Abstract
Punicalagin is a phenolic compound extracted from Lafoensia pacari A. St.-Hil (Lythraceae) leaves. It has demonstrated interesting activity against pathogenic fungi, e.g., Cryptococcus gattii and Candida albicans, by inhibiting fungi growth in a minimum inhibitory concentration (MIC) at 4 μg/mL. However, the mechanisms behind its antifungal action are not well understood. In this study, certain parameters were investigated, by transmission electron microscopy, ergosterol synthesis inhibition, and flow cytometry analyses, to gain insight into the possible biological targets of punicalagin (4 or 16 μg/mL) against yeast cells. Data showed that, in contrast to untreated cells, punicalagin triggered severe ultrastructural changes in C. gattii and C. albicans, such as disorganization of cytoplasmic content and/or thickened cell walls. In addition, it caused a decrease in yeast plasma membrane ergosterol content in a concentration-dependent manner. However, it was unable to bring about significant fungal cell membrane rupture. On the other hand, punicalagin (16 μg/mL) significantly arrested C. albicans and C. gattii cells at the G0/G1 phase, with a consequent reduction in cells at the G2/M phase in both fungi isolates, and thereby prevented progression of the normal yeast cell cycle. However, these alterations showed no involvement of reactive oxygen species overproduction in C. albicans and C. gattii cells, although punicalagin triggered a significant loss of mitochondrial membrane potential in C. albicans. These findings suggest that punicalagin is a promising plant-derived compound for use in developing new antifungal therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungal infections in immunocompromised patients have substantially increased in number and severity over the last five decades. The Cryptococcus neoformans species complex and Candida spp. continued to be the main yeast pathogens causing infections in humans [1]. Cryptococcal meningitis is the most common form of cryptococcosis [2], being C. neoformans and C. gattii are the major human pathogens causing these clinical infections [3]. Cases of candidemia have emerged, and this infection has become a severe worldwide public health problem with a mortality rate of around 50% [4, 5]. In Brazil, candidemia caused by Candida spp. is considered the 7th most prevalent [6].
A marked increase in drug resistance and the undesirable side effects caused by existing antifungal agents have greatly enhanced the interest in pursuing effective antimicrobial compounds [7, 8]. In this scenario, natural products and their derivatives are known to play a key role in pharmacological research for novel therapeutic entities. New compounds of plant origin provide a source of chemical scaffolds which present a variety of biological activities in drug development [9]. Of the medicinal plants, Lafoensia pacari A. St.-Hil (Lythraceae), a botanical species found in the Brazilian Cerrado biome, is noted for its popularly known medicinal properties (e.g., anti-ulcer, anti-cancer, and inflammatory activities) [10,11,12]. The phenolic compound punicalagin, extracted from the L. pacari leaf, has shown proven biological activities [13,14,15,16,17,18]. These include antifungal activities against the C. neoformans species complex and Candida albicans by inhibiting fungal growth in a minimum inhibitory concentration (MIC) at 4 μg/mL [16, 17].
However, the mechanisms behind the antifungal action of punicalagin against pathogenic fungi are not well understood. This study thus investigated certain parameters to gain insight into possible targets associated with the antifungal effects of punicalagin, such as the cytoplasmic membrane, cell cycle, and mitochondria activity in yeast cells. In this way, this study contributes to the development of a new antifungal therapy.
Materials and methods
Chemicals
The following chemicals, Roswell Park Memorial Institute (RPMI)-1640 medium, amphotericin B, propidium iodide (PI), RNAse, 2′-7’dichlorofluorescin diacetate (DCFH-DA), rhodamine 123, alcoholic potassium hydroxide solution, n-heptane, glutaraldehyde, cacodylate buffer, picric acid, osmium tetroxide, uranyl acetate, lead citrate, and sodium hydroxide solution were all acquired from Sigma-Aldrich, (St. Louis, MO, USA), while Epon resin was purchased from Hexion (Columbus, OH, USA).
Plant material
The punicalagin was extracted from leaves of L. pacari A. St.-Hil (Lythraceae) and characterized by HPLC/UV and ESI-TOF MS, 1D and 2D NMR spectroscopic evaluations as described by Carneiro et al. [19]. To carry out the assays, a stock solution of punicalagin was diluted in RPMI-1640 medium. The minimum inhibitory concentrations of punicalagin (MIC = 4 μg/mL) in each isolate were previously obtained to conduct the assays [16, 17].
Fungal strains
Cryptococcus gattii ATCC 24065 and Candida albicans ATCC 90028 yeast strains were used in this study.
Transmission electron microscopy analysis
This assay was performed in accordance with Brito et al. [20]. C. albicans ATCC 90028 and C. gattii ATCC 24065 were treated with punicalagin at 4 μg/mL for 72 h. After treatment, the yeasts were fixed in 2.5% glutaraldehyde, diluted in 0.1-M cacodylate buffer, pH 7.2 containing 0.2% picric acid, for 24 h at 4 °C. After two washes in cacodylate buffer for 10 min, the material was post-fixed in 1% osmium tetroxide for 2 h. Subsequently, the material was dehydrated in increasing concentrations of acetone and embedded in Epon resin. Ultrafine sections were stained with 2% uranyl acetate in distilled water and 0.2% lead citrate in a 1-N sodium hydroxide solution. Ultrastructural analysis of the slices was performed on a JEOL JEM-2100® scanning transmission electron microscope, equipped with EDS, Thermo Fisher Scientific (Waltham, MA, USA).
Determination of ergosterol content in the plasma membrane
Total intracellular sterols were determined according to the method used by Arthington-Skaggs et al. [21], with some modifications. Inocula were prepared from a single colony of C. gattii ATCC 24065 or C. albicans ATCC 90028. The yeasts were then treated with punicalagin at 4 μg/mL (MIC) or 2 μg/mL (1/2 MIC). Controls were performed using fluconazole at 8 μg/mL or 1 μg/mL for C. gatti and C. albicans, respectively. Samples of C. gattii were incubated for 48 h, and of C. albicans for 18 h under agitation at 200 rpm at 35 °C. Cells were harvested by centrifugation at 3700 rpm for 5 min and the net wet weight of the cell pellet was determined. An aliquot of 3 mL of 25% alcoholic potassium hydroxide solution was added and incubated in a water bath for 4 h at 85 °C for C. gattii and for 1 h for C. albicans. Sterol was extracted with the addition of 1 mL of sterile distilled water and 3 mL of n-heptane. The n-heptane layer was collected and diluted with ethanol and scanned spectrophotometrically between 240 and 300 nm (Varian Cary®50 UV-Vis spectrophotometer, Agilent Technologies, Santa Clara, CA, USA). Ergosterol content was calculated as described by Arthington-Skaggs et al. [21].
Evaluation of cytoplasmic membrane integrity
The effect of punicalagin on the integrity of fungal cells was investigated using PI staining. The action of punicalagin on the cell membrane of C. albicans ATCC 90028 and C. gattii ATCC 24065 was assessed based on a protocol described by Ahmad et al. [22], with some modifications. In brief, after the cells had been treated (1 × 106 cells/mL) with punicalagin at 4 (MIC) or 16 μg/mL (4X MIC) for 2 h, they were washed in PBS and then resuspended in 100 μl of PBS containing PI (2 μg/mL). After incubation in the dark for 30 min at room temperature, the cells were analyzed by BD FACSCanto II flow cytometer (Becton Dickinson, San Jose, CA, USA), by recording 10,000 events. Data were analyzed using BD FACSDiva software (version 6.0). PI+ cells with lesions of the cytoplasmic membrane were considered, while cells treated with amphotericin B (2 μg/mL) were used as positive controls.
Cell cycle analysis
In order to clarify the mechanisms of the growth inhibitory effects of punicalagin on yeasts, the cell cycle assessment was determined [23]. Cells (1 × 106 cells/mL) were treated with punicalagin (4 or 16 μg/mL) or amphotericin B (2 μg/mL) for 2 h. After that, they were centrifuged at 5000 rpm for 5 min, washed with 1 mL of ice-cold PBS, and fixed in 1 mL of 70% ice-cold ethanol overnight at 4 °C. After incubation, they were centrifuged at 5000 rpm for 5 min and washed twice with 1 mL of ice-cold PBS, and then resuspended in 400 μL of a solution containing RNAse (0.5 mg/mL). After a 1-h incubation at 37 °C, the cells were centrifuged at 5000 rpm for 5 min, and resuspended in 200 μL of PBS containing PI (5 μg/mL). After a further 15-min incubation at room temperature, a fluorescence intensity analysis of the PI–DNA complex was performed using a BD FACSCanto II flow cytometer.
Measurement of intracellular reactive oxygen species (ROS) production
ROS levels were determined using a 2′-7′dichlorofluorescin diacetate fluorescent probe (DCFH-DA). Inside the cells, the DCFH-DA is oxidized forming a 2′-7′-dichlorofluorescein (DCF), a highly fluorescent compound. Thus, the fluorescence intensity of the DCF is directly proportional to the ROS levels generated by cells [24]. In brief, cells (1 × 103–1 × 106 cells/mL) were treated with punicalagin (4 or 16 μg/mL) or amphotericin B (2 μg/mL) for 2, 6, and 24 h. They were then centrifuged at 5000 rpm for 5 min and resuspended in 300 μL of PBS containing DCFH-DA (10 μM). After 30 min of incubation at 35 °C, they were washed with PBS and analyzed by a BD FACSCanto II flow cytometer.
Assessment of mitochondrial membrane potential (ΔΨm)
The effects of punicalagin on the ΔΨm of C. albicans ATCC 90028 and C. gattii ATCC 24065 were investigated using rhodamine 123 dye [25]. In short, cells (1 × 106 cells/mL) were treated with punicalagin (4 or 16 μg/mL) or amphotericin B (2 μg/mL) for 2 h. They were then washed and incubated with PBS containing rhodamine 123 (10 μM) for 15 min at 35 °C. After that, they were washed twice with PBS and resuspended in 200 μL of PBS to conduct flow cytometry analysis.
Statistical analysis
A statistical analysis was performed using GraphPad Prism version 5.01 software for Windows (GraphPad Inc., San Diego, CA). Data are expressed as mean ± standard deviation (SD) of three independent assays. One-way analysis of variance (ANOVA) followed by Bonferroni’s test were used for intergroup variation analysis. Statistical significance was established as p < 0.05.
Results
Effect of punicalagin on yeast ultrastructure
Data obtained by transmission electron microscopy analysis (Fig. 1) showed that untreated yeast cells had normal and intact plasma membranes close to the cell wall and that there were no changes in the nucleus, cytoplasm, or cell wall. On the other hand, yeast cells treated with punicalagin showed profound changes. In C. gattii cells, punicalagin triggered a disorganization of the cytoplasmic content and a complete loss of the normal yeast cell form. The C. albicans yeast species showed a thickened cell wall, alterations in the space between cell wall and plasma membrane, an increase in the number and size of the vacuoles, and disorganization of the cytoplasmic content.
Effect of punicalagin on ultrastructure of C. gattii ATCC 24065 and C. albicans ATCC 90028. a Cells of C. gattii untreated; b Cells C. gattii treated; c cells of C. albicans untreated; d Cells C. albicans treated. Fungi cells were treated with punicalagin at 4 μg/mL (MIC) and the morphology was then analyzed by transmisson electron microscopy. Untreated yeast cells had normal plasma membranes and there were no changes in the nucleus, cytoplasm, or cell wall. In the treated cells, there was a disorganization of the cytoplasmic content and a complete loss of the normal yeast cell form
Effect of punicalagin on plasma membrane ergosterol content
Punicalagin caused a decrease in yeast plasma membrane ergosterol content in a concentration-dependent manner. In the C. gattii cells treated with punicalagin at 2 or 4 μg/mL, there was a reduction of 18.8% and 34.08%, respectively, when compared with the control cells. For the C. albicans, the reductions in ergosterol content were 3.66% and 7.88%, using the same punicalagin concentrations.
Effect of punicalagin on lesions in yeast cytoplasmic membrane
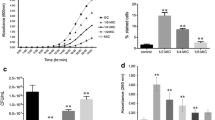
The results are summarized in Fig. 2a and b. As can be seen, amphotericin B (2 μg/mL) triggered an intense yeast cytoplasmic membrane rupture due to a significant increase in PI-stained cells (p < 0.0001) in both C. gattii (12.95 ± 1.75%) and C. albicans (20.40 ± 1.40%), when compared with untreated cells (1.0 ± 0.82% and 2.65 ± 0.15% in the same order). In contrast, punicalagin, at 4 (MIC) or 16 μg/mL (4X MIC), was incapable of causing significant cell membrane rupture in the fungi isolates tested, and showed a PI-stained cell percentage similar to that found for the controls.
Effect of punicalagin on cytoplasmic membrane of C. gattii ATCC 24065 and C. albicans ATCC 90028. Fungi cells were treated with punicalagin at 4 (MIC) or 16 μg/mL (4X MIC) for 2 h. Amphotericin B (2 μg/mL) was used as a positive control. After treatment, fungi cells were stained with propidium iodide (PI) and flow cytometry analysis followed. a Representative flow cytometry histograms. b Percentage of PI-stained. Each bar represents mean SD of three independent experiments (***p < 0.0001 vs. control; ###p < 0.0001 vs. amphotericin B)
Effect of punicalagin on the yeast cell cycle
The cell cycle phases analyzed in the presence of punicalagin presented two peaks, the first corresponding to the G0/G1 phase of the cell cycle (growth and formation of blastoconidia) and the second corresponding to the G2/M phase (cell division). The S phase, which corresponded to the area between these two peaks, was also visualized (Fig. 3a).
Effect of punicalagin on cell cycles of C. gattii ATCC 24065 and C. albicans ATCC 90028. Fungi cells were treated with amphotericin B (2 μg/mL) or punicalagin at 4 (MIC) or 16 μg/mL (4X MIC) for 2 h. Fungi cells were then processed, stained with propidium iodide (PI), and the DNA content was analyzed by flow cytometry. a Representative flow cytometry histograms showing cells at G0/G1, S, and G2/M stages. b Percentage of yeast cells at G0/G1, S, and G2/M phases. Each bar represents mean SD of three independent experiments (*p < 0.05, **p < 0.00,1 or ***p < 0.0001 vs. control)
The results demonstrated that the fungi isolates treated with punicalagin at 4 μg/mL (MIC) showed cell cycles similar to those of the control (Fig. 3a and b). On the other hand, punicalagin at 16 μg/mL (4X MIC), and also the treatment with amphotericin B (2 μg/mL), significantly increased the populations of C. albicans and C. gattii cells in the G0/G1 phase when compared with the control cells. In C. gattii, there was an increase of 67.93 ± 0.61% for amphotericin B (p < 0.001) and of 62.88 ± 5.0% for punicalagin at 16 μg/mL (p < 0.001); while these values were 66.73 ± 2.86% (p < 0.0001) and 63.41 ± 0.14% (p < 0.001) for C. albicans, when compared with the controls (41.13 ± 4.22% and 52.58 ± 5.13% for C. gattii and C. albicans isolates, respectively). (Fig. 3a and b).
As a consequence, it was seen that punicalagin and amphotericin B induced a significant reduction in cells at the G2/M phase in both fungi isolates. For the C. gattii, there was a decrease of 28.41 ± 1.19% for amphotericin B (p < 0.001) and of 28.65 ± 4.50% for punicalagin at 16 μg/mL (p < 0.001); while these values were 24.87 ± 7.57% (p < 0.0001) and 28.73 ± 0.33% (p < 0.05) for C. albicans, when compared with the controls (53.15 ± 6.24% and 38.94 ± 4.49% for C. gattii and C. albicans isolates, respectively). No treatment promoted significant changes in yeast cells at the S stage (Fig. 3a and b).
These results indicate that the antifungal effects of punicalagin could be related to the cell cycle arrest at the G0/G1 stage, which prevented progression of the normal yeast cell cycle.
Effect of punicalagin on ROS production
As shown in Fig. 4, there was no significant increase in ROS levels in C. neoformans or C. albicans treated with different concentrations of punicalagin (4 or 16 μg/mL) for 2, 6, or 24 h. In contrast, amphotericin B (2 μg/mL) led to a significant ROS overproduction in both fungi isolates (p < 0.0001), when compared with the control.
Effect of punicalagin on ROS production in C. gattii ATCC 24065 and C. albicans ATCC 90028. Fungi cells were treated with amphotericin B (2 μg/mL) or punicalagin at 4 (MIC) or 16 μg/mL (4X MIC) for 2, 6, or 24 h. Cells were then stained with DCFH-DA dye to monitor ROS levels by flow cytometry. Each bar represents mean SD of three independent experiments (***p < 0.0001 vs. control, ###p < 0.0001, or ##p < 0.001 vs. amphotericin B)
Effect of punicalagin on ΔΨm
Figure 5 shows results of the evaluation of the effects of punicalagin on ΔΨm of fungi isolates using rhodamine 123 staining. In comparison with the control, amphotericin B (2 μg/mL) triggered a ΔΨm loss of 29.89 ± 3.61% (p < 0.0001) and 82.18 ± 1.77% in C. gattii and C. albicans, respectively. On the other hand, punicalagin (4 or 16 μg/mL) had no reduction effects on ΔΨm of C. gattii, and showed a similar pattern to the control cells. However, punicalagin at 16 μg/mL led to a significant reduction in ΔΨm of 60.10 ± 18.88% (p < 0.05) in C. albicans, which was lower than that of the treatment with amphotericin B.
Effect of punicalagin on the ΔΨm in C. gattii ATCC 24065 and C. albicans ATCC 90028. Fungi cells were treated with amphotericin B (2 μg/mL) or punicalagin at 4 (MIC) or 16 μg/mL (4X MIC) for 2 h. Cells were then stained with rhodamine 123 dye and flow cytometry analysis followed. Each bar represents mean SD of three independent experiments (*p < 0.05 or ***p < 0.0001 vs. control; ###p < 0.0001 vs. amphotericin B; $p < 0.05 vs. punicalagin at 4 μg/mL)
Discussion
A promising antifungal activity of punicalagin on pathogenic yeasts, especially those of the C. neoformans complex and C. albicans, has already been seen in earlier findings [17]. Our study brought new insights into the mechanisms of action of this ellagitannin against yeast cells. The first step in understanding what occurred in punicalagin-treated yeast cells was checking the effect of treatment using ultramicroscopy since there are few reports on the ultrastructural effect of punicalagin on fungal cells [7, 18]. Although there are few studies on the punicalagin ultramicroscopy, the described authors also reported that the treated yeasts showed alteration of the cell wall, which became thicker, cytoplasmic membrane and intracellular content, leading to cell deformation and indicating that this compound causes serious damage to the yeast cell structure.
The data obtained by transmission electron microscopy showed that the yeasts underwent morphological changes when treated with punicalagin. These alterations involved membrane and cytoplasmic content. Given these results, we chose to study the cytoplasmic membrane and cellular analysis by flow cytometry in order to understand the possible mechanism of action of punicalagin, via different targets, such as interference in the cell cycle, injury to the plasma membrane, or mitochondrial damage to pathogenic species of fungus.
It was shown that punicalagin-induced damage to C. gattii and C. albicans is via a decrease in yeast plasma membrane ergosterol content with no total cell membrane rupture. Studies have shown that tannins and other natural polyphenols can inhibit the growth of yeasts due to their action on the cell, specifically through mechanisms involved in changes in cell membrane components [26,27,28]. Gallic acid, an endogenous precursor used by plants to synthesize tannin molecules, has shown antifungal activity against C. albicans, by binding to membrane ergosterol, and against Trichophyton rubrum through decreased ergosterol biosynthesis by inhibiting the sterol 14-α-demethylase and squalene epoxidase enzymes [27, 29]. In addition, gallic acid has shown in vivo effects against C. albicans in a murine systemic infection model [29]. The mechanism of primary action of azole antifungals occurs through inhibiting the 14-α-demethylase enzyme, which inhibits the synthesis of ergosterol, the main sterol component of the fungal cell membrane [28]. Ergosterol maintains cellular function and integrity [30]. According to various findings, products extracted from plants can cause a considerable reduction in the amount of yeast ergosterol [28, 31,32,33], similar to the results found here. Zorić et al. [31] showed that the phenolic compound, oleuropein, at the highest concentration studied, caused a 28% reduction in the total membrane sterol content of C. albicans. According to Reis et al. [32], the quantity of ergosterol observed in the C. neoformans species complex isolates was reduced in the presence of the plant polyphenol fisetin. In Aspergillus flavus, a reduction in the quantity of ergosterol was seen as a result of treatment with dill (Anethum graveolens L.) essential oil [33].
In addition, this study showed that punicalagin triggers yeast cell cycle arrest at the G0/G1 stage, and thereby prevents progression of the normal cell cycle. However, these alterations showed no involvement of ROS overproduction, although the punicalagin triggered a significant loss of ΔΨm in C. albicans isolate. Therefore, it seems that the concentrations of punicalagin used do not alter redox homeostasis to promote the cell death of the yeast isolates tested, probably due to its free radical scavenger property. The baicalin flavonoid showed antifungal activity against C. albicans by altering the cell cycle at the G0/G1 stage, culminating in intense irreversible cell damage, which brought about cell death through a possible apoptotic mechanism [34]. The furofuran type lignan (+)-Medioresinol has also shown cell cycle arrest in C. albicans [35]. In addition, these authors found that this phytochemical triggers intracellular ROS accumulation, which seems to be brought about by a mechanism other than punicalagin. A previous study has shown that gold nanoparticles induced apoptotic cell death in C. albicans through different pathways (e.g., yeast DNA damage and mitochondrial dysfunction); however, no involvement of the ROS signaling was seen [36]. Corroborating this, a novel equisetin-like compound, tetramic acid-289, has shown an interesting antifungal activity against Saccharomyces cerevisiae through mechanisms of cell cycle block involving mitochondrial morphological defects independently of the increase in oxidative stress [37]. Moreover, some findings have shown that natural polyphenols, such as myricetin, rhein, and gossypol, can induce ROS-independent cell death in cancer cells [38].
Conclusion
The present study presents the first attempts at uncovering the possible mechanism of action of punicalagin in C. gattii and C. albicans isolates. The results obtained showed that the antifungal activity of punicalagin seems to be related to its ability to alter the cell cycle of fungal cells and interfere in the ergosterol biosynthesis of the yeast plasma membrane. This suggests that punicalagin is a promising plant-derived compound for use in the development of new antifungal therapies. Further studies are needed to enhance the understanding of the mechanisms behind punicalagin-induced yeast cell death.
References
Pfaller MA, Diekema DJ (2011) Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 36:1–53. https://doi.org/10.3109/10408410903241444
Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids 23:525–530. https://doi.org/10.1097/QAD.0b013e328322ffac
Maziarz EK, Perfect JR (2016) Cryptococcosis. Infect Dis Clin N Am 30:179–206. https://doi.org/10.1016/j.idc.2015.10.006
Colombo AL, Guimarães T, Sukienik T, Pasqualotto AC, Andreotti R, Queiroz-Telles F, Nouér SA, Nucci M (2014) Prognostic factors and historical trends in the epidemiology of candidemia in critically ill patients: an analysis of five multicenter studies sequentially conducted over a 9-year period. Intensive Care Med 40:1489–1498. https://doi.org/10.1007/s00134-014-3400-y
Bassetti M, Righi E, Ansaldi F, Merelli M, Cecilia T, de Pascale G, Diaz-Martin A, Luzzati R, Rosin C, Lagunes L, Trecarichi EM, Sanguinetti M, Posteraro B, Garnacho-Montero J, Sartor A, Rello J, Rocca GD, Antonelli M, Tumbarello M (2014) A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med 40:839–845. https://doi.org/10.1007/s00134-014-3310-z
Doi AM, Pignatari ACC, Edmond MB, Marra AR, Camargo LFA, Siqueira RA, da Mota VP, Colombo AL (2016) Epidemiology and microbiologic characterization of nosocomial candidemia from a Brazilian National Surveillance Program. PLoS One 11:e0146909. https://doi.org/10.1371/journal.pone.0146909
Anibal PC, Peixoto ITA, Foglio MA, Hofling JF (2013) Antifungal activity of the ethanolic extracts of Punica granatum L. and evaluation of the morphological and structural modifications of its compounds upon the cells of Candida spp. Braz J Microbiol 44:839–848. https://doi.org/10.1590/S1517-83822013005000060
Xiao Y, Tang J, Guo H, Zhao Y, Tang R, Ouyang S, Zeng Q, Rappleye CA, Rajaram MVS, Schlesinger LS, Tao L, Brown GD, Langdon WY, Li BT, Zhang J (2016) Targeting CBLB as a potential therapeutic approach for disseminated candidiasis. Nat Med 22:906–914. https://doi.org/10.1038/nm.4141
Harvey AL, Edrada-Ebel R, Quinn RJ (2015) The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 14:111–129. https://doi.org/10.1038/nrd4510
de Ávila RI, Ferreira CC, Alvarenga CBM, Vieira MS, Cortez AP, Batista AC, Costa EA, Valadares MC (2019) Toxicological evaluation of Lafoensia pacari A. St.-Hil. (Lythraceae) stem bark extract: acute and subchronic studies in mice. Braz J Pharm Sci 55:e17289. https://doi.org/10.1590/s2175-97902019000217289
Müller V, Chávez JH, Reginatto FH, Zucolotto SM, Niero R, Navarro D, Yunes RA, Schenkel EP, Barardi CRM, Zanetti CR, Simões CMO (2007) Evaluation of antiviral activity of south American plant extracts against herpes simplex virus type 1 and rabies virus. Phytother Res 21:970–974. https://doi.org/10.1002/ptr.2198
Guimarães HA, Nascimento MVM, Tavares A, Galdino PM, De Paula JR, Costa EA (2010) Effects of ethanolic extract of leaves of Lafoensia pacari A. St.-Hil., Lythraceae (pacari), in pain and inflammation models. Rev Bras Farmacogn 20:328–333. https://doi.org/10.1590/S0102-695X2010000300007
Reddy BU, Mullick R, Kumar A, Sudha G, Srinivasan N, Das S (2014) Small molecule inhibitors of HCV replication from pomegranate. Sci Rep 4:5411. https://doi.org/10.1038/srep05411
Foss S, Nakamura C, Ueda-Nakamura T, Cortez D, Endo E, Dias Filho B (2014) Antifungal activity of pomegranate peel extract and isolated compound punicalagin against dermatophytes. Ann Clin Microbiol Antimicrob 13:32. https://doi.org/10.1186/s12941-014-0032-6
Howell AB, Souza DHD (2013) The pomegranate: effects on bacteria and viruses that influence human health. Evid Based Complement Alternat Med 2013:606212
Silva TC, Zara ALS, Sá FAS et al (2018) Antifungal potential of punicalagin against Cryptococcus neoformans species complex. Rev Inst Med Trop Sao Paulo 60. https://doi.org/10.1590/S1678-9946201860060
Silva TC (2017) Atividade Biológica da punicalagina sobre Leveduras do Complexo Cryptococcus neoformans e de espécies de Candida Tese. Programa de pós graduação em Medicina Tropical e Saúde Pública da Universidade Federal de Goiás, Brazil
Endo EH, Garcia Cortez DA, Ueda-Nakamura T, Nakamura CV, Dias Filho BP (2010) Potent antifungal activity of extracts and pure compound isolated from pomegranate peels and synergism with fluconazole against Candida albicans. Res Microbiol l161:534–540. https://doi.org/10.1016/j.resmic.2010.05.002
Carneiro CC, da Costa Santos S, de Souza LR et al (2016) Chemopreventive effect and angiogenic activity of punicalagin isolated from leaves of Lafoensia pacari a. St.-Hil. Toxicol Appl Pharmacol 310:1–8. https://doi.org/10.1016/j.taap.2016.08.015
Brito P, Lino-Neto J, Dolder H (2009) Sperm structure and ultrastructure of the Melittobia hawaiiensis, Perkins and M. australica, Girault (Chalcidoidea: Eulophidae). Tissue Cell 41:113–117. https://doi.org/10.1016/j.tice.2008.08.002
Arthington-Skaggs BA, Jradi H, Desai T, Morrison CJ (1999) Quantitation of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J Clin Microbiol 37:3332–3337. https://doi.org/10.1128/JCM.37.10.3332-3337.1999
Ahmad A, Khan A, Akhtar F, Yousuf S, Xess I, Khan LA, Manzoor N (2011) Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur J Clin Microbiol Infect Dis 30:41–50. https://doi.org/10.1007/s10096-010-1050-8
Riccardi C, Nicoletti I (2006) Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc 1(3):1458–1461. https://doi.org/10.1038/nprot.2006.238
Thannickal VJ, Fanburg BL (2000) Reactive oxygen species in cell signaling. Am J Physiol Cell Mol 279:L1005–L10028. https://doi.org/10.1165/ajrcmb.25.6.f213
Araújo AJ (2013) Mecanismos envolvidos na indução de morte celular por desacetilnemorona em células de câncer colorretal. Tese (Doutorado em Farmacologia) – Faculdade de Medicina, Universidade Federal do Ceará, Fortaleza
Vasconcelos LCDS, Sampaio MCC, Sampaio FC, Higino JS (2003) Use of Punica granatum as an antifungal agent against candidosis associated with denture stomatitis. Mycoses 46:192–196. https://doi.org/10.1046/j.1439-0507.2003.00884.x
Carvalho RS, Carollo CA, de Magalhães JC, Palumbo JMC, Boaretto AG, Nunes e Sá IC, Ferraz AC, Lima WG, de Siqueira JM, Ferreira JMS (2018) Antibacterial and antifungal activities of phenolic compound-enriched ethyl acetate fraction from Cochlospermum regium (mart. Et. Schr.) Pilger roots: mechanisms of action and synergism with tannin and gallic acid. S Afr J Bot 114:181–187. https://doi.org/10.1016/j.sajb.2017.11.010
de Oliveira Santos GC, Vasconcelos CC, Lopes AJO, de Sousa Cartágenes MDS, Filho AKDB, do Nascimento FRF, Ramos RM, Pires ERRB, de Andrade MS, Rocha FMG, de Andrade Monteiro C (2018) Candida infections and therapeutic strategies: mechanisms of action for traditional and alternative agents. Front Microbiol 9:1351. https://doi.org/10.3389/fmicb.2018.01351
Li ZJ, Liu M, Dawuti G, Dou Q, Ma Y, Liu HG, Aibai S (2017) Antifungal activity of gallic acid in vitro and in vivo. Phytother Res 31:1039–1045. https://doi.org/10.1002/ptr.5823
Rodriguez RJ, Low C, Bottema CD, Parks LW (1985) Multiple functions for sterols in Saccharomyces cerevisiae. Biochim Biophys Acta 837:336–343. https://doi.org/10.1016/0005-2760(85)90057-8
Zorić N, Kopjar N, Bobnjarić I, Horvat I, Tomić S, Kosalec I (2016) Antifungal activity of Oleuropein against Candida albicans - the in vitro study. Molecules 21:1631. https://doi.org/10.3390/molecules21121631
Reis MPC, Carvalho CRC, Andrade FA, Fernandes OFL, Arruda W, Silva MRR (2016) Fisetin as a promising antifungal agent against Cryptocococcus neoformans species complex. J Appl Microbiol 121:373–379. https://doi.org/10.1111/jam.13155
Tian J, Ban X, Zeng H, He J, Chen Y, Wang Y (2012) The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS One 7:e30147. https://doi.org/10.1371/journal.pone.0030147
Yang S, Fu Y, Wu X, Zhou Z, Xu J, Zeng X, Kuang N, Zeng Y (2014) Baicalin prevents Candida albicans infections via increasing its apoptosis rate. Biochem Biophys Res Commun 451:36–41. https://doi.org/10.1016/j.bbrc.2014.07.040
Hwang JH, Hwang IS, Liu QH, Woo ER, Lee DG (2012) (+)-Medioresinol leads to intracellular ROS accumulation and mitochondria-mediated apoptotic cell death in Candida albicans. Biochimie 94:1784–1793. https://doi.org/10.1016/j.biochi.2012.04.010
Seong M, Lee DG (2018) Reactive oxygen species-independent apoptotic pathway by gold nanoparticles in Candida albicans. Microbiol Res 207:33–40. https://doi.org/10.1016/j.micres.2017.11.003
Quek NC, Matthews JH, Bloor SJ, Jones DA, Bircham PW, Heathcott RW, Atkinson PH (2013) The novel equisetin-like compound, TA-289, causes aberrant mitochondrial morphology which is independent of the production of reactive oxygen species in Saccharomyces cerevisiae. Mol BioSyst 9:2125–2133. https://doi.org/10.1039/c3mb70056a
Ivanova D, Zhelev Z, Aoki I, Bakalova R, Higashi T (2016) Overproduction of reactive oxygen species – obligatory or not for induction of apoptosis by anticancer drugs. Chin J Cancer Res 28:383–396. https://doi.org/10.21147/j.issn.1000-9604.2016.04.01
Funding
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (finance code 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (process 454660/2014-6) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. TCS and ALSAZ received scholarships from CAPES/CNPq, which had no involvement in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical approval
Not applicable.
Consent for publication
All authors gave their consent for publication of the manuscript.
Additional information
Responsible Editor: Fernando R. Pavan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, T.C., de Ávila, R.I., Zara, A.L.S.A. et al. Punicalagin triggers ergosterol biosynthesis disruption and cell cycle arrest in Cryptococcus gattii and Candida albicans. Braz J Microbiol 51, 1719–1727 (2020). https://doi.org/10.1007/s42770-020-00364-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-020-00364-4