Abstract
Generally, plastics pose a variety of environmental impacts due to their increased use and non-biodegradability. End-of-life treatment is a viable way of recovering energy from plastics while at the same time reducing the amount of plastics disposed of in landfills. This paper studies the environmental impact of Non-Recycled Plastics (NRP)-to-energy processes. Three waste treatment processes were considered for NRP: pyrolysis, waste-to-energy (WtE), and landfill. The environmental impact assessment results indicated that conversion technologies such as pyrolysis and WtE are preferred over landfill. The total energy consumed in the pyrolysis process was 24635.7 MJ/tonne. The conversion technologies have a lower environmental impact and produced net positive energy from NRP. The global warming potential shows that pyrolysis (3.91 kg eq. CO2) contributes the least to global warming than waste-to-energy (18.56 kg eq. CO2) and landfill (17.5 kg eq. CO2). However, sensitivity analysis suggested that the inefficiencies of the current conversion technologies should be addressed. Between the two technologies studied, pyrolysis contributed less environmental burden, having a lower global warming potential, a higher efficiency in energy conversion, and less harmful emissions such as selenium and methane.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There has been a surge in growth in the use of plastics over the past few decades. In 1950, only two million metric tons of plastics were produced. The plastic production growth increased from 230 million metric tons in 2009 to 359 million metric tons in 2019 [1]. In 2017, over 17 percent of the plastics in the world were produced in Europe and 50 percent in Asia [2]. A large fraction of plastics present in municipal solid waste (MSW) is not recycled in the commercial market despite the efforts to increase recycling and reduce waste generation rates. Capital costs, low prices of products, plastic collection systems, plastic types, and technical knowledge are the various barriers facing the recycling of plastics. For example, in the US alone, 35.4 million tons of plastics were generated in 2017 and this accounted for 13.2 percent of the total generated municipal solid wastes that year. Of that 35.4 million, only 8.4 percent (3 million tons) was recycled, out of which 29.1 percent were polyethylene terephthalate (PET) bottles and jars, and 31.2 percent were high-density polyethylene (HDPE) natural bottles. 5.6 million tons of the plastics generated were combusted and 26.8 million tons were disposed of in landfills [3]. It is estimated that around three percent of the total plastic wastes produced in the world is disposed of in the ocean [4]. In the US, an estimated 4.5 to 13 million tons of plastics are disposed of in the ocean annually [5].
NRP refers to plastics that cannot be recycled. This is because the plastics contain toxic substances that may be released during recycling. These non-recycled plastics (NRP) constitute various challenges for ocean and land management causing significant pollution and a threat to aquatic life [6]. The transformation of non-recycled plastics (NRP) to valuable oils has gained attention over the past years because of the increased production rate of plastics [2]. This is because NRP is made up of high-energy hydrocarbons that can be converted to energy, fuel, and chemical feedstock [7]. The lightweight, low-cost, durable, and chemical resistant properties of plastics are some of the many inherent reasons for the increase in demand. Other factors causing an increase in demand for plastics include an increase in the human population, rapid economic growth, continuous urbanization, and lifestyle changes. However, plastics have a very short life span and its disposal in the environment attracts great concern because of its non-biodegradable nature [8]. The various uses of plastics are due to their existence in large varieties and forms. Therefore, it is necessary to look for different environmental disposal methods and possible energy recovery processes.
The physical and chemical properties of plastic wastes determine whether plastic wastes should undergo mechanical or chemical recycling, or energy recovery and landfill disposal. There is no particular method or chosen process for the treatment of all commercial plastic wastes in the world today. Nonetheless, the most important non-economic factor that affects the choice of what approach is more suitable for particular plastic waste is the composition of the type of polymer (low density polyethylene (LDPE), PET, polyvinyl chloride (PVC), HDPE, polypropylene (PP), etc.) and the non-polymeric material content of a plastic. Environmental disposal methods include landfills, sewage systems, and disposal into water bodies. Disposal of solid waste such as plastics in landfills releases harmful gases like methane, dioxins, hydrogen chloride, airborne particles, and carbon dioxide [8], while combustion of polymeric materials may cause serious air pollution problems. Due to the related health issues, environmental concerns, and very poor biodegradability of plastic wastes, disposal in landfills is no longer desirable [9]. Chemical recycling, a process that converts plastic wastes into feedstock or fuel has grown significantly as an ideal process to reduce the net cost of plastics [10]. Due to the high energy hydrocarbon present in NRP, there have been growing interests in converting NRP to chemical feedstock, fuel, and energy as an effort to mitigate waste management issues and at the same time produce valuable products. Pyrolysis, Waste-to-energy (WtE), and landfill gas recovery are the three main methods of treating non-recycled plastics.
Pyrolysis is an endothermic process involving the thermal decomposition of carbon-based materials in the absence of oxygen or air. The main products of pyrolysis are syngas, a gaseous mixture of carbon monoxide (CO) and hydrogen gas (H2) that can be used for heating or electricity generation, synthetic oil and char. The proportion of the byproducts of pyrolysis differs and depends on reactor design, feedstock, and reactor conditions [11]. In thermal pyrolysis, the feedstock is heated at high temperatures (350–900 °C) without the use of a catalyst. Catalytic pyrolysis involves the use of a catalyst to improve the pyrolytic process, promote process efficiency, and reduce the process temperature and time [12]. Thermal pyrolysis is the most common commercial method of treating NRP [2]. Examples of these catalysts include acidic materials (silica-alumina), zeolites (mordenite), and alkaline compounds (zinc oxide) [10]. The pyrolytic process can be used to PET, polystyrene (PS), polymethyl methacrylate (PMMA), and some other polyamides like nylon into their constitutive monomers [13].
WtE involves the combustion of solid wastes under controlled conditions. In the case of plastics, WtE is carried out at very high temperatures (850–100 °C). The WtE process produces superheated steam, which is used within a cogeneration system to produce heat and electricity. The emission of greenhouse gases (GHG) is the most felt environmental impact of combusting municipal solid waste [14]. The CO2 produced in the WtE process is the primary driver of global climate change. However, WtE can be utilized as a system for energy generation to replace fossil fuel. The emissions and amount of energy recovered depends on the efficiency of the WtE process and the source of energy being replaced [4]. Modern WtE processes tackled the problem of dioxin and other toxin releases to a large extent. The technologies employed include efficient combustion, end-of-pipe treatment, selective catalytic reduction, and the addition of suitable inhibitors [15]. In the case of the replacement of fossil fuel sources, emissions to the environment can be reduced. The primary concern of WtE is the release of toxic substances into the environment. Incomplete combustion of PE, PP, and PS can release carbon monoxide (CO) and noxious emissions while PVC can produce dioxins [16].
Landfill gas occurs naturally as a byproduct of the decomposition of organic materials in landfills. The gas is composed of approximately 50 percent methane and 50 percent carbon dioxide [17]. In the US, municipal solid waste landfills are the third-largest source of human-related methane, making up almost 15.1 percent in 2018 [17]. The primary issue with landfill is the question of the extent to which plastics decompose and the period to calculate it. As commonly used in life cycle analysis (LCA), a period of 100 years was considered as the time for the accumulated estimated emissions including gas recovery from landfills. The energy recovery from landfills has low efficiency compared to WtE. In addition, 70 percent of the plastics will emit methane with a yield of 25 kg methane per 1 tonne plastic [11].
Environmental impact assessments are considered to be one of the most effective management tools for assessing the performance of a product or process on the environment [18]. A typical LCA involves a cradle-to-grave analysis of products throughout its lifetime. Life cycle stages include the extraction of resources, material processing, manufacturing, transport, use, and end-of-life disposal [19]. LCA accounts for all uses of resources and all emissions from the system accumulated through the complete lifetime of the NRP. As a result, LCA can help direct policies and technological development to avoid environmental burden shifting among different stages of a product’s life cycle as well as the types of the impact it produces. Results largely depend on local characteristics of the solid waste management systems. Research studies have shown that recycling is mostly preferable to landfill disposal or incineration concerning life cycle energy use and the causation of global warming [20]. LCA has been successfully applied to waste thermal technologies in waste incineration with or without energy recovery [21]. The approach of LCA systematically covers all impacts related to waste management including all processes in the solid waste system as well as upstream and downstream of the waste management system.
This study aims to use an LCA to assess the environmental impact of non-recyclable plastics-to-energy processes. These processes are pyrolysis, WtE, and landfill. The focus of the analysis is to present a comparison of the environmental impacts of these three processes. LCA is often criticized for being time-consuming, challenging to apply [22], requiring the knowledge of experts and does not provide direct feedback, or lacks the flexibility to adjust to design changes in the short term [19]. However, using LCA software like OpenLCA allows the analysis to be conducted at a much higher rate, so that it can be applied on a live basis with constant updates as the design progresses or new data becomes available.
Methodology
The standard followed in this study is in accordance with the standard four-phase LCA procedure of ISO 14,040 [23], which is outlined below.
System boundary
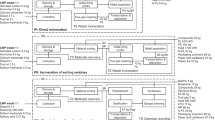
The system boundary starts with the collection of wastes and its transportation to a material recovery facility (MRF). The plastics are then sorted out for mechanical recycling and NRP are recovered and prepared for conversion to fuel/energy or disposal to landfills. As shown in Fig. 1, only the conversion of NRP to energy (pyrolysis and WtE) and disposal to landfill are considered in this study. However, municipal solid wastes may be mentioned at various points of the study when the generalization of wastes is being explained. Also, the transportation of MSW to the MRF site is not considered in the analysis as well as the transportation of NRP to the Plastics-to-fuel (PTF) conversion unit because whether the MSW or NRP would be disposed at landfill sites or used as PTF feedstock, transportation is inevitable. Plastics in MSW unlike other postindustrial plastics, which are largely homogeneous and slightly contaminated, usually bypass municipal recycling processes and are moved directly to energy recovery or recycling facilities. The recovered NRP is moved to the PTF conversion unit. In most cases, the NRP may undergo pretreatment for size or moisture content reduction before conversion [7]. The pyrolysis process produces synthetic oil and char as co-products while the WtE process produces heat, which is used to produce steam for steam turbines. Both processes produce emissions to the atmosphere. The char and solid residues were sent to landfills.
Goal and scope definition phase
The overall goal of this study is to compare the environmental impacts of several end-of-life treatments for NRP. In addition, to determine the impact of the conversion technologies of NRP on public health and the environment. The main goals are as follows: (1) evaluation of the environmental impact of the chosen processes (pyrolysis, WtE, and landfill) for waste treatment (2) comparison of the environmental impact of the three processes. The functional unit chosen for this study is 1 ton of NRP.
Life cycle inventory (LCI)
The LCI analysis involves the quantity of materials and energy inputs into the system and the resulting emission outputs of the product system. In this study, the data for the foreground processes including collection, separation, and treatment of the waste through pyrolysis, WtE, and landfill, were obtained from the literature. However, collection and separation were not included in the life cycle analysis, as the analysis was solely based on an impact assessment of NRP-to-energy processes. The data collected and compiled for the pyrolysis, WtE, and landfill processes were collected from Shonfield [24]. The background process data for upstream material use is from the EcoInvent 3.5 database [25]. After the compilation of all the unit process data, process models and life cycle inventories were constructed for the various NRP treatment using OpenLCA 1.10.4 LCA software.
Feedstock
The feedstock for the pyrolysis process includes plastics from municipal solid wastes and other non-recycled plastics as obtained from the literature. These plastics include PET, PVC, HDPE, LDPE, PP, PS, and other plastics. Heteroatoms such as chorine were present in the feedstock and therefore included in the final product. The variation in the feedstock for the WtE and landfill processes presented several complications. Therefore, the average values for the components in the feedstock were used. The feedstock composition for the WtE and landfill are LDPE, HDPE, PET, PP, PS, PVC. Chlorine and HCl are present in the emissions because of the presence of PVC in the feedstock as shown in Table 1. Therefore, chlorine was tracked although it is not shown on the system boundary.
The main feedstock sources are NRP derived from MSW (50 wt.%) and other NRP (50 wt.%). Other NRP wastes include 19 wt.% electrical and electronic plastic wastes, 17 wt.% industrial plastic wastes, and 14 wt.% coming from transportation plastic wastes and agricultural plastic wastes. The electrical and electronics plastic wastes include cable insulation from an aluminum core. The component data of the electrical and electronics plastic waste do not appear in the final data due to the limited use of this waste by most companies. The physical properties of the feedstock such as density, carbon content, and low heating values were based on the values reported in various literature [26, 27].
End of life treatments
Pyrolysis
The first conversion technology explored in this study to convert non-recycled plastic waste to fuel is pyrolysis. From the literature, the existing capacities have ranged from 20 to 641 tonnes of feedback stock per day [21]. The technology vendors considered operating facilities from pilot to commercial plant stages. Information from vendors as reported in literature shows that feedstock pre-processing required shredding, chipping, or pre-melting prior to the pyrolysis process. In addition, the feedstock composition is 100 percent plastic. The overall energy recovery efficiency of the different processes studied is for the indicated efficiency range of 75–92 percent [21]. It is assumed that the pyrolysis process only accepts plastic wastes and its main product is synthetic oil and byproduct is char. The heating value of the waste (plastics with dust and moisture) is 29.5 MJ/kg.
Waste-to-energy
In this study, WtE is seen as an energy recovery process considered in the absence of plastic-to-energy processes such as pyrolysis or gasification. In addition, the part of the plastic wastes sent to the WtE process were plastics that were not recycled. The waste stream sent to the WtE process was not sorted and pretreated. To maximize the usefulness of the incineration process, the heat produced would be recovered for energy. It is assumed that the conversion efficiency and internal energy usage of the WtE process were 18 percent and 15 percent of the electricity production, respectively [25].
Landfill
The environmental impact of landfill of plastic wastes is assessed in this study. The efficiency of gas collection from landfills for municipal solid waste is assumed to be 70 percent [17]. The gas recovered is used to drive gas turbines for electricity production. The emissions from the combustion of landfill gas include CH4, NOx, non-methane volatile organic compounds (NMVOC), CO and HCl, SO2 [17].
Part of the methane emission isis converted to carbon dioxide and released into the atmosphere. The material inputs for the landfill process were the same as those for pyrolysis and WtE obtained from various literature sources [24] and are referenced to the EcoInvent 3.5 database. The life cycle inventory data for three processes considered are shown in Table 2.
Impact assessment
The CML 2001 is an impact assessment method which restricts quantitative modelling to early stages in the cause-effect chain to limit uncertainties. Results are grouped in midpoint categories according to common mechanisms (e.g. climate change) or commonly accepted groupings (e.g. ecotoxicity). w/o Long-term (LT) method was used for the impact analysis. The impact categories assess are given below:
Global Warming Potential (GWP)
Acidification Potential (AP)
Photochemical Oxidants Creation Potential (POCP)
Eutrophication Potential (EP)
Ozone Layer Depletion Potential (OLDP)
Human Toxicity Potential (HTP)
Abiotic Depletion Potential (ADP)
CML impact indicators only concentrate on the midpoints of the cause-effect chain. These indicators sum up data on emissions (at the starting point of the cause-effect chain) to potential impacts in various impact categories such as eutrophication and ozone layer depletion. However, they do not assess the endpoints of the end of life treatments like damage to human health, which are caused by the impacts.
The impact categories in the CML assessment vary according to the different scales and range from global, regional, and local. The global impact categories are GWP, OLDP, and ADP. The regional impact category is AP, and the local impact categories are POCP, EP, and HTP.
Results and discussion
Normalization for the LCA models
The impact assessment of the end-of-life-treatment model is presented in normalization and characterization methods. These methods present the process impact contribution of potential environmental disturbances through seven impact categories. Characterization values are shown in Table 3. The least values indicate the least damaging effect of the impact categories on the environment while the high values show the environmental burden implications of the technologies. These positive values indicate negative effects on the environment through the impact categories assessed. Table 3 shows that the conversion technology (pyrolysis) studied has the lowest environmental burden from the conventional waste treatment processes. Landfill shows a higher environmental burden as seen in Fig. 2. The disposal of char and other solid residues from the pyrolysis and WtE processes to landfill sites contributes to this high effect of landfill emissions on the environment. The electricity generation of the WtE process contributed to a large extent of environmental benefits. In addition, as shown in the characterization table, pyrolysis emitted less carbon dioxide than WtE or landfill. This is because of the inefficiencies of the existing WtE processes in existence and the conversion of methane from landfills to carbon dioxide. Transportation and pretreatment of wastes were not considered for any of the treatments studied.
Energy consumption
Table 4 presents the total energy consumed by the pyrolysis process. The total energy covers the overall pyrolysis process including pretreatment processes such as size and moisture reduction. The consumption of fuels such as crude oil, lignite is reported in kg/tonne and energy consumption in MJ/tonne. Energy consumption was obtained using the equation below:
From Table 4, the total energy consumed for the pyrolysis of one tonne of plastic is 24635.7 MJ/tonne. The energy consumed is assumed to have the US conversion efficiency for the fuel to energy conversion modules and all transmission and distribution losses are also covered in the same process. In most cases, recovered energy from pyrolysis is used to replace purchased energy from the utility sector and the production of fuels. However, we only accounted for the energy consumed as part of the life cycle inventory [24].
Acidification potential
Acidification potential is concerned with emissions that increase the acidity of water and soil. It is constituted by sodium dioxide, nitrogen oxides, HCl, ammonia, and HF by their potential to form H+ ions. Acidification potential varies according to the characteristics of different regions and their atmospheric environments. It is an environmental issue that can affect quite a large region from where the acidifying gases are originally released. Acidification potential is not considered a global problem unlike global warming or ozone layer depletion. In LCA, acidification potential is a bit complicated because acid damage only occurs after exceeding a threshold limit [15].
The CML impact assessment method sums up all existing emissions regardless of location. Therefore, the results presented in this study cover the worst-case potential and not the impacts for any given location. As shown in Fig. 3 and Table 2, landfill (19.231 kg eq. SO2) and WtE (13.881 kg eq. SO2) have the worst performance because of their high acidifying emissions. Landfill emits more acidifying agents compared to WtE and nitrogen oxides alongside sulfur dioxide are the most important contributors to the acidification potential impact category. Pyrolysis generated the least acidification potential (4.774 kg eq. SO2). Other emissions like ammonia, hydrogen sulfide, and HCl contribute the least to acidification potential.
Global warming potential
The importance of the global warming potential is dependent on the period in which the global warming potential effect is assessed. Global warming potential factors for shorter times (20–50 years) indicate the short-term effect of greenhouse gases on the climate. Longer periods, 100–500 years, are used in the global warming potential prediction of cumulative effects on the global climate. GWP is used to determine the climatic impact of a substance.
It is the measure of the effect on the radiation of a particular quantity of substance over time relative to that of the same quantity of carbon dioxide. The time spent by the gas in the atmosphere and the ability of the gas to affect radiation are the factors on which GWP is dependent. The gases that contribute to global warming are CO2, CH4, and N2O. These gases have a global effect on the environment no matter where they are released. Carbon dioxide is the most important greenhouse gas, followed by methane. Other gases such as N2O have a negligible impact. Figure 4 shows that pyrolysis (3.906 kg eq. CO2) contributes less than WtE (18.565 kg eq. CO2) and landfill (17.503 kg eq. CO2) to global warming potential. The large positive value of WtE is because of the high emission of carbon dioxide.
Ozone layer depletion potential
Ozone layer depletion is a global problem similar to global warming. It indicates the presence of chlorofluorohydrocarbons (CFCs) and chlorinated hydrocarbons (HCs) in emissions. An emission of CFCs, HCs, and other ozone layer depletion chemicals anywhere will contribute to ozone depletion globally because of the long lifetimes of these chemicals in the atmosphere. The ozone layer filters UV-B radiation and its depletion can cause skin cancer, crop damage, and material damage. In this impact analysis, WtE (0.000278 kg eq. R11) and landfill (0.000341 kg eq. R11) contributed largely to ozone depletion potential compared to pyrolysis (0.0000667 kg eq. R11)as seen in Fig. 5. This is because of the energy consumption in the WtE process. The presence of PVC in the feedstock resulted in the emission of chlorinated hydrocarbons and compounds in the landfill process.
Photochemical oxidants creation potential
Photochemical oxidants or ozone creation is used to estimate the potential of forming atmospheric oxidants by some airborne substances. Its value for a hydrocarbon is measured relatively based on the ozone concentration measured at a single location and varies on the changing of the emission of the hydrocarbon in question by the same amount of reference hydrocarbon, ethylene. POCP, like acidification potential, is not a global issue. Ozone creation potential in the inventory analysis is made up largely of hydrocarbon emissions such as non-methane volatile organic compounds (NMVOC). Photochemical ozone creation and the photochemical smog that comes, as a result, depends on the chemical reaction between the volatile organic compounds and nitrogen with UV light from the sun as a catalyst. The degree of any photochemical oxidant created depends on the interrelationship between these factors—the amount of volatile organic compounds (VOC) emissions, the background level of nitrogen oxides, and the amount of received sunlight. Figure 6 shows the contribution of the processes studied. In this study, sulfur dioxide and nitrogen oxides contributed largely to the creation of photochemical oxidants. These emissions are seen significantly in the landfill process (0.251 kg eq. C2H4). Pyrolysis (0.0737 kg eq. C2H4) and incineration (0.0840 kg eq. C2H4) show less significant contributions.
Abiotic Depletion Potential
Abiotic depletion simply refers to the consumption of non-renewable resources such as fuels, metals, and minerals. It is regarded as a global issue and is shown in Fig. 7. The positive contribution of landfill in this category is because of the deposition of solid residues from the pyrolysis and WtE processes that end up in landfills.
However, this value is low due to the use of the heat from the WtE process to produce steam, which is used to produce electricity in steam-powered plants for the WtE process. Pyrolysis (0.00017 kg eq. Sb) and WtE (0.000227 kg eq. Sb) contributed less significantly to abiotic depletion potential. The abiotic depletion potential contribution for landfill (0.00086 kg eq. Sb) is more significant due to leachate from landfills to water bodies.
Human toxicity potential
Human toxicity deals with the effect of toxic emissions that go to air, water, and soil. Toxicity factors are calculated using the acceptable daily intake or daily tolerable intake of toxic substances. The human toxicity impact category is dependent on location and it is not a global issue. Human toxicity potential in LCA is difficult to calculate because people respond to toxic chemicals in different ways and chemicals can be very toxic. According to Fig. 8, WtE (174.23 kg eq. DCB) and pyrolysis (180.64 kg eq. dichloro benzene (DCB)) contribute less significantly to human toxicity. This impact is mostly contributed by arsenic, cadmium, selenium, nitrogen oxide, and hydrogen fluoride. Nonetheless, antimony and vanadium are the main cause of human toxicity by the WtE process because residues from WtE go to landfills. Landfill (697.48 kg eq. DCB) contributed more significantly to human toxicity potential. Dichlorobenzene is the key toxic component contributing to this impact category. Vanadium compounds are often used to provide smoke retardant properties to plastics.
Eutrophication potential
Eutrophication impact refers to the consumption of dissolved oxygen in water by waterborne emissions. These emissions include phosphorus and nitrogen. The CML impact category nitrifies these emissions regardless of location so the results presented here represent worst-case potential impacts rather than the actual impacts that would occur at a given location. It is immediately noticeable in Fig. 9 that landfill (0.242 kg eq. phosphate) and pyrolysis (0.196 kg eq. phosphate) has the largest contribution to eutrophication. This is because of the leaching of disposed of residues from pyrolysis and WtE in landfills that eventually go to bodies of water. Incineration (0.0569 kg eq. phosphate) is seen to contribute the least to eutrophication while the main contributor to eutrophication is phosphorus. Figure 10 shows a closer look at the impact categories. This is because of the leaching of disposed of residues from pyrolysis and WtE in landfills that eventually go to bodies of water. Incineration (0.0569 kg eq. phosphate) is seen to contribute the least to eutrophication while the main contributor to eutrophication is phosphorus.
Sensitivity analysis
Table 5 and Fig. 11 show the sensitivity analysis of the end-of-life treatments studied. The pyrolysis and WtE non-recycled plastic-to-energy processes can be improved by 30% in the next decade. The sensitivity analysis was based on the assumption of the inefficiencies of the end-of-life treatments studied. More than 50% of the carbon dioxide emission from the pyrolysis process can be reduced as seen in Table 3. This reduces the global warming effect of the pyrolysis process. This is achieved through the selection and pretreatment of plastics and the improvement of the pyrolysis process. Abiotic depletion potential showed a 100% significant contribution from the pyrolysis process due to the improvement of the pyrolysis process, as more resources would be consumed. The sensitivity report is based only on the assumption of process efficiency improvement and reduction of emissions through this improvement and therefore is not guaranteed to show significant results if the assumptions are not followed (Fig. 11).
Conclusion
This life cycle assessment covers only waste treatment options for non-recycled plastics as shown in the system boundary. The growing generation of plastics and the residuals of recycled plastics drives the need for improving treatment options such as the conversion technologies (pyrolysis and WtE) studied in this paper. Overall, landfill was the least environmentally friendly waste treatment process. However, the global warming potential impact category presents pyrolysis as the most favorable conversion technology process. This is because of the inefficiencies of the current pyrolysis process as a new technology for the treatment of non-recycled plastics. The results in this study suggest the need for non-recycled plastics to undergo the waste to energy processes before disposal to landfills. Landfill contributes an exceptionally large impact on the environmental burden (water and soil) through leachates. Carbon dioxide, sulfur dioxide phosphates, antimony, dichlorobenzene, selenium, heavy metals such as chromium and cadmium, and methane are the most common emissions reported in this study. Phosphorus and its compounds are the major emissions to water contributing to eutrophication. Transportation and other pretreatment processes such as removal of dirt and drying to reduce moisture were not considered in this study and therefore, were not discussed.
Change history
27 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42768-021-00078-9
References
Mastellone, M.L. 2019. A feasibility assessment of an integrated plastic waste system adopting mechanical and thermochemical conversion processes. Resources, Conservation & Recycling: X 4: 100–117.
Rodriguez, L. Valdiviezo, T. Harden, et al. 2019. Transforming non-recyclable plastics to fuel oil using thermal pyrolysis. http://ccnyeec.org/wp-content/uploads/2013/12/GroupH_FINALREPORT.pdf. Accessed 29 Oct 2020
Miandad, R., Rehan, M., Barakat, M.A., et al. 2019. Catalytic pyrolysis of plastic waste: moving toward pyrolysis based bio-refineries. Frontiers in Energy Research 7: 27.
Ritchie, H., Faqs on plastics, 2018. https://ourworldindata.org/faq-on-plastics.
United States Environmental Protection Agency, Municipal solid waste generation, recycling, and disposal in the united states: Facts and figures for 2008., http://www.epa.gov/waste/nonhaz/municipal/pubs/msw2008rpt.pdf.
United States Environmental Protection Agency, Facts and figures about materials, waste and recycling, 2019. https://www.epa.gov/facts-and-figures-about-materials-waste-andrecycling/plastics-material-specific-data
Benavides, P.T., Sun, P., Han J., et al. 2017. Life-cycle analysis of fuels from post-use non-recycled plastics. Fuel 203: 11–22.
Guerrero, L.A., Maas, G., Hogland, W. 2013. Solid waste management challenges for cities in developing countries. Waste management 33 (1): 220–232.
B. Bauman, How plastics contribute to climate change, 2019. https://yaleclimateconnections.org/2019/08/how-plastics-contribute-to-climate-change/.
Research Triangle Institute, Environmental and economic analysis of emerging plastics conversion technologies, 2012.
Eriksson, O., Finnveden, G. 2009. Plastic waste as a fuel-co2-neutral or not? Energy & Environmental Science 2 (9): 907–914.
Verma R., Vinoda, K. S., Papireddy, M., Gowda, A. N. S. 2016. Toxic pollutants from plastic waste-A review. International Conference on Solid Waste Management, 5IconSWM 2015. Procedia Environ. Sci, vol. 35, pp. 701– 708, 2016. Available at: https://doi.org/10.1016/j.proenv.2016.07.069
Chen, D., Yin, L., Wang, H., et al. 2014. Pyrolysis technologies for municipal solid waste: a review. Waste Management 34 (12): 2466–2486.
Lombardi, L., Carnevale, E., Corti, A. 2015. A review of technologies and performances of thermal treatment systems for energy recovery from waste. Waste Management 37: 26–44.
Mukherjee, B.D., Ghosh, S.K. 2016. A review on technologies of removal of dioxins and furans from incinerator flue gas, Procedia environmental sciences, vol. 35, pp. 528–540.
Barabad, M.L.M., Jung, W., Versoza, M.E., et al. 2018. Characteristics of particulate matter and volatile organic compound emissions from the combustion of waste vinyl. International Journal of Environmental Research and Public Health 15 (7): 1390.
United States Environmental Protection Agency, Basic information about landfill gas, 2019. https://www.epa.gov/lmop/basic-information-about-landfill-gas#%3A~%3Atext%3DMunicipal%20solid%20waste%20(MSW)%20landfills%2Cof%20these%20emissions%20in%202018
Jaunich, M.K., Levis, J.W., DeCarolis, J.F., et al. 2019. Solid waste management policy implications on waste process choices and system wide cost and greenhouse gas performance. Environmental Science & Technology 53 (4): 1766–1775.
Hetherington, C., Borrion, A.L., Griffiths, O.G., et al. 2014. Use of LCA as a development tool within early research: challenges and issues across different sectors. The International Journal of Life Cycle Assessment 19 (1): 130–143.
Wernet, G., Bauer, C., Steubing, B., et al. 2016. The ecoinvent database version 3 (part i): Overview and methodology. The International Journal of Life Cycle Assessment 21 (9): 1218–1230.
Gear, M., Sadhukhan, J., Thorpe, R., et al. 2018. A life cycle assessment data analysis toolkit for the design of novel processes–a case study for a thermal cracking process for mixed plastic waste. Journal of Cleaner Production 180: 735–747.
Hanes, R.J., Bakshi, B.R.. 2015. Sustainable process design by the process to planet framework. AIChE Journal 61 (10): 3320–3331.
E. International Organization for Standardization, 14040: 2006. environ. manag. cycle assessment and principles fram. European Committee for Standardization, 2006.
Shonfield, P. 2008. LCA of management options for mixed waste plastics. UK: WRAP.
Jeswani, A.H., Azapagic, A. 2016. Assessing the environmental sustainability of energy recovery from municipal solid waste in the uk. Waste Management 50: 346–363.
McDougall F.R. , White, P.R., Franke, M., et al. 2008. Integrated solid waste management: a life cycle inventory. Wiley, United States
Tsiamis, D.A., Castaldi, M.J. 2016. Determining Accurate Heating Values of Non-Recycled Plastics (NRP). Earth Engineering Center City College City University of New York, United States. March 23.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, H., Themelis, N.J. & Bourtsalas, A. Environmental impact assessment of emissions from non-recycled plastic-to-energy processes. Waste Dispos. Sustain. Energy 3, 1–11 (2021). https://doi.org/10.1007/s42768-020-00063-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42768-020-00063-8