Abstract
Fungal pathogens are important determinants of plant dynamics in the environment. These pathogens can cause plant death and occasionally yield losses in crops, even at low initial densities in the soil. The objective of this study was to select and evaluate fungal antagonistic bacteria and to determine their biological control capacity in soybean seedlings. A total of 877 strains from the genera Pseudomonas, Bacillus, and Paraburkholderia/Burkholderia were screened, and their antagonistic effects on fungi frequently found in seeds were evaluated using four methods: quadruple plating, paired culture confrontation, strain containment, and inoculation of soybean seeds. The experimental design was completely randomized, with three replications for the first three methods and five replications in a 3 × 9 factorial scheme for the fourth treatment. The strains with the highest biotechnological potential were inoculated into soybean seeds to evaluate the biological control of fungi that attack this crop at germination. Seventy-nine strains presented some type of antagonistic effect on the tested fungi, with two strains presenting a broader antagonistic action spectrum in the seed test. In addition to the antagonistic potential, strains BR 10788 and BR 11793, when simultaneously inoculated or alone, significantly increased the seedling dry matter mass, and promoted the growth of soybean seedlings even in the presence of most fungi. Thus, this study demonstrated the efficiency of the antagonistic activity of these strains in relation to the target fungi, which proved to be potential agents for biological control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungal pathogens are determinants of plant population dynamics in agricultural environments. For soybeans, examples of economically important pathogens include Colletotrichum spp., Fusarium spp., Aspergillus spp., Cercospora spp., Penicillium sp., Alternaria spp., Cladosporium sp., Sclerotinia spp., and Rhizoctonia spp., which can cause plant death and occasionally total yield loss even at low initial soil inoculum densities [1,2,3].
The use of biological agents to reduce pathogens has been shown to be an effective, promising, and widely studied method [4,5,6]. However, although the number of diseases that can be controlled biologically in practical terms is significant, few biological products have actually been used. This is because of the limitations imposed by the pathogens and failure of the selection procedure, environmental factors, technical challenges, and strategies in the experimental system [7].
The process of selecting microorganisms for use in new commercial products for the biocontrol of plant fungal pathogens is complex because there are several criteria to be analyzed that are crucial for the success of subsequent steps. Among these, the antagonistic efficacy, mechanisms of antagonism, growth in a vehicle that maintains an adequate population, and even procedures of legal property rights, and market insertion are crucial [8].
Cultural collections currently provide services to the scientific community, ensuring the considerable diversity of pure and authentic microorganisms already available. Microorganisms from these institutions have beneficial characteristics and can be used in various programs of agricultural interest [9]. Thus, the use of microorganisms belonging to the collections, isolated from native cultures, and already identified facilitates the development of processes and products of economic interest.
Different bacteria isolated from various hosts that have the ability to promote plant growth, and may also be antagonists to plant pathogens, have been studied for the development of commercial products [10]. Despite their high biotechnological potential, there are still a few products registered on the market for this purpose. However, the use of microorganisms as biocontrol agents is growing worldwide, albeit slowly. Trichoderma spp. is an example used to control soil-dwelling fungi [11].
Alternatively, other species and genera of bacteria are important groups of biological control agents, such as Pseudomonas, Bacillus, and Paraburkholderia. These have been shown to have the potential to control fungal and/or bacterial diseases because they occupy an ecological niche similar to that occupied by the pathogens [12,13,14]. In addition, bacteria of the genus Bacillus can produce a wide variety of antimicrobial compounds, such as iturine, surfactin, subtilosin, fengycin, and bacillomycin [15,16,17], giving it the ability to protect plants from phytopathogenic fungi [18,19,20,21]. Additionally, lipopeptide biosurfactants produced by Pseudomonas and Bacillus are effective in biocontrol because of their positive potential for competitive interactions with other organisms, including bacteria, fungi, protozoa, nematodes, and plants [22,23,24].
Similarly, the Paraburkholderia species mainly stand out due to their biocontrol and bioremediation properties [25,26,27]. This genus was recently reclassified as belonging to the genus Burkholderia based on molecular markers [25]. It comprises a versatile group consisting of taxonomic members with beneficial characteristics to the environment and associated plants [28]. In addition to promoting plant growth, it can improve nutrient absorption, increase tolerance to stress, induce systemic resistance, and confer resistance to plant pathogens [29]. For example, Glick et al. [30] revealed that a Paraburkholderia phytofirmans strain decreased the level of ethylene in host plants by producing the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase. Similarly, it was shown that Burkholderia strains could contribute to plant nutrition by producing plant hormones, indirectly leading to reduced disease susceptibility [31, 32].
Thus, the goals of this study were to select and evaluate bacteria antagonistic to phytopathogenic fungi and to determine their biological control capacity in soybean seedlings. The strategy used in this study was to screen bacteria isolated previously from healthy plants and deposited in culture collections.
Material and methods
Bacterial and fungal strains
The study was based on bacterial strains already deposited in the culture collection of the Embrapa Agrobiologia Johanna Dobereiner Center for Biological Resources (CRB-JD). Eight hundred and seventy-seven strains previously characterized by 16S rRNA in the genera Pseudomonas, Bacillus, and Paraburkholderia/Burkholderia were subjected to a phylogenetic analysis using the MEGA (version 7.0) program [33] based on the neighbor-joining method and the Tamura 3-parameter, which was the best substitution model. Subsequently, representative strains of specific phylogenetic groups within each genus were defined (Table S1). We used the name Paraburkholderia/Burkholderia throughout because of the uncertain taxonomic position of some strains related to these genera. The 16S rRNA of all bacteria was amplified with the primers 27F and 1492, as recommended elsewhere.
The fungi used have the potential to attack soybean, bean, rice, and cotton seeds during germination. These included Aspergillus flavus (F5), Rhizoctonia solani (F4), Corynespora cassiicola (F3), Fusarium piperis (F7), Fusarium semitectum (F1), Phomopsis sojae (F6), Sclerotinia sclerotiorium (F2), Cladosporium sp. (A104), and an isolate of the order Pleosporales (A103 and A105). The fungal strains originated from the Culture Collection of the Embrapa Agropecuária Oeste Seed Laboratory and from CRB-JD. The strains of the order Pleosporales (A103 and A105) and Cladosporium sp. (A104) can colonize plant roots, such as rice and tomato [34].
Phylogeny of the bacteria strains
Strain affiliation with different species groups within each genus was obtained by phylogenetic analysis based on the 16S rRNA gene sequences of the strains and type strains. The neighbor-joining method was used for the analysis and the models that best fit model for each genus (Pseudomonas: Kimura two parameters; Bacillus: Tamura-Nei; Paraburkholderia/Burkholderia: Tamura three parameters) using the MEGA program (version 7.0). The closest type strains within each genus described in the bacterio.net platform (http://www.bacterio.net/methylophilus.html) were considered. Based on clusters, species were placed within the appropriate groups.
Initial screening of antagonistic bacteria
The 101 strains selected were analyzed for antagonism against phytopathogenic fungi. As a first approach, a quadruple plating method was used, in which plates containing PDA medium were divided into quadrants. Fungi also grown in PDA (10 days at 28 °C in a growth chamber) were used as inoculant. Seven-millimeter discs of this culture medium containing the grown fungi were arranged in the center of a new plate containing BDA medium, and in each quadrant, a distinct strain was inoculated, totaling four in each Petri dish. This was followed by incubation (15 days at 28 °C in a growth chamber). At 7 and 15 days, visual evaluations were performed to identify the antagonism (absence of fungal growth and/or an inhibition halo) promoted by the bacterial strains. From this test, the strains were selected within each bacterial genus for further tests.
Specific antagonism tests for the five strains within each genus
The paired culture confrontation technique proposed by Mariano [35] was used, with minor adaptations to evaluate the antagonism of 15 (five for each of the three genera) strains. Briefly, the strains were plated on PDA medium in two bands spaced 40 mm apart. Seven-millimeter discs of the culture medium containing fungi, as mentioned above, were arranged in the center of the plate such that they were equidistant between the bacterial bands, followed by incubation at 28 °C for 15 days. Control plaques consisted of fungal discs in the absence of bacteria. A completely randomized design with three replications was used, and the data were analyzed by the Scott-Knott test at 5% probability.
In this second stage, antagonism was interpreted by analyzing three variables: the inhibition zone, colony area, and percentage of inhibition. The inhibition zone was evaluated at 15 days by measuring the distance between the fungal colony’s edges and the bacterial strip [35, 36]. The area of the fungal colony was obtained by measuring its radial growth on the orthogonal axes. The mean between these two measurements was calculated and assumed to be the value of the radius (r) for area calculation using the formula 2πr2. The results were expressed in mm2. The percentage inhibition was determined by the relationship between the area of growth of the pathogen in the presence of each bacterium and the area occupied by the pathogen in the control treatment. Using these values, the percent inhibition was calculated using formula (1) adapted from Tullio [36]: (% In = 100 − [(treatment area mm) × 100)/(control area mm)]. In addition, three levels of antagonism (below 40%, 40–80%, and above 80%) were defined for the obtained values.
In the third stage, a technique for fungus containment was adapted from Mariano [35]. A 7-mm disc of fungus on PDA medium (as described above) was arranged in the center of a Petri dish containing the same medium. Then, two bacterial strains that presented the best square-shaped results around the fungus disc were inoculated on the same plate. For the control treatment, the fungus was used without the presence of bacteria. The plates were then incubated for growth (28 °C), and each treatment consisted of three replicates. At 15 days, the fungus containment caused by the bacteria in the plaques was visually verified.
Bioassay to assess the bacterial antagonism to the fungus during soybean germination
Bacterial strains identified with higher antagonistic potential from the previous tests were selected and cultured in BP culture medium, optimized using glycerol as a carbon source [37], for 24 h at 28 °C, and under orbital agitation at 150 rpm. Individual growth curves were generated to determine the maximum cell production potential. Initially, each bacterial strain was grown in a test tube with 5 mL of medium (20 h; 28 °C; with shaking at 1500 rpm). Then, the growth broth was transferred to a 250-mL Erlenmeyer flask, and the volume was adjusted to 50 mL with the same medium, followed by a new growth cycle (18 h, 28 °C, and 1500 rpm agitation). After that, the growth broth was transferred to another Erlenmeyer flask, where the volume was adjusted to 250 mL and incubated (28 °C; with 1500 rpm agitation). Every 2 h, aliquots were taken to determine the optical density and also plated on “spiral plate” equipment at dilutions 10−5, 10−6, and 10−7 CFU/ml. After determining the point of maximum cell production, each strain (BR 10788 and BR 11793) and the mixture of the two were formulated according to Scheidt et al. [37] for further use in seed treatments.
The bioassay was conducted in a completely randomized design with five replications in a 3 × 9 factorial scheme, corresponding to three strains (BR 10788, BR 11793, and a mixture of both) and nine fungi (F. piperis, Pleosporales, Cladosporium sp., Ph. sojae, A. flavus, C. cassiicola, R. solani, F. semitectum, and S. sclerotiorum). Each plot corresponded to a Petri dish consisting of eight seeds, half of which were inoculated with one of the three inoculants, and the other half (four seeds) was not inoculated. All seeds were initially disinfected: immersion in 70% ethanol for 30 s and in 4% (v/v) H2O2 for 3 min and then rinsed three times in sterile distilled water [38] before inoculation.
For bacterial inoculation, 0.2 mL of each product was applied to 180 seeds (after being distributed in the plates), resulting in a cell concentration of approximately 108 bacterial cells per seed. Fungi inoculum discs (8 mm) were placed in the center of the plate, been the inoculated seeds on one side of the plate and the control without inoculant on the other side. After sowing the plates, they were incubated (28 °C; 12 h photoperiod; 7 days).
The variable for antagonistic potential (PA) was evaluated by using formula (2): PA = [(number of injured control treatment seedlings-number of injured inoculated seedlings)/number of injured control treatment seedlings) × 100]. For the fresh mass (g), dry mass (g), number of germinated seeds (NSG), and number of injured seedlings (NPL) of all four seedlings for each treatment in the plate were joined as a plot, and an analysis of variance (ANOVA) was performed. A P value ≤ 0.05 was defined as statistically significant.
Results and discussion
Evaluation of bacterial antagonism to fungi in Petri dishes
A total of 877 strains were phylogenetically studied based on the 16S rRNA within the genera Pseudomonas, Bacillus, and Paraburkholderia/Burkholderia. The strains were isolated in Brazil and originated from both hosts and substrates, such as roots, legume nodules, and the plant rhizosphere (Table S1). From the groupings, 32 strains were selected from the genera Pseudomonas and Bacillus and 37 from the genus Paraburkholderia/Burkholderia to represent distinct phylogenetic groups, totaling 101 strains.
The formation of six major phylogenetic groups was observed for Pseudomonas, seven for Bacillus, and ten groups for Paraburkholderia-type strains, wherein new strains were distributed (Fig. 1). The Pseudomonas and Bacillus species comprised of 272 and 380 species, respectively. The genus Paraburkholderia, recently defined by a reclassification of the genus Burkholderia, had 74 recognized species. Burkholderia is a large and complex group containing pathogenic, non-pathogenic, symbiotic, and non-symbiotic strains from a wide variety of habitats. Thus, its taxonomy has been reevaluated and divided into six genera: Burkholderia, Caballeronia, Mycetohabitans, Paraburkholderia, Robbisia, and Trinickia [39].
Phylogenetic tree of isolates based on 16S rRNA (1250 nt) of the genera Pseudomonas, Bacillus, and Burkholderia/Paraburkholderia tested for the biological control of the fungi Fusarium piperis, Fusarium semitectum, Pleosporales, Cladosporium sp., Phomopsis sojae, Rhizoctonia solani, Corynespora cassiicola, Sclerotinia sclerotiorum, and Aspergillus flavus. The numbers shown before the strains indicate the type of strain groups to which they belong and the number in parentheses indicates the number of fungi controlled by the strain and the scale
The Pseudomonas and Paraburkholderia/Burkholderia groups’ strains showed biological control potential for almost all fungal isolates tested. For Pseudomonas, 26 strains show some type of antagonism, similar to all isolates of the Paraburkholderia/Burkholderia group (Fig. 1). Sixteen isolates of Bacillus controlled the fungi evaluated. Although the number of isolates with control capacity was smaller than that of the other genera, they show a greater antagonistic capacity to the fungi (Fig. 1). Thus, the results showed that Pseudomonas and the Paraburkholderia/Burkholderia group had a broader spectrum of control, but Bacillus spp. had more pronounced antagonistic effects.
After the initial screening for visual identification of bacterial control ability of fungi and identification of different groups of strains, five strains from each genus showed greater antagonism and were studied in more detail using the paired culture challenge test. In this test, it is observed that the control-efficient Pseudomonas strains were mostly grouped with species groups 1, 3, 4, and 6, in which those belonging to the type 1 and 4 strain groups presented inhibition potential higher than 80% for Pleosporales, Cladosporium sp., and S. sclerotiorum (Fig. 1). It was also observed that the only strain among the 101 selected (considered for this test), capable of inhibiting the growth of the phytopathogen A. flavus, was BR 10843 belonging to group 3, represented by Pseudomonas species, Ps. baetica and Ps. helmanticensis. This inhibitory effect may have been expressed more intensely because of this strain’s growth rate, resulting in a greater ability to inhibit sporulation.
Within the five Bacillus strains, those showing antagonism are those represented in groups 3, 5, 6, and 7 (Fig. 1). Strains of this genus showed a narrower spectrum of control than the Paraburkholderia/Burkholderia genera; however, strains with higher antagonism (> 80%) were obtained from Pleosporales, Cladosporium sp., C. cassiicola, Ph. sojae, and S. sclerotiorum.
The strains of the genus Paraburkholderia/Burkholderia had the broadest spectrum of antagonism, controlling a large number of fungi, ranging from 4 to 7. Most strains presented antagonistic levels of 40–80% (groups 1, 4, and 10), highlighting the species groups P. caballeronis, P. kururiensis (group 4), and P. andropogonis and B. vietnamiensis (group 10), which presented levels above 80% for S. sclerotiorum (Fig. 1).
The ability to produce and release one or more compounds; for example, lytic enzymes against compounds, such as chitin, proteins, cellulose, hemicellulose, and DNA, may contribute to the suppression of pathogen activity [40,41,42]. In addition, when a bacterial strain is in contact with the pathogen, competition for space and nutrients may occur, which may have occurred in this situation.
From the analysis of the paired culture challenge test data, two strains were selected, one from the genus Bacillus (BR 10788) and one from the Paraburkholderia/Burkholderia group (BR 11793), which presented the broadest spectrum of action and the highest level of antagonism against the fungi.
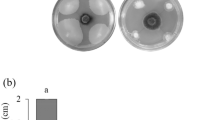
In the challenge test, strain BR 11793 performed better, as it restricted the development of all phytopathogens within the square and was even able to significantly inhibit the growth of A. flavus (Fig. 2; C1). On the other hand, BR 10788 inhibits all fungi, except A. flavus (Fig. 2; B1) and F. semitectum (Fig. 2; B8).
Containment test for the control evaluation of the BR 10788 and BR 11793 strains with the fungal pathogens Aspergillus flavus, Pleosporales, Fusarium semitectum, Phomopsis. sojae, Sclerotinia sclerotiorum, Corynespora cassiicola, Cladosporium sp., Fusarium piperis, and Rhizoctonia solani. Column (A) Control; column (B) BR 10788; column (C) BR 11793. Row 1, Aspergillus flavus; row 2, Pleosporales; row 3, Fusarium semitectum; row 4, Phomopsis. sojae; row 5, Sclerotinia sclerotiorum; row 6, Corynespora cassiicola; row 7, Cladosporium sp.; row 8, Fusarium piperis; row 9, Rhizoctonia solani
Both strains show high antagonistic potential for S. sclerotiorum (Fig. 2; B5 and C5), especially when compared to other fungi tested (Fig. 2). The ability of bacteria to parasitize and degrade spores and hyphae and produce compounds that inhibit pathogenic fungi’s development is widely described in the literature [43]. The action of bacteria leads to an inhibition of fungal growth and can range from simply fixing cells to hyphae to the complete breakdown and degradation of the fungi. Bacterial cell adherence to hyphae often occurs because of biofilm production. Zucchi [44] demonstrated that strains of Bacillus subtilis and Paenibacillus lentimorbus, chitinase producers, are capable of parasitizing Aspergillus parasiticus. Due to their ability to degrade the cell wall of filamentous fungi, including Aspergillus, Penicillium, Rhizoctonia, and Colletotrichum species—chitinases are considered an important ally in the control of phytopathogenic fungi.
Lima et al. [45] evaluated the antagonistic effect of ten isolates of Bacillus spp., on Fusarium oxysporum, the causative agent of tomato fusariosis, using the containment technique, observed that a large number of isolates had an inhibitory effect on the pathogen, indicating that the characteristic of fungal inhibition is frequent within certain groups of Bacillus species. B. subilis isolates were also effective in inhibiting mycelial growth of pathogenic fungi Fusarium subglutinans, Curvularia lunata, and Bipolaris when evaluated by circular (or containment) methods with a high percentage of mycelial growth inhibition [3].
Therefore, the results presented here corroborate the results of previous studies, especially because the phylogenetically isolated isolates close to B. subtilis presented a high level of antagonism. In addition, the challenge test indicated that the selected strains did not show preferential action on a specific fungus, which allows their use for different plant-pathogen systems.
Bioassay for fungal strain antagonism evaluation on soybean seed germination
In the bioassay to evaluate the antagonism of strains against fungi on seeds, it is observed that strain BR 10788 was able to effectively control the fungi in the genus Fusarium and S. sclerotiorum compared to that of the other fungi with an antagonistic potential from 80 to 100%, which is highly efficient in the control of the latter (Table 1). Strain BR 10788 also presented antagonistic potential superior to 50% for Ph. sojae and C. cassiicola. On the other hand, BR 11793 significantly controlled the fungi of the genera Fusarium and Ph. sojae, A. flavus, and S. sclerotiorum, ranging from 66 to 100%. In particular, it exhibited strong control of pathogens in the genus Fusarium, with a potential above 90%. There was variation in the antagonistic potential of other fungi, between 5 and 44%, with no significant differences between them for the different bacterial compositions. When the bacterial mixture was used, the observed results are similar to the individualized bacteria, except for S. sclerotiorum, for which the antagonistic potential was lower (Table 1).
Considering the 100% antagonistic potential presented by BR 10788 for the fungus S. sclerotiorum, it can be concluded that it has the potential to control this fungus on seeds effectively. It is also noteworthy that treatment with this bacterium led to better seed germination because it controlled the development of the fungus (Table 2).
Bacillus subtilis, one of the species in the group to which BR 10788 belongs, is classified as an efficient biological disease control agent in plants and has been widely studied and used in agriculture for soil phytopathogen control [46,47,48]. For example, in the USA, products formulated from B. subtilis have been used since 1983 for peanut seed treatment and foliar and soil applications. Moreover, B. subtilis is also used as an active seed treatment against Fusarium wilt, P. damping-off, and leaf blotch caused by Cercospora, Colletotrichum, Alternaria, Ascochyta, Myrothecium, Ramularia, Xanthomonas, and Erysiphe polygoni in cotton, cereals, vegetables, fruit, and ornamentals in India. Its action is mainly due to the production of antibiotics, competition for space and nutrients, antibiosis, and cell wall degradation. Thus, products based on antagonistic microorganisms serve as a tool for the control of phytopathogens.
Studies by Araújo [49] also showed that the treatment of soybean seeds with Bacillus spp. reduced the incidence of fungi in the seeds and improved the plants’ nodulation and development in the presence of Bradyrhizobium japonicum. These additional effects of B. subtilis strains may be caused by the bacteria’s ability to act on the synthesis of auxins, gibberellins, and cytokines leading to better root system development [50, 51].
Regarding germination, higher NSG is observed compared with the control, which did not receive the bacterial treatment for seeds inoculated with strain BR 11793 against A. flavus and Cladosporium sp. (Table 2). In the case of strain BR 10788, the NSG was significantly higher when tested against R. solani. The other treatments did not differ statistically from each other (P < 0.05) (Table 2).
Rhizoctonia solani is an optional phytopathogenic fungus, naturally inhabiting the soil and lives saprophytically, which, although not specific to a host, deserves attention. It is an aggressive pathogen that, through enzyme production, degrades the cell wall and rapidly kills the plant, promoting decomposition and reproducing rapidly at the expense of available nutrients [52]. Thus, the seeds are attacked soon after absorbing water and starting germination. Given the softened integument and soaked interior tissues, the seeds favor pathogen action. In this sense, BR 10788 was efficient against R. solani germination by providing rapid germination and emergence of seedlings, which led to an acceleration in the differentiation and maturation of plant tissues, thereby increasing their resistance to both penetration and colonization by this pathogen. Studies by Kondoh et al. [53], also using a B. subtilis isolate plus the fungicide flutolanil, found a synergistic effect for R. solani control in tomatoes. Therefore, growth promotion provided by B. subtilis or other Bacillus species can lead to rapid germination.
Regarding the biomass accumulation of soybean seedlings, strain BR 10788 also provided higher values occurring prominently in the presence of the fungi F. piperis, F. semitectum, Ph. sojae, and S. sclerotiorum. This further highlights the bacteria’s ability to control fungi and have an additive effect on the promotion of plant growth (Table 3).
Regarding BR 11793, seedlings originating from seeds inoculated with this strain accumulated greater biomass than the control without bacteria in most treatments, as occurred for strain BR 10788. This effect is observed for all fungi, except Cladosporium and F. semitectum (Table 3). For the mixture of the two bacterial strains, an increase in seedling biomass is also observed, mainly for F. piperis, Cladosporium, Ph. sojae, and F. semitectum representing an additional effect on Cladosporium sp., not observed with the individualized strains (Table 3).
In the NPL evaluation, when inoculated with BR 10788, there was a reduction in lesions in the presence of the fungi F. piperis, Cladosporium sp., A. flavus, Ph. sojae, and F. semitectum, with a tendency of reduction in the lesions for the other treatments.
The success of Bacillus and Paraburkholderia in promoting plant growth and controlling fungal attacks on seeds is intrinsically related to the biological characteristics of these microorganisms. Thus, treatment of seeds before planting with biofungicides is an additional guarantee for the establishment of plants in the field, because they protect seedlings from pathogen attack early in seedling development.
BR 11793, including the P. andropogonis species group, showed a broad spectrum of action, presenting biocontrol capacity above 60% for most of the pathogens tested. Recently, comparative genomic analyses of the genome of P. kururiensis strain KP23T, M130, and ATSSB13T revealed important traits, such as genes involved in plant growth, including ACC deaminase, genes for AIA biosynthesis, and genes involved in the breakdown of aromatic compounds. These findings indicate important mechanisms that require further investigation of these strains for environmentally strategic applications, bioremediation, biofertilization, and biocontrol of plant pathogens [28]. However, although they have shown positive results in the applied tests, little is known about the molecular mechanisms involved in the bacterium-plant relationship, and further research is needed for their elucidation.
Conclusions
Strains BR 10788 and BR 11793 showed efficiency in the treatment of soybean seeds against phytopathogenic fungi. The combination of bacteria BR 10788 and BR 11793 showed fungal control ability equal to or superior to individual strains. The studied bioagents can reduce the use of fungicides in the treatment of seeds of agricultural crops.
Data Availability
The data are stored and available in our institution following the own rules
References
Deacon JW (1991) Significance of ecology in the development of biocontrol agents against soil-borne plant pathogens. Biocontrol Sci Technol 1:5–20
Pliego C, Ramos C, de Vicente A, Cazorla F (2011) Screening for candidate bacterial biocontrol agents against soilborne fungal plant pathogens. Plant Soil 340:505–520
Braga Junior GM, Chagas Junior AF, Chagas LFB, de Carvalho Filho MR, Miller LO, dos Santos GR (2017) Controle biológico de fitopatógenos por Bacillus subtilis in vitro. Biota Amazônia Open Journal System – Macapá 7(3):45–51
Bettiol W (1999) Controle biológico de doenças. Ação Ambiental 2:30–33
Santos MSB, Silva AACR (2014) Sanidade de sementes de arroz, biocontrole, caracterização e transmissão de Curvularia lunata em semente-plântula de arroz. Revista Ceres 61(4):511–517
Vinale F, Abadi K, Ruocco M, Marra R, Scala F, Zoina A, Woo S, Lorito M (2003) Remediation of pollution by using biological systems based on beneficial plant-microorganisms interactions. Journal of Plant Pathology 85(4):301 https://scholar.google.com.br/scholar?start=70&q=Vinale&hl=pt-BR&as_sdt=0,5#d=gs_qabs&u=%23p%3DGygbAOdjotYJ
Melo IS (1998) Agentes Microbianos de Controle de Fungos Fitopatogênicos. In: Melo IS, Azevedo JL (eds) Controle Biológico. 1. Embrapa Meio Ambiente, Jaguariúna, pp 17–67
Blum BJ (2007) Concepts and Strategies for a successful product development: the industry’s development concept. In: Cost Action 850 – Conference Schloss Salzau, Germany
Guedes, AC, Goedert CO, Bustamante PG, Moreira JRA, Mariante AS, Walter BMT, Brandão CRF, Proença CEB, Munhoz CBR, Magalhães C, Silva GP, Colli GR, Branchetti L, Mendes MS, Veiga R, Mendonça RC, Silva SR, Cavalcanti, TB, Pereira TS (2004) Estratégia Nacional de Diversidade Biológica. Conservação ex situ
Weller DM, Raaijmakers JM, Gardener BBM, Thomashow LS (2002) Microbial populations responsible for specific soil suppressiveness to plant pathogens. Ann Rev Phytopathol 40:309–348
Morandi MAB, Paula Júnior TJ, Bettiol W, Teixeira H (2009) Controle biológico de pragas, doenças e plantas invasoras. Informe Agropecuário, Belo Horizonte 30(251):73–82 jul/ago
Bressan W (2003) Biological control of maize seed pathogenic fungi by use of actinomycetes. Biocontrol 48:233–240
Fravel DR (2005) Commercialization and implementation of biocontrol. Annu Rev Phytopathol 43:337–359
Peixoto-Neto PAS, Azevedo JL, Araújo WL (2002) Microrganismos endofíticos: interação com plantas e potencial biotecnológico. Biotecnologia, Ciência & Desenvolvimento 29:62–76
Pérez C, Muñoz-Garay C, Portugal LC, Sánchez J, Gill SS, Soberón M, Bravo A (2007) Bacillus thuringiensis ssp. israelensis Cyt1Aa enhances activity of Cry11Aa toxin by facilitating the formation of a pre-pore oligomeric structure. Cell Microbiol 9:2931–2937
Govindasamy V, Senthilkumar M, Magheshwaran V, Kumar U, Bose P, Sharma V, Annapurna K (2011) Bacillus and Paenibacillus spp: Potential PGPR for Sustainable Agriculture. In: Maheshwari DK (ed) Plant Growth and Health Promoting Bacteria. Springer, Berlin Heidelberg, pp 333–364
Velho RV, Caldas DGG, Medina LFC, Tsai SM, Brandelli A (2011) Real-time PCR investigation on the expression. Of sboA and ituD genes in Bacillus spp. Letters in Applied Microbiology 52:660–666
Rückert C, Blom J, Chen X, Reva O, Borriss R (2011) Genome sequence of B. amyloliquefaciens type strain DSM7T reveals differences to plant-associated B. amyloliquefaciens FZB42. J Biotechnol 155:78–85
Kumar KVK, Yellareddygari SKR, Reddy MS, Kloepper JW, Lawrence KS, Zhou XG, Sudini H, Groth DE, Raju SK, Miller ME (2012) Efficacy of Bacillus subtilis MBI 600 against shealth blight caused by Rhizoctonia solani and on growth and yield of rice. Rice Science 19(1):55–63
Chen J, Wang X, Han H (2013) A new function of graphene oxide emerges: inactivating phytopathogenic bacterium Xanthomonas oryzae pv. Oryzae. Journal of Nanoparticle Research 15(5):1658 https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s11051-013-1658-6
Chowdhury SP, Dietel K, Rändler M, Schmid M, Junge H, Borriss R (2013) Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS ONE 8:e68818. https://doi.org/10.1371/journal.pone.0068818
Raaijmakers JM, De Bruijn I, Nybroe O, Ongena M (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 34:1037–1062. https://doi.org/10.1111/j.1574-6976.2010.00221.x
Beneduzi A, Ambrosini A, Passaglia LM (2012) Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genetics and Molecular Biology 35(suppl 4):1044–1051
Khan N, Maymon M, Hirsch AM (2017) Combating Fusarium Infection Using Bacillus-Based Antimicrobials. Microorganisms 5:75. https://doi.org/10.3390/microorganisms5040075
Sawana A, Adeolu M, Gupta RS (2014) Molecular signatures and phylogenomic analysis of the genus Burkholderia: Proposal for division of this genus into the emended genus Burkholderia containing pathogenic organisms and a new genus Paraburkholderia gen. nov. harboring environmental species. Frontiers in Genetics 5:429
Miotto-Vilanova L, Jacquard C, Courteaux B, Wortham L, Michel J, Clément C, Barka EA, Sanchez L (2016) Burkholderia phytofirmans PsJN confers grapevine resistance against Botrytis cinerea via a direct antimicrobial effect combined with a better resource mobilization. Front Plant Sci 7:1236. https://doi.org/10.3389/fpls.2016.01236
Eberl L, Vandamme P (2016) Members of the genus Burkholderia: Good and bad guys. F1000. Research 5:1007
Dias GM, Pires AS, Grilo VS, Castro MR, Vilela LF, Neves BC (2019) Comparative genomics of Paraburkholderia kururiensis and its potential in bioremediation, biofertilization, and biocontrole of plant pathogens. Microbiol Open
Vitorino LC, Bessa LA (2017) Technological microbiology: Development and applications. Frontiers in Microbiology 8:827
Glick BR, Todorovic B, Czarny J, Cheng Z, Duan J, McConkey B (2007) Promotion of Plant Growth by Bacterial ACC Deaminase. Critical Reviews in Plant Sciences 26:227–242
Yang JO, Kim WY, Bhak J (2009) ssSNPTarget: genome-wide splice-site single nucleotide polymorphism database. Human Mutation 30:E1010E1020
Andreolli M, Lampis S, Zapparoli G, Angelini E, Vallini G (2016) Diversity of bacterial endophytes in 3 and 15 years-old grapevines of Vitis vinífera cv. Corvina and their potential for plant growth promotion and phytophatogen control. Microbiol Res 183:42–52
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30:2725–2729
Vergara C, Araújo KEC, Urquiara S, Schultz, N, Balieiro FC, Medeiros PS, Santos LA, Xavier GR, Zilli JE (2017) Dark septate endophytic fungi help tomato to arquire nutrients from ground plant material. Front Microbiol 8
Mariano RLR (1993) Métodos de seleção “in vitro” para controle microbiológico. Revista Anual Patologia de Plantas 1:369–409
Tullio HE (2017) Potencial de bactérias endofíticas do cacau para o controle de fungos de solo e promoção de crescimento radicular na cultura da soja. Dissertação, Universidade Estadual de Ponta Grossa, Paraná, Brazil
Scheidt W, Pedroza ICPS, Fontana J, Meleiro LAC, Soares LHB, Reis VM (2019) Optimization of culture medium and growth conditions of the plant growth-promoting bacterium Herbaspirillum seropedicae BR11417 for its use as an agricultural inoculant using response surface methodology (RSM). Plant Soil 451:75–87
Luz WC (2001) Efeito de bioprotetores em patógenos de sementes e na emergência e rendimento de grãos de milho. Fitopatologia Brasileira 26:1
los Santos P E-d, Palmer M, Chávez-Ramírez B, Beukes C, Steenkamp ET, Bricoe L, Khan N, Mulak M, Lafos M, Humm E, Arrabit M, Crook M, Gross E, Simon MF, dos Reis FB Jr, Whitman WB, Nicole S, Poole PS, Hirsch AM, Venter SN, James EK (2018) Whole Genome Analyses Suggests thar Burkholderia sensu lato Contains Two Additional Novel Genra (Mycetohabitans gen. nov., and Trinickia gen. nov.): Implication for the Evolution of Diazotrophy and Nodulation in the Burkholderiaceae. Genes 9:389
Kobayashi DY, Reedy RM, Bick JA, Oudemans PV (2002) Characterization of a chitinase gene from Stenotrophomonas maltophilia strain 34 S1 and its involvement in biological control. Appl Environ Microbiol 68:1047–1054
Raaijmakers JM, Vlami M, de Souza JT (2002) Antibiotic production by bacterial biocontrol agents. Antonie van Leeuwenhoek 81:537–547
Gross H, Loper JE (2009) Genomic of secondary metabolite production by Pseudomonas spp. Nat Prod Rep 26:1408–1446
Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. Journal of Experimental Botany 52:487–511
Zucchi TD (2007) Potencial de linhagens de Bacillus subtilis, Paenibacillus lentimorbus e Streptomyces sp. no controle de fungos aflatoxigênicos em amendoim (Arachis hypogaea) e aspectos de biossegurança. Tese, Universidade de São Paulo, São Paulo, Brazil
Lima ODDR, dos Santos MSB, Rodrigues AAC (2014) Ação antifúngica in vitro de isolados de Bacillus ssp. sobre Fusarium oxysporum f. sp. lycopersici. Revista Caatinga 27(4):57–64
Matsuno Y, Ano T, Shoda M (1992) Cloning of a gene responsible for the specific production of an antifungal antibiotic iturin with n-C16-b-amino acid residue. Journal of General and Applied Microbiology 38:505–509
Bettiol W, Kimati H (1990) Efeito de Bacillus subtilis subtilis sobre Pyricularia oryzae agente causal de bruzone do arroz. Pesquisa Agropecuária Brasileiro 25:1165–1174
Furlan SH, Verchaito MH (2005) Efeito de Bacillus subtilis e Trichoderma sp. no tratamento de sementes de feijão visando o controle de Colletotrichum lindemuthianum. In: Congresso Nacional de Pesquisa de Feijão, 7, Santo Antônio de Goiás. Anais... Santo Antônio de Goiás: Embrapa Arroz e Feijão, Documentos 182, vol 1, pp 182–185
Araújo FF, Henning A, Hungria M, de Lima J (1995) Caracterização do potencial antifúngico de Bacillus spp. isolados de solos do Paraná. In: Hungria M, Balota EL, Colozzi-Filho A, Andrade DS (eds) Microbiologia do solo: desafios para o século XXI. Iapar/Embrapa-CNPSo, Londrina, pp 450–455
Persello-Cartieaux F, Nussaume L, Robaglia C (2003) Tales from the underground: Molecular plant-rhizobacteria interactions. Plant Cell and Environment 26:186–199
Tsavkelova EA, Klimova SY, Cherdyntseva TA, Netrusov AI (2006) Microbial producers of plant growth stimulators and their practical use: a review. Applied Biochemistry and Microbiology, New York 42(2):117–126
Kimati H, Gimenez-Fernandes N, Soave J, Kurozawa C, Brignani Neto F, Bettiol W (1997) Guia de Fungicidas Agrícolas – Recomendações por Cultura, v. 1, 2ª ed. Jaboticabal, Grupo Paulista de Fitopatologia, 225p
Kondoh M, Hirai M, Shoda M (2001) Integrated biological and chemical control of damping-off caused by Rhizoctonia solaniu using Bacillus subtilis IXB14-C and Flutolanil. Journal of Bioengineering 91(2):173–177
Code availability
Not applicable
Funding
We thank Dr. Goulart, ACP (Embrapa Agropecuária Oeste) for providing the fungi for the study. We also thank the CNPq (Brazilian National Council for Scientific and Technological Development) and Capes (Coordination for the Improvement of Higher Education Personnel) for the grants awarded to some of the authors and financial support of projects, especially INCT Plant Growth–Promoting Microorganisms for Agricultural Sustainability and Environmental Responsibility (465133/2014-2).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the study and manuscript preparation
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
All authors consent to participate in the paper.
Consent for publication
All authors consent to participate in the paper publication.
Conflicts of interest/Competing interests
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Fernandes, M.F.R., Ribeiro, T.G., Rouws, J.R. et al. Biotechnological potential of bacteria from genera Bacillus Paraburkholderia and Pseudomonas to control seed fungal pathogens. Braz J Microbiol 52, 705–714 (2021). https://doi.org/10.1007/s42770-021-00448-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00448-9