Abstract
Blast fungus (Magnaporthe oryzae B.C. Couch) is an imminent threat to global food security because it causes serious yield losses in rice (Oryza sativa L.) and wheat (Triticum aestivum L.). The investigation of infection processes in resistant and susceptible varieties, as well as the cellular responses involved in resistance, can help us to better understand the process of interaction of the M. oryzae-Poaceae pathosystems. Thus, the objectives of this study were to evaluate the processes of pre- and post-infection of M. oryzae in leaves of wheat varieties with different levels of resistance. The percentage of germinated conidia, appressorium formed, tissue penetration and colonization, and the reaction of leaf tissue to infection were evaluated. A decrease was observed in the percentage of germinated conidia, appressorium formation, tissue penetration and colonization, especially in the tissues of resistant varieties, in addition to an increase in the plant’s response to infection, with cell wall reinforcement, cell death, and autofluorescent cytoplasm aggregation. Nevertheless, our data produced a different temporal perspective regarding the expression of the known types of resistance. We found that, for a single genotype, recognition can start as early as 6 h after inoculation and continue to evolve until very late during the infection cycle, culminating in cell death. The combined and overlapping pre- and post-haustorial resistance mechanisms were sufficient to prevent disease symptoms, with a few punctual lesions observed in one of the resistant varieties (BR 18) and no visible symptoms in the other two (Ônix or BRS229) as opposed to susceptible variety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fungus Magnaporthe oryzae B.C. Couch is an imminent threat to global food security. It causes serious disease that imposes yield losses on rice (Oryza sativa L.) and wheat (Triticum aestivum L.), the two more important crops in terms of sources of calories and protein for a major part of the world’s population, thereby constituting a threat to global food security [14, 39]. In wheat, M. oryzae causes the disease called blast or “brusone” that first emerged in Brazil in 1985 [20] and spread during subsequent decades into neighboring countries in South America [5, 29, 41]. Depending on the host specificity, the pathogen has been classified in subpopulations within the M. oryzae species, with pathotypes named according to the preferential host [10].
Increasing concern for a worldwide dissemination of the pathogen has been underscored since the first outbreak outside South America was reported in Bangladesh [25]. The capacity for long-distance of genotype flow combined with a mixed reproductive system place M. oryzae in the category of pathogens with the highest evolutionary potential [24].
Most wheat varieties available in Brazil are highly susceptible to disease [12]. We believe that many wheat cultivars in various parts of the world are also susceptible to disease due to the aggressiveness of the pathogen. In addition to the scarcity of sources of resistance, breeding resistant varieties remains a challenge because M. oryzae also infects barley (Hordeum vulgare L.), rye (Secale cereale L.), triticale (× Triticosecale Wittmack), and a wide range of plants within the Poaceae family [24].
Because little is known about the cellular responses involved in resistance within the pathosystems of M. oryzae × wheat, its understanding may help wheat breeding programs to define microphenotypes to select more effective and durable forms of resistance.
The implantation of resistant material is the most efficient and viable method to control M. oryzae in wheat. Some varieties of wheat resistant to blast disease have been identified [12], but the mechanisms that confer resistance to fungal infection have not yet been elucidated in detail, nor the specificity of this interaction, if it follows Flor’s gene-for-gene model or not. The infectious process of M. oryzae and host responses can be investigated through histopathological and histochemical analyses of infected plants of wheat varieties with different levels of resistance. The identification of structures and/or biochemical mechanisms that confer resistance can be important for obtaining new productive and long-term resistant varieties originated from breeding programs.
The Brazilian wheat varieties Ônix, BR 18, and BRS 229 were reported by Cunha et al. [12] to be among the few commercial cultivars showing moderate field resistance to M. oryzae, representing invaluable genetic resources for the solution of this puzzle. In this article, we investigated cytological events over time during host resistance in seedlings of these three cultivars infected with one isolate of a M. oryzae Oryza pathotype and compared them with responses observed in the susceptible variety Anahuac, which was previously tested for its response. Our findings help to understand some important mechanisms of resistance in wheat to its relatively new pathogen.
Material and methods
Plant materials and growth
All experiments were conducted in the Department of Fitossanidade of the Federal University of Rio Grande do Sul, south Brazil. Four wheat varieties were used in the study: BR 18, Ônix, BRS 229, and Anahuac. Ten to 12 seeds of each variety were sown in 300-mL plastic pots containing field soil accordingly fertilized as recommended, totaling 10 pots per wheat variety. Plants were grown for 12 to 14 days in a growth chamber at 24 ± 2 °C, 80% relative humidity, and a light and dark cycle of 16 h and 8 h, respectively. When plants reached the five-to-six true leaves stage, a thinning was carried out, remaining four plants per pot.
Fungal inoculum
The isolate Py145 of Magnaporthe oryzae Oryza pathotype (MoO) used in this study was given by the Embrapa Rice and Bean unit, in Santo Antônio de Goiás city, Brazil, which was previously identified based on its morphological and molecular characteristics and stored in dry cellulose filter disc papers [18]. The isolate was originally collected from panicles of rice cultivar SCS 114 Andosan in Nova Veneza county (Santa Catarina State, Brazil—28°38′12″S,49°29′52″W). To grow it, dried cellulose filter disk papers were placed in Petri dishes containing oatmeal agar culture medium (40 g of oat flakes, 15 g agar, 15 g dextrose, and 1 L distilled water + 500 µg/mL of streptomycin) for 7 days in an incubator at 24 ± 1 °C with a photoperiod light and dark of 16 and 8 h, respectively. Hyphal tips were transferred to oatmeal agar and incubated again for 12 days under near-ultraviolet light at 22 °C to induce conidia production. For harvesting of conidia, sterile water containing 1% Tween 20 was added to the plates and the surface of the colony was scraped with a sterile Drigalski loop. The spore suspension was filtered through a double layer of gauze and adjusted to a concentration of 105 conidia mL−1.
Inoculation protocol and incubation conditions
The ten pots containing four plants of each variety were transferred to a growth chamber (model CCP1200, Instalafrio®, Pinhais, PR) at 26 ± 2 °C with a photoperiod light and dark of 16 and 8 h, respectively. The adaxial surface of all the sixth fully expanded leaves was inoculated with 20 mL of a suspension of 105 conidia mL−1. After the inoculation process, the plants were covered with transparent plastic bags for 24 h to maintain high humidity and incubated at 28 °C in the dark. After incubation, the plastic bags were removed, and the temperature in the growth chamber restored to 26 °C.
Symptom’s evaluation
Five and twenty days after inoculation, the disease was scored according to the classification proposed by Valent et al. [40] as follows: type 0, no visible evidence of infection,type 1, uniform dark brown pinpoint lesions without visible centers; type 2, small lesions with distinct tan centers surrounded by a darker brown margin; type 3, small eyespot lesions approximately 2 mm in length with tan centers surrounded by dark brown margins; type 4, intermediate size eyespot lesions, approximately 3–4 mm in length; and type 5, large eyespot lesions that attain the maximum size seen for a particular cultivar. Types 0 and 1 were considered incompatible interactions, because affected tissue cannot produce conidia under high humidity conditions. Types 2, 3, 4, and 5 were considered compatible, because conidia can be produced from such infected tissues under high humidity conditions.
Histological observation
Segments of the inoculated leaves were collected at 6, 12, 18, 24, 36, 48, and 72 h after inoculation (hai) for cytological analysis. From each time evaluated, four wheat leaves of each cultivar were collected. At least 10 segments of 2.5 cm in length were collected for each treatment (variety × collection time). Leaf samples were fixed and bleached, mounted on glass slides, and observed with a fluorescence microscope as described previously [17]. Briefly, samples were fixed for 24 h in 3:1 (v:v) ethanol:dichloromethane containing 0.15% (w/v) trichloroacetic acid, stained by boiling for 5 min in 1:2 (v:v) lactophenol:ethanol containing 0.05% (w/v) trypan blue, and cleared in a 5:2 (w:v) chloral hydrate:water mixture for 24 h. The stained samples were mounted on glass slides with 50% glycerol and analyzed under bright field or phase contrast microscopy using Olympus BX 41 microscope (Olympus Corporation, Tokyo, Japan). To detect autofluorescence, the same samples were dehydrated with ethanol (80% (v:v for 30 min; 90% for 30 min; and 100% twice × 30 min and stained for 5 min with a saturated solution of picric acid in methyl salicylate. Excess picric acid was removed by 15-min clearing in methyl salicylate [17]. Cell wall strengthening was assessed by the accumulation of stain beneath appressoria associated with cell wall appositions as previously reported [46]. The samples were mounted on glass slides containing methyl salicylate under cover slips sealed with nail polish and examined using blue-light epifluorescence microscopy (Olympus BX 41) fitted with a UMWB2 excitation filter set, consisting of a 460- to 490-nm dichroic beamsplitter 500-nm BA520 barrier filter. The percentages of germinated conidia, appressorium formed, tissue penetration, and colony formation were evaluated, as well as the occurrence of events associated with plant resistance including cell death, autofluorescent cytoplasmic aggregation, and cell wall strengthening.
The model of efficiency of infection
To illustrate the differences observed along the infection process, we used a theoretical model, as proposed by Wesp-Guterres et al. [44], based on the assumption that 103 conidia were deposited on the leaves of susceptible and resistant plants. In this model, numbers in each stage were calculated with respect to the previous event and were based on the data collected, starting from spore germination, and following to appressorium differentiation, colony formation, cell death, and colonization.
Experimental design and statistical analysis
Experiments were conducted in a 4 × 7 factorial arrangement of treatments in a completely random design. The number of conidia found in the beginning of infectious process varied according with each treatment (Table 1). The response variables were measured as the percentage of spore germination, appressorium formation, penetration, and colony formation. The occurrence of events associated with plant resistance including cell death (recorded as percentages), cell wall strengthening, and autofluorescent cytoplasm aggregation was also observed. Treatments were compared using the 2 × 2 Fisher’s exact test of independence for pairwise comparisons [22] with the Bonferroni correction for multiple tests [23].
Results
Symptom’s evaluation
Five days after inoculation, Anahuac variety showed typical disease lesions type 4 or 5 and BR 18 showed few pinpoint lesions type 1; the varieties Ônix and BRS229 did not exhibit any visible symptom of the disease (Fig. 1). Disease symptoms evolved in Anahuac with coalescence of lesions recorded at 20 days after inoculations; however, the other three, resistant varieties, remained with their typical initial symptoms.
Histological observation
Conidia germination, appressorium formation, and penetration varied across wheat varieties and evaluated times (Tables 2, 3, and 4; Fig. 3). A progressive increase in the germination of MoO pathotype conidia on the leaf tissues of the four evaluated wheat varieties was observed over 72 h (Table 2). In Anahuac and BR 18, spore germination significantly increased from 6 to 12 hai, and from 12 and 18 hai, when it reached a maximum. In Ônix and BRS229, spore germination was faster, significantly increasing from 6 to 12 hai, when maximum values were reached. However, the greatest difference in the percentage of conidia germination between the varieties was observed at 6 hai. The percentage of conidia germinated on Anahuac was 84%, significantly higher than that of BRS229 (73%) or BR 18 (64%), but it did not differ from the Ônix variety (77%).
At 6 hai, appressoria formation and melanization were low, varying from 15 to 30%, but not differing among the cultivars (Table 3). At 12 hai, appressorium formation increased significantly in all varieties, except for BRS 229 that had the lowest percentage of formed appressorium (17%). At 18 hai, the percentages of appressorium differentiation in Anahuac, BR 18, and Ônix reached 88%, 87%, and 80%, respectively, while in BRS 229, only 38% of germinated conidia showed appressorium formation. Over the evaluation periods, appressorium formation continued increasing on all varieties, peaking at 24 hai on Anahuac (92%) and at 48 hai for BR 18 (96%), Ônix (93%), and BRS 229 (93%).
The penetration rate of MoO hyphae on the leaf tissues varied between the varieties and in relation to the evaluated times (Table 4). At 6 hai, no hyphae penetration was observed in any wheat variety. In the susceptible variety Anahuac, rates of germinated conidia that had differentiated appressoria and succeeded in penetrating leaf tissue were low until 24 hai. The highest percentage of penetration occurred at 36 hai, when they abruptly reached a peak of 30%. In Ônix, the maximum penetration rate (around 26%) occurred at 12, 24, 48, and 72 hai. At 18 and 36 hai, a low percentage of hyphae penetration was observed. In BRS 229 and BR 18, the maximum values of differentiated appressoria whose hyphae successfully penetrated leaf tissues were observed at 24 hai (20%) and 72 hai (27%), respectively.
Colonization, defined as invasion of hyphae into various neighboring cells from the point of penetration below the appressorium, was analyzed only at 48 hai, because this was the only time point at which the event could be most clearly observed. After this time, the necrotrophic growth of the fungus over dead cells turned critical the microscopic observation. The four wheat varieties significantly differed from one another (Table 5). The highest percentage of colony formation was observed in the susceptible variety Anahuac (71%), followed by Ônix (43%), BRS 229 (18%), and BR18 (2%).
A reaction in the leaf tissues to MoO infection was also observed (Table 6 and Figs. 2 and 3). A hypersensitivity (HR) response due to epidermal cell death under appressoria was observed with greater intensity in resistant varieties (Onyx, BRS 229, and BR18) compared to the susceptible (Anahuac). Cell death could be visualized as soon as 24 hai in all four cultivars; however, this response was significantly higher in BR 18 (70%) of cell death associated with melanized appressoria (Table 6). In Ônix (5%) and BRS229 (3%), it was not significantly different from that observed in Anahuac (4%). However, in Ônix and BRS229, this response reached a peak at 48 hai, with 61% and 84% of cell death associated with melanized appressoria, respectively, while in the susceptible variety, it was only 39%. In all four wheat varieties, the observed cell death clearly could be categorized in two types: single that consisted of collapse restricted to only one cell (Fig. 2A and B) and multiple cell death that consisted of cell death spanning several adjacent cells (Fig. 2C and D). In addition to cell death, strengthening of cell wall in the penetration sites of the fungus was observed in resistant varieties BR18, Ônix, and BRS229 (Fig. 2A and C; Fig. 3). In Ônix, in addition to the hypersensitive cell death and cell wall strengthening, infection attempts were also blocked by an additional resistance response that could only be observed at 72 hai. At this time point, 55% of cells undergoing HR also showed dense cytoplasm aggregation (Fig. 2E and G) with intense lemon-yellow coloration under fluorescence light (Fig. 2F and H).
Hypersensitive cell death in four wheat varieties in response to infection of Magnaporthe oryzae Oryza pathotype. Images obtained by phase contrast light microscopy (A–D), brightfield microscopy (E and G), and fluorescence light microscopy (F and H). Variety BR 18 cell death shown at 24 h after inoculation (hai) (A and B); variety BRS 229 with cell death peaked at 48 hai (C and D); and variety Ônix with cell death peaked at 48 hai (E and G) with cytoplasm aggregation visible at 48 and 72 hai (F and H). Images captured using an Olympus BX-41 microscope, 200 × magnification. Con—Conidium; Ap—appressorium; CWS—cell wall strengthening; CAA—cytoplasm aggregation, autofluorescent under UMWB2 (blue excitation filter set), consisting of a 460- to 490-nm dichroic beamsplitter 500-nm BA520 barrier filter
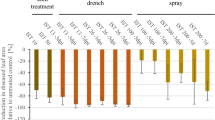
Theoretical model adapted from Wesp-Guterrez et al. [44]of a pre- and post-infection cycle of the Magnaporthe oryzae Oryza pathotype in leaf tissues of resistant wheat varieties BR 18, Ônix, BRS 229 compared to the susceptible variety Anahuac. Based on the results obtained, the bars indicate the subsequent efficiency in the events of infection, starting from an equal number of 1000 conidia landed on leaf surface. Pictures of the infection cycle were adapted from Ribot et al. [31]
The projected numbers of infective events taking place in the studied varieties, starting with a theoretical 1000 conidia landing on a leaf surface, are shown in Fig. 3. Numbers in each stage were calculated with respect to the previous event and were based on the data collected, as displayed in Tables 2 through 6. At each stage of the infection cycle, the efficiency of infection decreased, with BR 18, Ônix, and BRS 229 showing fewer established infection events than those of Anahuac. Although for all varieties the infection process started to be delayed as early as 6 hai, during the germination stage, for BRS 229, most pronounced reductions in infection efficiency were seen during both the appressorium formation and penetration stages, where of the 725 germinated conidia, only 277 succeeded to differentiate appressoria; and of these, only 57 successfully penetrated the leaf epidermal cells. For BR 18 and Ônix, infection was more efficiently delayed during the penetration stage: where of the 560 and 675 melanized appressoria, only 73 and 185 successfully penetrated, respectively.
Discussion
In the present study, we investigated the cytological events occurring in seedling leaves of Brazilian field-resistant wheat varieties in response to one isolate of M. oryzae Oryza pathotype from 6 to 72 hai. The data presented in this paper provide evidence that resistant wheat plants possess a few defense mechanisms against M. oryzae Oryza pathotype, such as inhibitors of germination, infection structure growth, haustorium formation and functioning, physical and chemical barriers. To date, only few studies have demonstrated the pre- and post-infection processes of M. oryzae in wheat and the plant’s reaction mechanisms to its infection.
Among the four varieties studied, the susceptible variety Anahuac also showed a reduction in infection efficiency, especially in the penetration stage; of the 743 conidia that differentiated appressoria, only 226 successfully penetrated; and of these, 136 were able to colonize leaf tissues. This was also observed in wheat leaves inoculated with 1000 urediniospores of Puccinia triticina Erikss. & Henn., in which only 20 urediniospores managed to colonize the susceptible variety, reaching a ratio of only 1000:1 for the partially resistant variety [44].
The type of cellular responses observed in the Brazilian wheat varieties BR 18, Ônix, and BRS229 was similar to those reported previously for many Magnaporthe × Poaceae interactions. Tufan et al. [37] reported that wheat variety Renan exhibited autofluorescence in several cells in the epidermis and mesophyll at 72 hai with a Triticum isolate of M. oryzae, occasionally accompanied of the formation of denser papilla-like structures. Resistance to Digitaria isolates produced no visible macroscopic symptoms, and most infection sites were arrested from 24 hai, with formation of cell wall appositions (autofluorescent halo). Hyphae in first invaded epidermal cell were associated with epidermal and mesophyll fluorescence by 72 hai, occasionally accompanied of the formation of denser papilla-like structures. Our findings regarding the occurrence of single or multiple epidermal cell autofluorescence were similar to those observed in both host and non-host resistance of rice and wheat against M. oryzae isolated from Digitaria, Triticum, and Oryza [3, 13]. So far, the role of autofluorescence response concerning cell resistance, however, has been mainly associated with cell death [21].
Despite that our work has observed resistance expressed on leaves of young plants, it may be of epidemiological significance to reduce epidemics. In another interaction, it has been reported that with the M. oryzae Triticum pathotype (MoT) only 57% of the head reaction could be explained by the seedling reaction [8], and that the reduction of inocula on lower leaves could be a factor contributing to disease management [9]. Mechanisms of cellular resistance responses in the M. oryzae × Poaceae pathosystems, once well characterized, can be an efficient breeding tool for achieving durable, effective forms of disease management. Knowing the mechanisms of resistance of wheat varieties to this pathogen is of significant scientific importance, particularly the pre- and post-infection mechanisms in different genetic materials with different levels of resistance.
Our results suggest that recognition can start in the very early hours after inoculation with a sequence of events occurring in order to prevent infection that continues evolving until very late, culminating in cell death, which would involve combined and overlapping mechanisms of resistance, such as pathogen-triggered immune (PTI), by pathogen or microbe-associated molecular patterns (PAMP or MAMP) that are conserved between species of a microbial group, and that triggered by isolate-specific pathogen effectors (ETI) [32]. The current understanding is that PTI is an important factor in non-host resistance and may contribute to quantitative resistance,ETI forms the basis of qualitative resistance [16, 27].
Although the concepts of qualitative and quantitative resistance often have been presented as a dichotomy, a continuum of scenarios can exist [27, 30] and overlapping mechanisms mediating both non-host resistance and quantitative resistance have been hypothesized [16, 27]. More recently, Yuan et al. [45] propose a revised model in which potentiation of PTI is an indispensable component of ETI during bacterial infection. This model conceptually unites the two major signaling cascades in plants and mechanistically explains some of the long-observed similarities in downstream defense outputs between PTI and ETI. The differences found among the levels of resistance among the varieties BR 18, Ônix, and BRS 229 and the susceptible variety Anahuac occurring sequentially and in all stages of the infection cycle (Fig. 3) may suggest a combined and overlapping PAMP-triggered immunity (PTI) and effector-triggered immunity (ETI). This hypothesis is also in accordance with the results reported by Casassola et al. [6] who analyzed the differential expression of genes involved in adult plant resistance for wheat variety Toropi-P. triticina pathosystem. They found that classical defense genes, including peroxidases, β-1,3-glucanases, and an endochitinases, were expressed both early (pre-haustorial) and late (post-haustorial) over the 72-h infection time course. The response seen in Toropi indicated a possible PTI resistance response at 24 hai, associated with an ETI resistance response, leading to the hypersensitive cell death at 120 hai. The authors pointed out that pre-haustorial leaf rust resistance in adult plants of Toropi is unusual. Phenotypically, it resembles non-host resistance in Arabidopsis to barley powdery mildew (Blumeria graminis DC. Speer f.sp. hordei Marchal) [4, 7] and in barley to non-adapted rust species [48],in these cases, infection is suppressed early by pre-haustorial mechanisms without cell necrosis, with the few haustoria that may elicit a post-haustorial hypersensitivity response.
The combined events related to plant resistance, including cell death (which was faster in BR 18), cell wall strengthening, and autofluorescent cytoplasm aggregation (Fig. 2) contributed to prevent symptoms of infection in the studied varieties. The few pinpoint lesions type 1 were the only symptoms observed in BR 18; however, cultivars Ônix and BRS229 did not exhibit any visible symptom of the disease (Fig. 1). Sherwood and Vance [33] reported that grasses have constitutive and inducible resistance mechanisms associated with the epidermis. The first mechanism restricts the frequency of penetration and the second is correlated with appositional cell wall formation (papillae), a general mechanism of resistance to fungal penetration in the Gramineae. Deposition of papillae at sites of pathogen detection is thought to act as a physical barrier to limit access of pathogens to the underlying protoplast and chemical due to the accumulation of antimicrobial compounds [38]. In addition to two avirulence loci, Pwt1 and Pwt2, conditioning HR and papilla formation, respectively, were postulated in wheat Norin 4 inoculated with hybrid isolates of M. grisea from Setaria and Triticum [26] and inoculated with hybrid Oryza × Triticum isolates of M. oryzae [36].
To date, 10 Rmg blast R genes have been identified in wheat [11, 28, 34, 35, 42, 43, 47]. More recently, Anh et al. [2] suggested that Rmg7 and Rmg8 appear to participate in gene-for-genes (plural) interactions [15], in which one avirulence gene corresponds to more than one resistance gene, and these two resistance genes would be equivalent to a single gene from the viewpoint of resistance breeding. Some varieties were found to carry more than one Rmg gene; for example, Norin 4 and Shin-chunaga carry Rmg1, Rmg4, and Rmg6 [19, 28, 35, 42],Norin 26 carries Rmg1 and Rmg4 [28, 35],and Thatcher carries Rmg2 and Rmg3 [47]. Since both Ônix and BRS229 have Norin 26 and Thatcher in their genetic background (http://www.wheatpedigree.net), it can be hypothesized that one or more Rmg genes identified in these genotypes could be present in the varieties of wheat plants in Brazil. Alternatively, these varieties may carry new R genes, since none of the Rmg genes previously identified in wheat has been found against an avirulent isolate of M. oryzae from rice (MoO pathotype), but from Digitaria sanguinalis (L.) Scop. [28], Lolium perenne L. [42], Avena sativa L. [11, 19, 35], and Triticum aestivum L. [1, 34, 43, 47]. Nevertheless, the resistance response in each variety results from combined mechanisms. Further studies will determine whether Ônix, BR 18, and BRS 229 carry new or already known resistance genes to M. oryzae.
The work presented in this paper, by testing the interaction of MoO on young leaves of wheat varying in their levels of resistance, offers a suitable and helpful model to understand some mechanisms by which plants can reduce infection and, therefore, the epidemic impact of wheat blast. The two most remarkable effects were seen on the rate of successful penetration and on the colonization, although not alone but along with other mechanisms they can be of significance.
References
Anh VL, Anh NT, Tagle AG, Vy TTP, Inoue Y, Takumi S, Chuma I, Tosa Y (2015) Rmg8, a new gene for resistance to Triticum isolates of Pyricularia oryzae in hexaploid wheat. Phytopathology 105:1568–1572. https://doi.org/10.1094/PHYTO-02-15-0034-R
Anh VL, Inoue Y, Asuke S, Vy TTP, Anh NT, Wang S, Chuma I, Tosa Y (2017) Rmg8 and Rmg7, wheat genes for resistance to the wheat blast fungus, recognize the same avirulence gene AVR-Rmg8. Mol Plant Pathol 19:1252–1256. https://doi.org/10.1111/mpp.12609
Araújo L, Soares JM, De Filippi MCC, Rodrigues FA (2016) Cytological aspects of incompatible and compatible interactions between rice, wheat and the blast pathogen Pyricularia oryzae. Sci Agric 73:177–183. https://doi.org/10.1590/0103-9016-2015-0169
Assaad FF, Qiu J, Youngs H, Ehrhardt D, Zimmerli L, Kalde M (2004) The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol Biol Cell 15:5118–5129. https://doi.org/10.1091/mbc.e04-02-0140
Barea G, Toledo J (1996) Identificación y zonificación de piricularia o bruzone (Pyricularia oryzae) en el cultivo del trigo en el dpto. de Santa Cruz. CIAT. Informe Técnico. Proyecto de Investigación Trigo, Santa Cruz, pp 76–86.
Casassola A, Brammer SP, Chaves MS, Martinelli JÁ, Stefanato F, Boyd LA (2015) Changes in gene expression profiles as they relate to the adult plant leaf rust resistance in the wheat cv. Toropi Physiol Mol Plant Pathol 89:49–54. https://doi.org/10.1016/j.pmpp.2014.12.004
Collins NC, Thordal-Christensen H, Lipka V, Bau S, Kombrinck E, Qiu JL, Hückelhoven R, Stein M, Freialdenhoven A, Somerville SC, Schulze-Lefert P (2003) Snare-protein-mediated disease resistance at plant cell wall. Nature 425:973–1007
Cruz CD, Bockus WW, Stack JP, Tang X, Valent B, Pedley KF, Peterson GL (2012) Preliminary assessment of resistance among U.S. wheat cultivars to the Triticum pathotype of Magnaporthe oryzae. Plant Dis 96:1501–1505. https://doi.org/10.1094/PDIS-11-11-0944-RE
Cruz CD, Kiyuna J, Bockus WW, Todd TC, Stack JP, Valent B (2015) Magnaporthe oryzae conidia on basal wheat leaves as a potential source of wheat blast inoculum. Plant Pathol 64:1491–1498. https://doi.org/10.1111/ppa.12414
Cruz CD, Valent B (2017) Wheat blast disease: danger on the move. Trop Plant Pathol 42:210–222. https://doi.org/10.1007/s40858-017-0159-z
Cumagun CJR, Anh VL, Vy TTP, Inoue Y, Asano H, Hyon GS, Chuma I, Tosa Y (2014) Identification of a hidden resistance gene in tetraploid wheat using laboratory strains of Pyricularia oryzae produced by backcrossing. Phytopathology 104:634–640. https://doi.org/10.1094/PHYTO-04-13-0106-R
Cunha GR, Caierão E, Rosa AC (eds) (2016) Informações técnicas para trigo e triticale – safra 2016. Biotrigo Genética, Passo Fundo, RS, Brasil.
Faivre-Rampant O, Thomas J, Allègre M, Morel JB, Tharreau D, Nottéghem JL, Lebrun MH, Schaffrath U, Piffanelli P (2008) Characterization of the model system rice – Magnaporthe for the study of nonhost resistance in cereals. New Phytol 180:899–910. https://doi.org/10.1111/j.1469-8137.2008.02621.x
FAO (2011) Economic and Social Development Department> Food Security Statistics>Data> Food securitydata and definitions>Diet composition> Food consumption pattern of main food items. http://www.fao.org/economic/ess/ess-fs/fs-data/ess-fadata/en/ Accessed 15 June 2011
Feyter RD, Yang Y, Gabriel DW (1993) Gene-for-genes interactions between cotton R genes and Xanthomonas campestris pv. malvacearum avr genes. Mol Plant-Microbe Interact 6:225–237. https://doi.org/10.1094/mpmi-6-225
Gill US, Lee S, Mysore KS (2015) Host versus nonhost resistance: distinct wars with similar arsenals. Phytopathology 105:580–587. https://doi.org/10.1094/PHYTO-11-14-0298-RVW
Graichen FAS, Martinelli JA, de Lima Wesp C, Federizzi LC, Chaves MS (2011) Epidemiological and histological components of crown rust resistance in oat genotypes. Eur J Plant Pathol 131:497–510. https://doi.org/10.1007/s10658-011-9825-z
Gupta DR, Surovy MZ, Mahmud NU, Chakraborty M, Paul SK, Hossain MS, Bhattacharjee P, Mehebub MS, Rani K, Yeasmin R, Rahman M, Islam MT (2020) Suitable methods for isolation, culture, storage and identification of wheat blast fungus Magnaporthe oryzae Triticum pathotype. Phytopathol Res 2:30. https://doi.org/10.1186/s42483-020-00070-x
Hirata K, Tosa Y, Nakayashiki H, Mayama S (2005) Significance of PWT4–Rwt4 interaction in the species specificity of Avena isolates of Magnaporthe oryzae on wheat. J Gen Plant Pathol 71:340. https://doi.org/10.1007/s10327-005-0215-2
Igarashi S, Utiamada CM, Igarashi LC, Kazuma AH, Lopes RS (1986) Pyricularia em trigo. I. Ocorrência de Pyricularia sp. no estado do Paraná. Fitopatol Bras 11:351–352 (Occurrence of Pyricularia sp. in wheat (Triticum aestivum L.) in the State of Paraná, Brazil. Abstract in Portuguese).
Jones K, Kim DW, Park JS, Khang CH (2016) Live-cell fluorescence imaging to investigate the dynamics of plant cell death during infection by the rice blast fungus Magnaporthe oryzae. BMC Plant Biol 16:69. https://doi.org/10.1186/s12870-016-0756-x
McDonald JH (2014) Paired t-test. Handbook of biological statistics. 3rd ed. Sparky House Publishing, Baltimore, MD, USA. Pp. 180–185
MacDonald PL, Gardner RC (2000) Type I error rate comparisons of post hoc procedures for I×J chi-square tables. Educ Psychol Meas 60:735–754. https://doi.org/10.1177/00131640021970871
Maciel JLN, Ceresini PC, Castroagudin VL, Zala M, Kema GHJ, Mcdonald BA (2014) Population structure and pathotype diversity of the wheat blast pathogen Magnaporthe oryzae 25 years after its emergence in Brazil. Phytopathology 104:95–107. https://doi.org/10.1094/PHYTO-11-12-0294-R
Malaker PK, Barma NCD, Tiwari TP, Collis WJ, Duveiller E, Singh PK, Joshi AK, Singh RP, Braun HJ, Peterson GL, Pedley KF, Farman ML, Valent B (2016) First report of wheat blast caused by Magnaporthe oryzae pathotype triticum in Bangladesh. Plant Dis 100:2330–2330
Murakami J, Tosa Y, Kataoka T, Tomita R, Kawasaki J, Chuma I, Sesumi Y, Kusaba M, Nakayashiki H, Mayama S (2000) Analysis of host species specificity of Magnaporthe grisea toward wheat using a genetic cross between isolates from wheat and foxtail millet. Phytopathology 90:1060–1067. https://doi.org/10.1094/PHYTO.2000.90.10.1060
Nelson R, Wiesner-Hanks T, Wisser R, Balint-Kurti P (2018) Navigating complexity to breed disease-resistant crops. Nat Rev Genet 19:21–33. https://doi.org/10.1038/nrg.2017.82
Nga NTT, Hau VTB, Tosa Y (2009) Identification of genes for resistance to a Digitaria isolate of Magnaporthe grisea in common wheat cultivars. Genome 52:801–809. https://doi.org/10.1139/G09-054
Perello A, Martinez L, Molina M (2015) First report of virulence and effects of Magnaporthe oryzae isolates causing wheat blast in Argentina. Plant Dis 99:1177–1178. https://doi.org/10.1094/PDIS-11-14-1182-PDN
Poland JA, Balint-Kurti PJ, Wisser RJ, Pratt RC, Nelson RJ (2009) Shades of gray: the world of quantitative disease resistance. Trends Plant Sci 14:21–29. https://doi.org/10.1016/j.tplants.2008.10.006
Ribot C, Hirsch J, Balzergue S, Tharreau D, Nottéghem JL, Lebrun MH, Morel JB (2008) Susceptibility of rice to the blast fungus, Magnaporthe grisea. J Plant Physiol 165:114–124. https://doi.org/10.1016/j.jplph.2007.06.013
Schulze-Lefert P, Panstruga R (2011) A molecular evolutionary concept connecting nonhost resistance, pathogen host range, and pathogen speciation. Trends Plant Sci 16:117–125. https://doi.org/10.1016/j.tplants.2011.01.001
Sherwood RT, Vance CP (1980) Resistance to fungal penetration in Gramineae. Phytopathology 70:273–279
Tagle AG, Chuma I, Tosa Y (2015) Rmg7, a new gene for resistance to Triticum isolates of Pyricularia oryzae identified in tetraploid wheat. Phytopathology 105:495–499. https://doi.org/10.1094/PHYTO-06-14-0182-R
Takabayashi N, Tosa Y, Oh HS, Mayama S (2002) A gene for gene relationship underlying the species-specific parasitism of Avena/Triticum isolates of Magnaporthe grisea on wheat cultivars. Phytopathology 92(1182):1188. https://doi.org/10.1094/PHYTO.2002.92.11.1182
Tosa Y, Tamba H, Tanaka K, Mayama S (2006) Genetic analysis of host species specificity of Magnaporthe oryzae isolates from rice and wheat. Phytopathology 96:480–484. https://doi.org/10.1094/PHYTO-96-0480
Tufan HA, McGrann GRD, Magusin A, Morel JB, Miche L, Boyd LA (2009) Wheat blast: histopathology and transcriptome reprogramming in response to adapted and nonadapted Magnaporthe isolates. New Phytol 184:476–484. https://doi.org/10.1111/j.1469-8137.2009.02970.x
Underwood W (2012) The plant cell wall: a dynamic barrier against pathogen invasion. Front Plant Sci 3:85. https://doi.org/10.3389/fpls.2012.00085
United Nations (2009) World population prospects: the 2008 revision. United Nations, Department of Economic and Social Affairs, Population Division, New York, USA. CDROM
Valent B, Farrall L, Chumley FG (1991) Magnaporthe grisea genes for pathogenicity and virulence identified through a series of backcrosses. Genetics 127:87–101
Viedma LQ (2005) Wheat blast occurrence in Paraguay. Phytopathology 95:S152
Vy TTP, Hyon GS, Nga NTT, Inoue Y, Chuma I, Tosa Y (2014) Genetic analysis of host-parasite incompatibility between Lolium isolates of Pyricularia oryzae and wheat. J Gen Plant Pathol 80:59–65. https://doi.org/10.1007/s10327-013-0478-y
Wang S, Asuke S, Vy TTP, Inoue Y, Chuma I, Win J, Kato K, Tosa Y (2018) A new resistance gene in combination with Rmg8 confers strong resistance against Triticum isolates of Pyricularia oryzae in a common wheat landrace. Phytopathology 108:1299–1306. https://doi.org/10.1094/PHYTO-12-17-0400-R
Wesp-Guterres C, Martinelli JA, Graichen FAS, Chaves MS (2013) Histopathology of durable adult plant resistance to leaf rust in the Brazilian wheat variety Toropi. Eur J Plant Pathol 137:181–196. https://doi.org/10.1007/s10658-013-0232-5
Yuan M, Jiang Z, Bi G, Nomura K, Liu M, Wang Y, Cai B, Zhou JM, He SY, Xin XF (2021) Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 592(7852):105–109. https://doi.org/10.1038/s41586-021-03316-6
Zellerhoff N, Jansen M, Schaffrath U (2008) Barley Rom1 antagonizes Rar1 function in Magnaporthe oryzae infected leaves by enhancing epidermal and diminishing mesophyll defence. New Phytol 180:702–710. https://doi.org/10.1111/j.1469-8137.2008.02597.x
Zhan SW, Mayama S, Tosa Y (2008) Identification of two genes for resistance to Triticum isolates of Magnaporthe oryzae in wheat. Genome 51:216–221. https://doi.org/10.1139/G07-094
Zhang HS, De La Rosa R, Rubiales D, Lubbers HH, Molenveld JW, Niks RE (1994) Role of partial resistance to Puccinia hordei in barley in the defence of barley to inappropriate rust fungi. Physiol Mol Plant Pathol 45:219–228. https://doi.org/10.1016/S0885-5765(05)80079-7
Acknowledgements
Thanks are given to Dr. John H. McDonald (University of Delaware, USA) for the assistance in the statistical analysis.
Funding
The authors wish to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Empresa Brasileira de Pesquisa Agropecuária (Embrapa- (Project SEG 02.11.08.004.00.00)) for financial support.
Author information
Authors and Affiliations
Contributions
Conceptualization: Chaves, M.S.; Martinelli, J.A.; Barbieri, M. Data acquisition: Barbieri, M. Data analysis: Barbieri, M.; Chaves, M.S.; Silva, G.B.P. Design of methodology: Chaves, M.S.; Graichen, F.A.S. Writing and editing: Barbieri, M.; Chaves, M.S.; Martinelli, J.A.; Silva, G.B.P.; Graichen, F.A.S.; Torres., G.A.M.
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Aleksander Westphal Muniz
Rights and permissions
About this article
Cite this article
Chaves, M.S., Antunes, M.B., da Silva, G.B.P. et al. Pre and post stage of infection of Magnaporthe oryzae Oryza in wheat leaves with different resistance levels. Braz J Microbiol 53, 1091–1100 (2022). https://doi.org/10.1007/s42770-022-00749-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00749-7