only 12 strains demonstrated a discernible unambiguous zone of inhibition.
Stability of bacteriocin activity against proteolytic enzymes
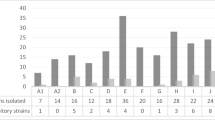
From the obtained results, we observed the complete disappearance of antimicrobial activity in the presence of proteinase K for the supernatants of strains AE13, AE14, AE21, AE24, AE72, AE89, AE97, AE99, AE113, and AE127. These results indicate that the antimicrobial activity of these strains is due to proteinaceous substances with bacteriocin-like inhibitory activity (Fig. 1).
Results of biochemical and molecular identification of isolated lactic acid bacteria
The phenotypic identification using API 50CH and API 20 STREP galleries was conducted on 12 LAB with the highest antagonistic activity. The results of the fermentation test were positive for 99.9% of the strains, and they were identified as Enterococcus mundtii, Lactococcus lactis, and Latilactobacillus sakei. These results were further confirmed by 16s rRNA sequencing, which showed a similarity to the strains deposited in the GenBank DNA database (Fig. 2). Enterococcus hirae (AE13, and AE14) isolated from King prawn (Farfantepenaeus kerathurus), Enterococcus mundtii (AE21, AE24) isolated from Sepia (Sepia officinalis), Lactococcus lactis (AE72, AE89, AE97, AE99) isolated from Mackerel (Rastrelliger brachysoma), Red mullet (Mullus), and whiting fish (Merlangius merlangus), Lactococcus cremoris (AE113) isolated from Palomino fish (Trachinotus ovatus), and Latilactobacillus sakei (AE126, AE127, AE128) isolated from Gray sea bream (Gymnocranius griseus), the accession numbers of these strains are shown in Table 4.
Determination of the antibacterial spectrum of the chosen producing strains
Table 5 presents the inhibitory spectra of the isolates obtained from fish. These strains exhibited strong inhibitory activity against a wide range of Gram-positive bacteria, including food-borne pathogens such as L. monocytogenes CECT (7467 and 4032), S. aureus CECT 976, and P. aeruginosa CECT 118, as well as other bacteria including E. coli ATCC 25922, and B. subtilis DSMZ 6633, and the well-known enterocin-producing enterococci: E. faecium F58 and E. hirae F420. Additionally, the tested strains showed antagonistic activity against a Gram-negative bacterium, E. coli K12. The inhibitory spectra of these isolates in a liquid medium are shown in Table 6, which also demonstrated strong inhibitory activity against a variety of Gram-positive bacteria, including the previously mentioned food-borne diseases and other bacteria.
Cross-activity between selected strains
Based on the cross-activity study of the isolates, it can be concluded that E. hirae AE13 and AE14, as well as E. mundtii AE21 and AE24, have similar activity spectra and were isolated from the same sample, suggesting that they are likely the same strains. Furthermore, the study also showed that isolates AE13 and AE14 exhibit a strong inhibitory activity against isolates AE21, and AE24, with inhibition diameters of 25 mm, and 21 mm, respectively. Additionally, specie Latilactobacillus sakei AE127 demonstrated strong activity against most of the tested isolates, with inhibition diameters ranging between 3 and 18 mm (Table 7).
Safety characteristics of selected LAB
Haemolytic and gelatinase activities and histamine production
No haemolysis or gelatinase activity was observed in any of the LAB isolates when cultured on blood agar and nutrient gelatin agar media, as evidenced by the absence of clear halo zones. Additionally, when tested for the decarboxylation of the amino acid histidine, these isolates did not produce histamine.
Antibiotic susceptibility test
Table 8 presents the findings of antibiotic susceptibility testing conducted on the selected isolates in the current study. A total of twelve antibiotics were evaluated, and the results indicated that the majority of the isolates were highly susceptible (S) to eight of the antibiotics (erythromycin, tetracycline, chloramphenicol, vancomycin, fosfomycin, gentamicin, ampicillin, netilmicin). However, specie AE126 demonstrated resistance (R) to ciprofloxacin, vancomycin, and fosfomycin, while also displaying moderate susceptibility (MS) to kanamycin, tetracycline, and chloramphenicol. Meanwhile, specie AE89, AE97, and AE113 exhibited only moderately susceptible (MS) to ciprofloxacin. These results suggest a varied susceptibility pattern among the tested species, with some displaying resistance or lower susceptibility to certain antibiotics.
Technology characteristics
Proteolytic and lipolytic activities, and gas production
The ability of isolated strains to produce gas, and lipolytic activity were not detected in any of the strains tested. However, all producing strains displayed proteolytic activity.
Auto-aggregation and co-aggregation
The ability of LAB isolates to auto-aggregate and co-aggregate was evaluated in this study. The auto-aggregation ability of the isolates ranged from 32.28 ± 0.15% to 51.32 ± 0.15%, with isolates AE14 and AE99 showing the highest (51,32 ± 0.15%) and lowest values (32,28 ± 0.15%), respectively (Fig. 3). These results indicate that selected LAB species/strains possess good auto-aggregation ability, a crucial property for the formation of biofilms. Co-aggregation ability, which is an essential host defense mechanism against the colonization of pathogenic microorganisms in the gastrointestinal tract don’t use abbreviations for the first time cited in the text tract, was assessed by measuring the average values for selected isolates with L. monocytogenes. The co-aggregation values for L. monocytogenes ranged from 34.26 ± 0.22% to 46.65 ± 2.84%, with significant differences (p < 0.05) among the tested isolates (Fig. 4).
Discussion
In this study, a pool of bacterial isolates from fish products was obtained and characterized for their inhibitory activity against pathogenic bacteria. Among the screened bacterial isolates (n = 720), we found that 1.67% (12 isolates) displayed antagonistic activity against one of the tested pathogenic bacteria. These findings support previous studies suggesting that the antagonism between endogenous microorganisms of fish and bacterial pathogens is a natural occurrence, and that the establishment of a normal or protective microbiota may be crucial for the defensive barrier function [7, 22].
Notably, several LAB strains isolated from fish and aquatic animals have been shown to exhibit antagonistic activity against fish pathogenic agents [7, 22, 23]. In particular, previous studies have reported the antagonistic effects of various bacterial strains against specific pathogens. For instance, Sarika et al. [24, 25] demonstrated the antagonistic effects of L. lactis PSY2 isolated from marine perch fish (Perca flavescens) against L. monocytogenes. Indira et al. [26] showed that L. fermentum isolated from Gray mullet (gut) and prawn (Penaeus monodon) (muscle) exhibited antagonistic effects against E. coli. Annamalai et al. [27] reported the antagonistic effects of E. faecium isolated from mangrove against L. monocytogenes. Pinto et al. [28] demonstrated the antagonistic effects of E. faecium isolated from marine shellfish against L. monocytogenes.
Additionally, various other bacterial strains have been reported to exhibit antagonistic effects against other pathogens, including L. casei AP8 isolated from Persian sturgeon (Acipenser persicus) (gut) [29], L. brevis FPTLB3 isolated from Mrigala (Cirrhinus mrigala) [30], L. acidophilus isolated from gut of marine prawn (Penaeus monodon) [31], L. lactis isolated from marine sediments (Chennai Harbor, India) [32], L. murinus AU06 isolated from marine sediments [33], and L. fermentum strain SBS001 from estuarine water [34]. These bacterial strains were found to exhibit antagonistic effects against various pathogens, including E. coli, S. aureus, B. subtilis. These findings suggest that the use of bacterial isolates as potential bioprotective could be a promising approach for controlling fish diseases caused by bacterial pathogens.
The auto-aggregation and co-aggregation abilities of LAB strains are essential for their survival and persistence in various environments, particularly in the gut. Auto-aggregation facilitates the formation of biofilms, which are necessary for the colonization and adhesion of LAB strains to intestinal epithelial cells. The ability of LAB strains to co-aggregate with pathogenic microorganisms in the gut, such as L. monocytogenes, is a desirable trait for the development of functional foods containing probiotics. Our results demonstrate that the LAB isolates evaluated in this study possess good auto-aggregation and co-aggregation abilities, which are desirable properties for probiotics. These findings are consistent with previous studies that have reported on the importance of auto-aggregation and co-aggregation abilities in the selection of probiotic LAB strains [35,36,37]. Further research is necessary to explore the potential applications of these LAB strains in the development of functional foods with health benefits.
Conclusion
Marine fish are found to have diverse bacterial populations that have the potential to be a source of antimicrobial agents. The results of the present study showed that only 12 strains of bacteria exhibited inhibitory effects against pathogenic bacteria. The selected strains were identified through biochemical and molecular tests and were found to belong to the following LAB species: E. hirae, E. mundtii, L. lactis, and L. sakei. These strains were found to be effective against a wide range of Gram-positive bacteria, including food-borne pathogens such as L. monocytogenes CECT 7467, S. aureus CECT 976, and P. aeruginosa CECT 118, as well as other bacteria. Additionally, these strains did not show any hemolytic or hemolytic or gelatinase activity, nor did they produce histamine. The study provides useful insights into the potential of marine bacteria as a source of antimicrobial/or probiotics agents for future studies.
Data Availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
FAO (2020) Consumption of Fish and Fishery Products. Available from: http://www.fao.org/documents/card/en/c/ca9229en. Accessed 24 Apr 2023
World-Bank (2014) Fish to 2030: Prospects for Fisheries and Aquaculture. Available from: http://documents.worldbank.org/curated/en/458631468152376668/Fish-to-2030-prospects-for-fisheries-and-aquaculture. Accessed 24 Apr 2023
ONP (Maroc) (2023) Rapport Statistiques P. maritime, Editor. http://www.onp.ma/wp-content/uploads/2023/03/RAPPORT-STATISTIQUE-FEVRIER-2023.pdf. Accessed 24 Apr 2023
Elbashir S, Parveen S, Schwarz J et al (2018) Seafood pathogens and information on antimicrobial resistance: A review. Food Microbiol 70:85–93. https://doi.org/10.1016/j.fm.2017.09.011
Ishikawa M, Tanasupawat S, Nakajima K et al (2009) Alkalibacteriumthalassium sp. nov., Alkalibacteriumpelagium sp. nov., Alkalibacteriumputridalgicola sp. nov. and Alkalibacteriumkapii sp. nov., slightly halophilic and alkaliphilic marine lactic acid bacteria isolated from marine organisms and salted foods collected in Japan and Thailand. IJSEM 59(5):1215–1226. https://doi.org/10.1099/ijs.0.65602-0
Matamoros S, Pilet MF, Gigout F et al (2009) Selection and evaluation of seafood-borne psychrotrophic lactic acid bacteria as inhibitors of pathogenic and spoilage bacteria. Food Microbiol 26(6):638–644. https://doi.org/10.1016/j.fm.2009.04.011
Balcázar JL, Vendrell D, de Blas I et al (2008) Characterization of probiotic properties of lactic acid bacteria isolated from intestinal microbiota of fish. Aquac 278(1–4):188–191. https://doi.org/10.1016/j.aquaculture.2008.03.014
Ahn B-K, Min K-C, Cho S-H et al (2021) Isolation of Lactic Acid Bacteria with Anti-MRSA Bacteriocin Activity and Characterization of the Bacteriocin Product. Kor J Microbiol Biotechnol 49(2):131–137. https://doi.org/10.48022/mbl.2012.12008
Achemchem F, Martínez-Bueno M, Abrini J et al (2005) <i>Enterococcus faecium</i> F58, a bacteriocinogenic strain naturally occurring in Jben, a soft, farmhouse goat’s cheese made in Morocco. J Appl Microbiol 99(1):141–150. https://doi.org/10.1111/j.1365-2672.2005.02586.x
Achemchem F, Cebrián R, Abrini J et al (2012) Antimicrobial characterization and safety aspects of the bacteriocinogenic Enterococcus hirae F420 isolated from Moroccan raw goat milk. Can J Microbiol 58(5):596–604. https://doi.org/10.1139/w2012-027
Ennahar S, Cai Y, Fujita Y (2003) Phylogenetic Diversity of Lactic Acid Bacteria Associated with Paddy Rice Silage as Determined by 16S Ribosomal DNA Analysis. Appl Environ Microbiol 69(1):444–451. https://doi.org/10.1128/AEM.69.1.444-451.2003
Kadri Z, Amar M, Ouadghiri M et al (2014) Streptococcus moroccensis sp. nov. and Streptococcus rifensis sp. nov., isolated from raw camel milk. IJSEM 64(7):2480–2485. https://doi.org/10.1099/ijs.0.062893-0
Gupta S, Mohanty U, Majumdar RK (2021) Isolation and characterization of lactic acid bacteria from traditional fermented fish product Shidal of India with reference to their probiotic potential. LWT 146:111641. https://doi.org/10.1016/j.lwt.2021.111641
Khay EO, Idaomar M, El Moussaoui N et al (2014) Application of a bacteriocin-like inhibitory substance producing Enterococcus durans E204 strain, isolated from camel milk, to control Listeria monocytogenes CECT 4032 in goat jben. Ann Microbiol 64(1):313–319. https://doi.org/10.1007/s13213-013-0666-1
Marcobal A, de las Rivas B, Muñoz R (2006) Methods for the Detection of Bacteria Producing Biogenic Amines on Foods: A Survey. J Verbrauch Lebensm 1(3):187–196. https://doi.org/10.1007/s00003-006-0035-0
Belgacem ZB, Abriouel H, Omar NB et al (2010) Antimicrobial activity, safety aspects, and some technological properties of bacteriocinogenic Enterococcus faecium from artisanal Tunisian fermented meat. Food Control 21(4):462–470. https://doi.org/10.1016/j.foodcont.2009.07.007
Elmoslih A, Zanzan M, Aissa R et al (2017) Isolation and Characterization of Bacteriocinogenic Enterococcal and Lactococcal Strains from South of Morocco Dairy Product. BJI 18(4):1–16. https://doi.org/10.9734/BJI/2017/32919
Rivas FP, Castro MP, Vallejo M et al (2012) Antibacterial potential of Enterococcus faecium strains isolated from ewes’ milk and cheese. LWT Food Sci Technol 46(2):428–436. https://doi.org/10.1016/j.lwt.2011.12.005
Mohd-Zubri NS, Ramasamy K, Abdul-Rahman NZ (2022) Characterization and potential oral probiotic properties of Lactobacillus plantarum FT 12 and Lactobacillus brevis FT 6 isolated from Malaysian fermented food. Arch Oral Biol 143:105515. https://doi.org/10.1016/j.archoralbio.2022.105515
Guo X-H, Kim J-M, Nam H-M et al (2010) Screening lactic acid bacteria from swine origins for multistrain probiotics based on in vitro functional properties. Anaerobe 16(4):321–326. https://doi.org/10.1016/j.anaerobe.2010.03.006
Govindaraj K, Samayanpaulraj V, Narayanadoss V et al (2021) Isolation of Lactic Acid Bacteria from Intestine of Freshwater Fishes and Elucidation of Probiotic Potential for Aquaculture Application. Probiotics Antimicro Prot 13(6):1598–1610. https://doi.org/10.1007/s12602-021-09811-6
Pérez-Sánchez T, Balcázar JL, García Y et al (2011) Identification and characterization of lactic acid bacteria isolated from rainbow trout, Oncorhynchus mykiss (Walbaum), with inhibitory activity against Lactococcus garvieae. J Fish Dis 34(7):499–507. https://doi.org/10.1111/j.1365-2761.2011.01260.x
Ringø E, Gatesoupe F-J (1998) Lactic acid bacteria in fish: a review. Aquac 160(3–4):177–203. https://doi.org/10.1016/S0044-8486(97)00299-8
Sarika AR, Lipton AP, Aishwarya MS et al (2012) Isolation of a bacteriocin-producing lactococcus lactis and application of its bacteriocin to manage spoilage bacteria in high-value marine fish under different storage temperatures. Appl Biochem Biotechnol 167(5):1280–1289. https://doi.org/10.1007/s12010-012-9701-0
Sarika AR, Lipton AP, Aishwarya MS et al (2018) Lactic Acid Bacteria from Marine Fish: Antimicrobial Resistance and Production of Bacteriocin Effective Against L. monocytogenes In Situ. JFSH 03:1–10. https://doi.org/10.4172/2476-2059.1000128
Indira K, Jayalakshmi S, Gopalakrishnan A et al (2011) Biopreservative potential of marine lactobacillus spp. Afr J Microbiol Res 5(16):2287–2296. https://doi.org/10.5897/AJMR11.613
Annamalai N et al (2009) Enterocin from Enterococcus faecium isolated from mangrove environment. Afr J Biotechnol 8(22):6311–6316. https://doi.org/10.5897/AJB2009.000-9478
Pinto AL, Fernandes M, Pinto C et al (2009) Characterization of anti-Listeria bacteriocins isolated from shellfish: potential antimicrobials to control non-fermented seafood. Int J Food Microbiol 129(1):50–58. https://doi.org/10.1016/j.ijfoodmicro.2008.11.005
Ghanbari M, Jami M, Kneifel W et al (2013) Antimicrobial activity and partial characterization of bacteriocins produced by lactobacilli isolated from Sturgeon fish. Food Control 32(2):379–385. https://doi.org/10.1016/j.foodcont.2012.12.024
Banerjee SP, Dora KC, Chowdhury S (2013) Detection, partial purification and characterization of bacteriocin produced by Lactobacillus brevis FPTLB3 isolated from freshwater fish: Bacteriocin from Lb. brevis FPTLB3. J Food Sci Technol 50(1):17–25. https://doi.org/10.1007/s13197-011-0240-4
Karthikeyan V, Santhosh SW (2009) Study of Bacteriocin as a Food Preservative and the L. acidophilus Strain as Probiotic. Pak J Nutr 8:335–340
Rajaram G, Manivasagan P, Thilagavathi B et al (2010) Purification and Characterization of a Bacteriocin Produced by Lactobacillus lactis Isolated from Marine Environment. Adv J Food Sci Technol 2:138–144. http://maxwellsci.com/print/ajfst/v2-138-144.pdf. Accessed April 24, 2023.
Elayaraja S, Annamalai N, Mayavu P et al (2014) Production, purification and characterization of bacteriocin from Lactobacillus murinus AU06 and its broad antibacterial spectrum. Asian Pac J Trop Biomed 4:S305–S311. https://doi.org/10.12980/APJTB.4.2014C537
Singh R, Sivasubramani K, Jayalakshmi S et al (2013) Isolation and production of bacteriocin by marine Lactobacillus fermentum SBS001. Int J Curr Microbiol Appl Sci (IJCMAS) 2(4):67–73. https://www.ijcmas.com/Archives/vol-2-4/Rajesh%20Singh%20et%20al.pdf. Accessed 24 Apr 2023.
Ahmadova A, Todorov SD, Hadji-Sfaxi I et al (2013) Antimicrobial and antifungal activities of Lactobacillus curvatus strain isolated from homemade Azerbaijani cheese. Anaerobe 20:42–49. https://doi.org/10.1016/j.anaerobe.2013.01.003
Collado MC, Meriluoto J, Salminen S (2008) Adhesion and aggregation properties of probiotic and pathogen strains. Eur Food Res Technol 226(5):1065–1073. https://doi.org/10.1007/s00217-007-0632-x
Nami Y, Vaseghi Bakhshayesh R, Mohammadzadeh Jalaly H et al (2019) Probiotic properties of Enterococcus isolated from artisanal dairy products. Front Microbiol 10:300. https://doi.org/10.3389/fmicb.2019.00300
Acknowledgements
The authors would like to thank the National Centre for Scientific and Technical Research (CNRST) of Morocco for providing them with the technical facilities of the UATRS division.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest related to this publication, and no significant financial support has influenced its outcome.
Additional information
Responsible Editor: Luis Augusto Nero
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elidrissi, A., Ezzaky, Y., Boussif, K. et al. Isolation and characterization of bioprotective lactic acid bacteria from Moroccan fish and seafood. Braz J Microbiol 54, 2117–2127 (2023). https://doi.org/10.1007/s42770-023-01077-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01077-0