Abstract
Newcastle disease (ND) is a highly contagious infection of many avian species, mainly chickens and turkeys, with a devastating impact on worldwide poultry production. This study was designed to examine the effect of virulent ND infection in turkey’s tissues and the tissue tropism of the virus. During the previous study period, poults were inoculated at 32 days of age with 105 EID50 virulent Newcastle disease virus. Three poults on days 0, 1, 2, 3, 4, 6, 7, and 14 postinoculations (PI) were selected from each group. They were euthanized by intravenous sodium pentobarbital injection. After macroscopic observation, to histopathological and immunohistochemical studies, the spleen, bursa, cecal tonsils, intestine, proventriculus, lung, kidney, and brain were sampled. Clinically, the infected turkeys exhibited loss of appetite, severe depression, down on hock joint, white to greenish (sometimes bloody) diarrhea, nervous signs, and mild respiratory problems. Out of 45 birds inoculated, 9 (20%) died. Histopathological effects in lymphoid tissues included necrosis and penetration of mononuclear cells on day 4 PI, and subsequent follicular lymphoid depletion on days 6 and 8 PI was observed. Based on the immunohistochemical test, on day 3 in cecal tonsils and spleen, and on day 8 PI, all of them were positive for virus antigen. In conclusion, the NDV circulating in Iranian chicken flocks has the potential to cause severe illness in commercial turkeys.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Newcastle disease is a contagious viral disease that affects approximately 236 species of domestic and industrial birds, including turkeys. Symptoms of the disease that result from infection with the Newcastle disease virus may vary considerably depending on the virus pathotype. Also, factors such as bird species, age and breed, immune status, environmental and nutritional conditions, and other concurrent infections can affect the severity of the disease. The first occurrence of Newcastle disease in turkey occurred in 1948 [1]. Since then, except the year 1990, its incidence has gradually declined each year. In the year 1999, there was a significant increase in the rate of Newcastle disease outbreaks in turkeys. In addition to type 1 of paramyxovirus, types 2, 3, 6, and 7 are also causative agents of mild respiratory diseases [2]. The sensitivity of turkeys and poultry is similar, but the clinical symptoms are less severe in turkeys. There has been a great deal of research and study on Newcastle disease and its pathogenicity in the world and Iran. Still, the importance of this disease in turkeys has not been studied in our country. We evaluated the virus shedding through turkeys’ respiratory and gastrointestinal tracts by RT-PCR following the experimental infection of turkeys with a velogenic chicken isolate of the Newcastle disease virus [3]. Here, following our previous study, we examined the tissue lesions of turkeys and the tissue tropism of the virus using immunohistochemistry.
Material and methods
During the previous research period [3], the virus with the accession number of NDA: KP347437 was isolated from an infected broiler chicken farm in southwest Iran in 2013 [4]. The virus was inoculated twice in the chorioallantoic membrane of 9-day-old embryonated chicken eggs, and the allantoic fluid was used in this experiment as inoculum. This experiment was done at the Animal Research Unit of Shahid Chamran University of Ahvaz, Iran. Seventy-day-old Wishart bronze unsexed poults were randomly divided into 45 poults as an infected group and 25 poults as control. Birds had access to feed and water was ad libitum. The infected group received 0.1 ml of viral inoculum (105 EID50) through the ocular route at 32 days of age, while the control group received 0.1 ml of PBS (phosphate-buffered saline) by the same route [3].
Sampling
Poults were inoculated at 32 days of age with 105 EID50 acute Newcastle disease virus. On days 0, 1, 2, 3, 4, 6, 7, and 14 PI, three poults from each group were selected and euthanized by intravenous sodium pentobarbital injection [5]. It should be noted that in addition to the days mentioned, birds that died during the experiment were also necropsied and sampled. After the autopsy, the observed lesions were recorded in different tissues. Histopathologic studies were performed on the spleen, bursa of Fabricius, cecal tonsils, intestine, proventriculus, lung, kidney, and brain. Some samples were placed in 10% formalin buffer and transferred to the pathology laboratory of the Faculty of Veterinary Medicine. Other tissues were embedded in aluminum foil and stored at − 70 C until immunohistochemical testing.
Histopathology
Samples of mentioned organs were fixed in 10% formal saline for a minimum of 24 h. They were processed, embedded, cut, and stained with hematoxylin and eosin and studied under a light microscope.
Immunohistochemical examination
Immunohistochemical staining was used to evaluate the presence of Newcastle virus antigen in some tissues. For this purpose, frozen samples were prepared by cryostat and transferred onto chromatin gelatin slides. The temperature of the machine when cutting was − 15 C. Then, it was cooled in acetone for 10 min and then, the slides were placed at room temperature to dry. For washing at all stages, two buffer containers were used, and slides were placed in each container for 5 min. Endogenous peroxidase was quenched by 10% hydrogen peroxide in methanol for 10 min. The sections were incubated with a primary antibody against Newcastle disease virus ribonucleoprotein (isotype IgG2a) (Abcam, England) diluted with PBS (1:250) for 180 min. Secondary antibody was conjugated with HRP (Dako, Denmark), added with a dilution of 1:100 for 45 min in a humid chamber, and then washed. Peroxidase activity was exposed by diaminobenzidine (DAB). Sections were counterstained with hematoxylin and then dehydrated and coverslipped. At each staining step, one slide of samples confirmed by molecular analysis of virus presence was considered a positive control, and one slide was considered a negative control. Negative controls were prepared by substituting the primary antibody with normal rabbit or mouse serum.
Results
Gross findings
Hemorrhages and ulcerations of cecal tonsils were detected as the first necropsy observed at 3rd dpi and intensified on the following days. At four dpi, enlarged, mottled, and brittle spleens were observed (Fig. 1A), whereas they were changed to rounded and shrunken shape at 6th dpi (Fig. 1E). Also, in 6 and 8 dpi, hyperemia and hemorrhage in the proventriculus, cecal tonsils (Fig. 1B), brain (Fig. 1C), pancreas, pectoral muscles, and skin (Fig. 1F) were seen. Also, gizzard was filled with greenish fluid (bile stained). Pale foci were seen over the cortical surface of the kidney and pale appearance due to the deposition of urate (Fig. 1D). Lymphoid organs, thymus, spleen, and bursa Fabricius underwent atrophy (Fig. 1E). Moreover, on the same day, ulcers were visible on proventricular glands and intestinal mucosa, especially in the cecum, which could be visualized through the serosal surface. Furthermore, the lungs were congested and edematous, and accumulation of serous fluid was detected in the tracheas. Turkeys developed varying degrees of air sacculitis at this day. Affected bird’s brains mostly suffered from hyperemia and hemorrhage in eight dpi (Fig. 1G). In 14 dpi, affected birds and their organs, e.g., proventriculus, gizzard (Fig. 1H), liver, and heart (Fig. 1I), were smaller compared to the control group.
Macroscopic pictures of different organs of SPF turkey inoculated with virulent Newcastle disease virus. A Spleen, 4 dpi. Enlarged and mottled spleen. B Cecal tonsils, 6 dpi. Hemorrhages are obvious in the cecum, which could be visualized through serosal surface. C Brain, 6 dpi. Hemorrhage and hyperemia in meninges. D Kidney, 6 dpi. Mosaic shape of kidney and urate deposits in ureter (arrow). E Spleen, 6 dpi. Note the smaller size of spleen compared to the control group (right side). F Pectoral muscle, 6 dpi. Hemorrhage on pectoral muscle and skin. G Brain, 8 dpi. Note hemorrhage and hyperemia in meninges compared to the control group (right side). H Proventriculus, gizzard and intestine, 14 dpi. Note smaller size compared to the control group (left side). I Heart, 14 dpi. Smaller size of heart compared to the control group (left side)
Microscopic findings
Bursa Fabricius
No significant microscopic changes were observed on days 1 and 2 PI compared to the control group. Necrosis of lymphocytes, infiltration of heterophils, and some large cells with eosinophilic inclusions were observed in some follicles on day 3PI. Numerous reticuloendothelial cells were also found in the centers of some lymphatic follicles. The microscopic features of infected bursa at day 6 PI contained the intense depletion of lymphoid follicles, multiple reticuloendothelial cells in their medulla, and large cells with multiple eosinophilic inclusions in the cytoplasm. Eight days after challenge, the lesions were seen on day 6. The proliferation of lymphocyte cells was observed on day 14 after inoculation (Fig. 2).
Microphotographs of the bursa of Fabricius, spleen, and cecal tonsil of SPF turkey inoculated with virulent Newcastle disease virus (H&E). Bursa of Fabricius: Note the shrinkage of the follicles by day 8 and then the appearance of multiple lymph follicles along with some degenerated follicles on day 14. Spleen: Note the degeneration of the white pulp (WP) and red pulp (RP) by day 8 and their reappearance on day 14. Cecal tonsils: Note the emptying of lymph follicles under the mucosa by day 8 and the reappearance of follicles on day 14
Spleen
Two days PI, microscopic observations of the spleen showed white and red pulp structures similar to those in the control group. Then, 5 days PI, macrophages infiltrated into the white pulp and necrosis of some lymphocytes. On day 3 PI, numerous macrophages were observed in the spleen white pulp along with lymphocytes and plasma cells. On day 6 PI, extensive infiltration of multinuclear and mononuclear inflammatory cells was seen in the spleen, which made the red pulp structure indistinguishable from the white pulp. Large foamy macrophages were also found that had eosinophilic inclusions in their cytoplasm. On day 6 PI, the number of erythrocytes in the red pulp also decreased. The dead birds on this day had cells that showed brown pigment in their cytoplasm. In Prussian blue staining, the cells were blue. On day 8, the above lesions on day 6 were more strongly observed. On day 14 PI, the lymphocyte proliferation in the white pulp was clearly marked, and the border between the white and red pulp reappeared. The red pulp was also accumulated by the erythrocytes (Fig. 2). Immunohistochemical examination revealed the presence of the virus as small brown spots. On day 3, these foci were less than day 8 (Fig. 4).
Cecal tonsils and intestine
On day 2 after challenge, hypertension and low hemorrhage were observed along with the infiltration of multinucleated inflammatory cells. On day 3, the lymphocytes in the tonsils showed necrosis with extensive infiltration of heterophils and large cells with eosinophilic cytoplasmic inclusions. Another bird slaughtered on this day had cells containing brown pigments that had accumulated in the lamina propria. On day 6 PI, the cecal tonsils were completely devoid of lymphocytes and filled with foamy macrophages. The mucosa covering the tonsils was also excised (Fig. 3A). In the small intestine, intestinal villi also suffered severe villous atrophy, as well as infiltration of inflammatory cells and cells containing brown pigment in the lamina propria that were seen in Prussian blue staining (Fig. 3A, inset). The lesions observed on day 8 were more severe than those on day 6. On day 14, the formation of small follicles in the cecal tonsils was the most significant change. On days 3 and 8 PI, the presence of the viral antigen was confirmed in the cecal tonsils by immunohistochemical examination.
Microphotographs of different organs of SPF turkey, post-inoculation with virulent Newcastle disease virus. A Intestine, 6 dpi. Note: Atrophy of villous and sloughing the mucosa (arrows) (H&E, bar: 100 µm). Inset: Blue area in the villous (Prussian blue, bar: 100 µm). B Proventriculus, 3 dpi; necrosis of mucosal epithelial cells (asterisk) (H&E, bar: 100 µm). Inset: Eosinophilic intracytoplasmic inclusion bodies (arrows) were observed at the end of the esophagus (H&E, bar: 100 µm). C Proventriculus, 6 dpi. Note to mucosal gland dilatation (asterisks) (H&E, bar: 100 µm). D Lung, 2 dpi; hemorrhages (green asterisk), pink exudates in some parabronchus, and infiltration of granulocytes (white asterisk) were seen (H&E, bar: 100 µm). E Kidney, 6 dpi; infiltration of mononuclear inflammatory cells into the interstitial tissue (asterisk) and urinary tracts (arrows) was observed (H&E, bar: 20 µm). F Cerebellum, 6 dpi; note to hemorrhages in white and gray matter (arrows) (H&E, bar: 100 µm). G Cerebellum, 6 dpi; accumulation of inflammatory cells of lymphocytes and plasma cells around the vessels in the white matter (perivascular cuffing) (arrow) is obvious (H&E, bar: 20 µm). H Medulla oblongata, 6 dpi; note to gliosis (focal accumulation of microglial cells) (asterisk) and spongy white matter that correlates with myelin degradation and axonal swelling (arrows) (H&E, bar: 20 µm). I Cerebrum, 6 dpi; note to perivascular cuffing of lymphocytes and plasma cells (red arrows), diffuse infiltration of inflammatory cells in the gray matter in the cerebrum (black arrow), and increased Virchow–Robin space (asterisk) (H&E, bar: 20 µm)
Proventriculus
The proventriculus of infected turkeys at days 1, 2, and 3 did not show any specific tissue damage compared to the control group. On day 4, necrosis of mucosal epithelial cells with eosinophilic intracellular and cytoplasmic inclusion bodies was observed at the end of the esophagus (the proventriculus junction) (Fig. 3B). Proventriculus mucosal necrosis was found in dead birds on day 6 dpi. Also, mucosal gland dilatation was observed in 6 and 8 dpi (Fig. 3C). On day 14, the gastric structure was normal. In immunohistochemical studies on days 3 and 8 PI, the virus was detected only on day 8 inside the mucosal gland cells (Fig. 4D).
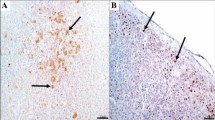
Microphotographs of different organs of SPF turkey, post-inoculation with virulent Newcastle disease virus (immunohistochemical staining with ND virus antibody and counterstain with hematoxylin). A Spleen, 3 dpi; ND virus antigens were observed in the white pulp (bar: 20 µm). B, C Spleen, 8 dpi; ND virus antigens were observed in the white pulp (bar: 20 and 10 µm). D Proventriculus, 8 dpi; Note to the virus existence inside the mucosal gland cells (bar: 10 µm). E Cerebellum, 8 dpi; Note to brown area between granule cells (bar: 10 µm). F Medulla oblongata, 8 dpi; virus accumulation spongy spaces in the white matter and around the swollen axons (bar: 10 µm)
Lung
On day 2 PI, areas of hyperemic with some hemorrhages, infiltration of granulocytes (especially heterophils), and pink exudates were seen in some parabronchus in the lung and continued until day 8 PI (Fig. 3D). On day 14, hyperemic and hemorrhagic and heterophil penetration decreased and partly resulted in partial improvement of lung tissue.
Kidney
All turkeys slaughtered on days 2 to 4 PI showed no specific microscopic lesion. At day 6 PI, infiltration of mononuclear inflammatory cells into the interstitial tissue of the urinary tract was observed (Fig. 3E). On day 8, necrosis of tubule epithelial cells was observed. Numerous heterophils were also seen in the urethra and interstitial tissue.
Brain
No specific lesion was found on day 4 PI in the cerebrum, brain stem, and cerebellum. Hemorrhages and meningoencephalitis were diagnosed at day 6 PI (Fig. 3F). This was associated with the accumulation of inflammatory cells around the vessels (perivascular cuffing) (Fig. 3G, I), and increased Virchow–Robin space (Fig. 3I). Also, gliosis (focal accumulation of microglial cells), the spongy white matter that correlates with myelin degradation, and axonal swelling were also seen (Fig. 3H). Immunohistochemical analysis also revealed the accumulation of virus within the cerebellar gray matter (Fig. 4E). Immunohistochemical examination on days 3 and 8 after challenge showed only virus accumulation within the gray matter of the cerebellum and within the degenerated cell bodies and spongy spaces in the white matter and around the swollen axons (Fig. 4F).
Discussion
The pathogenicity of the Newcastle disease virus and determining the extent of damage to turkey farms are an essential task, and it can be of great help in proper detection of this disease at the farm level and in rapid response to infection. Therefore, in this study, we investigated the precise injuries caused by the ND virus isolated from infected poultry flocks in the southwest of Iran, Ahvaz.
Histological lesions in the lymphatic tissues (spleen, bursa Fabricius, and cecal tonsils) included necrosis and infiltration of mononuclear cells on day 6 PI and then lymphoid depletion of follicles on days 6 and 8 PI. It was also associated with a partial recovery of these tissues and lymphoid hyperplasia at day 14 PI. This finding indicates that the immune system of turkeys was attenuated by the destruction of lymphatic structures and by the active proliferation of the Newcastle virus in the lymphatic organs. Also, the decrease in the size of lymphatic organs also reflects lymphatic depletion by the Newcastle disease virus and consequently suppressed avian immunity due to their role in producing antibodies [6]. These results are similar to the study by Wakamatsu et al. [7] regarding the effects of Newcastle virus on the suppression of immunity of turkeys. One of the prominent features of lymphoid necrosis in lymphoid tissues has been the existence of pyknosis (wrinkled nucleus) and karyorrhexis (disintegration of small nuclei). Ballooning degeneration of bursa cells in the early stages of infection and then a proliferation of reticular cells and growth of germ cells at stages of disease recovery were similar to the results of Okoye et al. [8]. In this study, degeneration of the spleen hematopoietic tissue in the ellipsoid region indicates trapping of antigens by reticular cells such as large foamy macrophages. Igwe et al. [9] reported that, in Newcastle disease, reticuloendothelial and erythrophagocytic cells accumulated around the arterioles in the spleen. These lesions were not found in the ellipsoid region by Beard and Hanson [10] but were reported by Hamid et al. [11]. Brown pigments which were blue in Prussian blue staining were hemosiderin-laden macrophages in splenic red pulps. The presence of these cells was also confirmed in a study by De Kock [12]. The presence of these pigments indicated extensive lysis of erythrocytes, which resulted in a small red pulp.
Microscopic examination of different tissues revealed intra-nuclear or intracytoplasmic inclusion bodies in different tissues. Inclusion bodies are frequently referred to in various references in relation to viruses such as herpesviruses (laryngotracheitis), poxviruses (pox disease), adenoviruses (inclusion body hepatitis), rotaviruses, parvoviruses, and circoviruses. In addition, inclusion bodies are a prominent feature of the family of paramyxoviruses, such as distemper and plague. But in the studies that have been done so far on Newcastle disease, inclusion bodies have rarely been mentioned. De Kock [12] noted the presence of inclusion bodies in splenic reticular cells infected with Newcastle disease virus. They were multisided and larger than the nucleus, and were eosinophilic in the staining of hematoxylin and eosin. Das and Goldberg [13] indicated that inclusion bodies were observed in cells that survived the acute phase of Newcastle disease, which may be the result of Newcastle disease virus activity. They referred to these objects as inclusion bodies in their study.
The microscopic signs observed in the nervous system were meningoencephalitis (the accumulation of inflammatory cells around the arteries and under the meninges, edema, gliosis, and sponging). The main brain lesions in a study by Okoye et al. [8] after challenge with Newcastle virus in chicken were lymphocyte aggregation around the cerebellum and cerebellar, and edema under the meninge. Meningitis and gliosis were consistent with the present study [8]. Also, sponge changes in the brain due to necrosis and loss of neurons and axons as well as axonal rupture and ellipsoid formation (chromatolysis) are other results of brain lesions similar to previous studies such as Shivaprasad et al. [14]. One of the main triggering factors for clinical neurological manifestations is the presence of neuronal infection and direct necrosis of the neuron by the virus, which in this study confirmed the presence of virus antigens by immunohistochemistry. In this study, to confirm the presence of virus antigen, immunohistochemical tests were performed on four organs (spleen, cecal tonsils, proventriculus, and brain) with prominent and specific histopathologic and autopsy lesions, on days 3 and 8 PI. The results of this staining, on day 3 in two organs (cecal tonsils and spleen) and on day 8 PI, showed the virus antigen was indicated in all the tissues. In a 2007 study by Wakamatsu et al., the presence of Newcastle virus antigen in the spleen was detected by immunohistochemistry on days 3 to 5 PI [7]. Virus antigen detection in tissues is directly related to autopsy lesions and clinical signs [15].
Previously, we detected the virus RNA in tracheal and cloacal swabs by RT-PCR [3]. Virus excretion was observed through the trachea on days 3, 4, 6, and 8 after challenge and through cloaca only on day 8 after challenge. On the 14th and 21st days PI, no positive samples were seen. Late detection of the virus by cloaca can be due to the low sampling rate, but it seems that the isolate has first been excreted from the respiratory tract and then over time through the cloaca. The results of this test were inconsistent with macroscopic and microscopic observations. The first autopsy and pathological lesions in the gastrointestinal tract were associated, in particular, with cecal tonsils, indicating that its verification was no evidence of virus shedding and positive PCR for cloacal swab samples [3]. Besides, in our study, the onset of positive virus detection (day 3 PI) was associated with earlier clinical symptoms (day 5 PI). Differences in the timing of virus transmission also depend on the immune status of birds [16]. Based on previous findings, the latency period of Newcastle disease varies from 2 to 15 days (6–5 days on average) in poultry and 6 to 7 days in humans. The duration of the latency period of infectious virus in Newcastle disease has been shown in previous reports that interspecific susceptibilities such as chicken, turkey, pigeon, and guinea fowl are one of the influencing factors in the onset of clinical manifestations [17]. It is also more severe in younger birds [18]. In this study, mortality was observed 1 day after the onset of clinical symptoms in turkeys. In susceptible birds exposed to high levels of acute Newcastle virus, mortality may occur without showing any clinical symptoms. Mortality rates in unvaccinated fields by acute isolates can be as high as 100%. The severity of the disease in turkeys is less than in chickens, but its effect on egg production is similar to chickens. There have been reports of high mortality in turkey farms [19]. In this study, experimental infection with EID105 Newcastle disease virus into poults resulted in lower mortality than field infections. Disorders of the central nervous system in this study, such as tremors, and unilateral or bilateral paralysis of the neck, wings, and feet, indicate a high tissue tropism for the brain and spinal cord (neurotropic). In this study, despite the presence of pathological lesions in the trachea and lung specimens and autopsy lesions in the lungs and air sacs, no respiratory symptoms such as respiratory rales and crushing were observed in the bed. Differences in the type of clinical manifestations of turkeys in different studies can be due to the method of virus inoculation and the pathogenicity of the virus [20]. In this study, gastrointestinal hemorrhages such as cecal tonsils, intestines, and proventriculus were prominent features of post-inoculation symptoms. There was less severity of hemorrhages in the proventriculus and delayed onset. One of the reasons for the widespread hemorrhages in the gastrointestinal epithelium may be due to the proliferation of Newcastle virus in the lymphatic organs. In this study, despite the macroscopic manifestations of both the nervous system and the gastrointestinal tract, the BJ2013 clone Nda isolate can be described as a viscerotropic strain.
Conclusion
According to the results of this study and the large population of industrial and local turkeys and the continuous observation of Newcastle disease in them, and, on the other hand, the possibility of exposure to commercial poultry flocks, awareness of the Newcastle disease virus infection in turkeys seems necessary. Monitoring of Iranian turkey farms is important to determine the appropriate vaccination program against the Newcastle disease virus.
References
Abdoshah M, Pourbakhsh S, Peighambari S, Shojadoost B, Momayez R, Mojahedi Z (2012) Pathogenicity indices of Newcastle disease viruses isolated from Iranian poultry flocks in Iran. J Vet Res 67(2):159–164
Saif Y, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE (2011) Diseases of poultry. John Wiley & Sons
Jafari R, Broomand Z, Rezaie A, Mayahi M, Nejati AS (2019) Experimental infection of turkeys with a virulent Newcastle disease virus isolated from broiler chickens. Arch Razi Inst 74(1):51–57
Boroomand Z, Jafari RA, Mayahi M (2016) Molecular characterization and phylogenetic study of the fusion genes of Newcastle disease virus from the recent outbreaks in Ahvaz, Iran. Virusdisease 27(1):102–105
Trapp A, Taylor R (1986) Methods of euthanasia in poultry and food-producing animals. Vet Clin North Am Food Anim Pract 2(1):31–41
Reynolds D, Maraqa A (2000) Protective immunity against Newcastle disease: the role of antibodies specific to Newcastle disease virus polypeptides. Avian diseases:138–144
Wakamatsu N, King DJ, Seal BS, Brown CC (2007) Detection of Newcastle disease virus RNA by reverse transcription-polymerase chain reaction using formalin-fixed, paraffin-embedded tissue and comparison with immunohistochemistry and in situ hybridization. J Vet Diagn Invest 19(4):396–400
Okoye J, Agu A, Chineme C, Echeonwu G (2000) Pathological characterization in chickens of a velogenic Newcastle disease virus isolated from guinea fowl. Revue d Elevage et de Medicine Veterinaire des Pays Tropicaux 53(4):325–330
Igwe OA, Ezema SW, Eze CD, Okoye O (2014) Experimental velogenic Newcastle disease can be very severe and viscerotropic in chickens but moderate and neurotropic in guinea fowls. Int J Poult Sci 13(10):589–590
Beard C, Hanson R (1984) In Disease of poultry, ed. MS Hofstad. Iowa State University Press, Ames Iowa
Hamid H, Campbell R, Parede L (1991) Studies of the pathology of velogenic Newcastle disease: virus infection in non-immune and immune birds. Avian Pathol 20(4):561–575
De Kock G (1954) Studies on the histopathology and pathogenesis of Newcastle disease of fowls in South Africa, with special reference to the lymphoid tissue A preliminary report Onderstepoort. J Vet Res 26(4):599–629
Das M, Goldberg HS (1961) Inclusion bodies from Newcastle disease virus in HeLa cells. J Bacteriol 82(1):151
Shivaprasad H, Rupiper D, Woolcock P, Woods L outbreak of Newcastle disease in exotic pheasants and doves. In: Western Poultry Disease Conference, 1999.
Nakamura K, Ohtsu N, Nakamura T, Yamamoto Y, Yamada M, Mase M, Imai K (2008) Pathologic and immunohistochemical studies of Newcastle disease (ND) in broiler chickens vaccinated with ND: severe nonpurulent encephalitis and necrotizing pancreatitis. Vet Pathol 45(6):928–933
Eze C, Okoye J, Ogbonna I, Ezema W, Eze D, Okwor E, Ibu J, Salihu E (2014) Comparative study of the pathology and pathogenesis of a local velogenic Newcastle disease virus infection in ducks and chickens. Int J Poult Sci 13(1):52–61
Mishra S, Kataria J, Sah R, Verma K, Mishra J (2001) Studies on the pathogenicity of Newcastle disease virus isolates in guinea fowl. Trop Anim Health Prod 33(4):313–320
Nwanta J, Abdu P, Ezema W (2008) Epidemiology, challenges and prospects for control of Newcastle disease in village poultry in Nigeria. World’s Poult Sci J 64(1):119–127
McFerran J, McCracken R (1988) Newcastle disease. In: Newcastle disease. Springer, pp 161–183
Beach J, Bankowski R, Quortrup E (1948) A preliminary report on the modification of avian pneumoencephalitis (Newcastle disease) virus by cultural methods. Cornell Veterinarian 38(4):341–357
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical
We hereby declare all ethical standards have been respected in preparation of the submitted article, and approved by the committee of ethics in research at Shahid Chamran University of Ahvaz.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Mariana X Byndloss
Rights and permissions
About this article
Cite this article
Saravi, A.N., Jafari, R.A., Boroomand, Z. et al. A histopathological and immunohistochemical study of experimental infected turkeys with a virulent Newcastle disease virus. Braz J Microbiol 52, 1677–1685 (2021). https://doi.org/10.1007/s42770-021-00623-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00623-y