Abstract
The repair of tendon to bone junction (TBJ) remains a tremendous challenge in tissue engineering due to the complicated structure, components, mechanical properties, and cell types. In order to reconstruct the tissue and restore its functionality, biomedical scaffolds with hierarchical and gradient structures have been fabricated by various strategies. In recent decades, electrospinning has become one of the most popular methods in fabricating TBJ scaffolds due to easy fabrication, high porosity, and ECM-like nano-scale structure. However, mechanical properties are the pain point of electrospun biomedical scaffolds. Traditional textile technology can be exploited to compensate for this weakness, which will be deeply discussed here. This review will start with a brief introduction to the structure and function of the native TBJ tissue and a short overview of electrospinning technology. Then, different electrospun biomedical scaffolds for TBJ repair will be summarized and compared. Furthermore, some advanced technologies and modification methods in fabricating functionalized electrospun TBJ scaffolds are discussed. In the end, current challenges and solutions are being proposed, which would provide instruction for the research of electrospun textile TBJ scaffolds.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The human body is composed of multiple functional tissues that work in tandem to support our daily activities. The musculoskeletal system is the most representative multi-tissue unit in human bodies, which is composed of skeletal muscle, tendon, bone, and their interfaces (Fig. 1a). According to the research report, tendon and ligament injuries account for up to 30% of all musculoskeletal consultations with 4 million new incidences among the annual musculoskeletal clinical cases [1], while physical training accounts for up to 80% of injuries [2]. The rotator cuff tendon of the shoulder and the anterior cruciate ligament (ACL) of the knee are two of the most common injury sites. Failures in the repair site of the musculoskeletal system usually occur with the generation of scar tissue, which results in defects in the regenerated tissue. They may unable to withstand the external load to the same degree as the native tissue [3, 4]. The interface, tendon to bone junction (TBJ), is relatively complicated in structure, components, and mechanical properties [5]. TBJ is the place for tendon and bone connection and withstands higher pressure and tension during movement. It plays the role of dissipating energy, and the mechanical properties are extremely difficult to restore after injury. Collagen fibers in tendons and ligaments show very strong tension and withstand tensile stress, while bone tissues are excellent in anti-compression loading. Therefore, TBJ exhibits a gradient in collagen fiber alignment, compositions, and mechanical properties. For the tissue engineering strategy in the TBJ regeneration, challenges still exist to replicate the intricate gradient micro- and nano-structure, as well as the gradient components and mineralization.
a The cross-section overview of the native TBJ tissue; Reproduced with permission from ref [16], Copyright 2015, Elsevier. And a schematic illustration of gradient biomimetic scaffold for TBJ. b A summary of research outputs for electrospun TBJ scaffold from 2000 until 2022 (data from Google Scholar search)
Traditional clinical therapeutic methods to repair the injury of TBJ include surgical sutures and autologous transplantation. However, scar tissue tends to generate in the process of tissue repair, and this defective tissue is hard to bear force loading as much as the native one. So, the repaired site is easy to be damaged during exercise. Autologous transplantation also requires suturing process. In the meantime, the autologous graft might cause immune rejection and inflammation, which make it worse at the injured tendon. Using biomedical scaffolds to repair and reconstruct the injured TBJ has been studied a lot, and it has been testified to be an important and effective way for the repair. Figure 1a shows the native TBJ tissue, the biomimetic design of the gradient structure, as well as the biomimetic design of the scaffold. Such scaffolds include single-layer or multi-layered films, hydrogels, foams, electrospun nanofibers, and textiles. However, all of the films, hydrogels and foams show relatively poor mechanical properties, which are difficult to withstand the force loading during scaffold implantation and tissue regeneration. In this way, electrospun textile is a better format for constructing scaffolds.
Nanofibers show great potential in clinical applications for the following reasons: (1) Nanofibers could be easily fabricated using different top-down or bottom-up methods, with different structures and sizes according to the clinical need. (2) Nanofiber scaffolds resemble the ECM, which is suitable for cell adhesion and guiding tissue regeneration. (3) The large specific area provided by the nano-size fiber offers more places for cells to adhere and migrate. In the meantime, the high porosity benefits cell migration and nutrition exchange. Thus, nanofibers show enormous potential for clinical applications such as wound dressings, sutures, tissue engineering, etc.
Among the diverse methods to fabricate nanofibers, electrospinning is a simple and convenient approach to fabricate 2D and 3D microfibers and nanofibers bulk scaffolds. It has emerged as a facile method in fabricating tissue engineering scaffolds due to the ECM-like structure, which is suitable for constructing tissue resembling native structure and function [6]. As one of the main methods to fabricate tissue engineering scaffolds for musculoskeletal injury repair, many researchers have designed and fabricated scaffolds for single tissue repair, including tendon, ligament, bone, and cartilage [7, 8]. These scaffolds showed positive effect on reconstructing the injury site with bio function and structure. Nevertheless, the repair of the tendon to bone junction (TBJ) remains a challenge because of its complexity in structures and components. Many efforts have been made to fabricate scaffolds that mimic the native TBJ tissue to facilitate the reconstruction. Electrospinning could easily achieve the gradient and hierarchical structure in the 2D or 3D scaffolds, which has been reported to be applied for TBJ healing [9, 10]. However, traditional electrospun nanofiber membranes hardly meet the dimensional need and mechanical properties in the repair of the native tissue. This defect could be compensated by combining the electrospun nanofiber into the textile structure. Moreover, a textile structure composed of electrospun nanofiber yarns could be made with a hierarchical structure to mimic the native tissue better. In order to promote the regeneration of TBJ, growth factors and mechanical stimulation are also important.
The number of research work on electrospun nanofiber scaffolds for TBJ treatment is increasing remarkably in the recent 20 years, as summarized in Fig. 1b. Electrospun scaffolds for the bone, cartilage, and tendon have been reported earlier than the TBJ. In 2009, electrospun nanofiber scaffolds with gradient and mineral structure were reported by Li et al., which were used for connecting soft and hard tissue at TBJ [11]. The fabrication and construction of scaffolds to mimic the native insertion to facilitate the connection of tendon (soft) and bone (hard) tissue still is a big challenge for researchers. Hence, it is important to summarize and overview the current research works, which would instruct future work. However, although there are some review papers focused on the electrospun scaffolds for tissue engineering [12], textile technologies for tissue engineering [13], nanofiber-based and textile scaffolds for bone and tendon [14, 15], gradient fabrication strategy for TBJ treatment [16,17,18], an overview on the research works on electrospun scaffolds for TBJ treatment cannot be found.
This review aims to present a brief overview of current research on fabricating tissue engineering scaffolds using electrospinning and textile strategy for tendon to bone junction regeneration. Meanwhile, it also aims to introduce and promote the strategies of constructing biomimetic micro- and nano-hierarchical and gradient textile structure. This review will start with a brief discussion of the structure and traditional therapeutic methods of TBJ injury and followed by a short elaboration of electrospinning technology. The fourth part covered the fabrication method of electrospun nanofibers in different formats, including membranes, yarns, and textiles. Furthermore, current challenges in the fabrication of electrospun textile scaffolds for TBJ treatment with advanced functions will be discussed.
Structure and Repair Strategy of Tendon–Bone Junction (TBJ) REPAIR
Structure of TBJ
The main function of tendons is to transfer force between muscles and bones. In this case, tendons must remain elastic and flexible enough to require high mechanical properties [17, 19]. Therefore, tendons have the following three main characteristics: first, there are uniaxially oriented nanometric collagen fibrils. Second, collagen fibrils are organized into fiber bundles according to a hierarchical structure. The collagen fibrils in the relaxed state appear wavy, and when the tendon is stretched, the curly collagen fibrils begin to straighten. And the muscle forces are transferred to the bone by the fiber bundles and ultimately cause movement. Third, the gradual transition of tendon–bone junction (TBJ) and tendon–muscle junction (TMJ) can achieve effective load transfer and minimize stress concentration [16, 19, 20]. The tensile modulus of the tendon in the direction of muscle force is only ~ 0.4 GPa and of bone is ~ 20 GPa. Tendons are much stronger than TBJ or TMJ. So, in overload, it is more common for MTJ or TBJ to be damaged before the tendon [16, 17].
The specific morphology of the TBJ is called the enthesis (also known as fibrocartilage entheses). To reduce these mechanical stress concentrations and allow for efficient stress transfer at such scales, it is necessary for effective force transfer between soft tissue, such as ligaments, tendons, joint capsules, and hard bones [16, 20]. Thus, the enthesis shows special gradients at all levels, including matrix composition, cell phenotype, tissue structure, and mechanical properties, while realizing the purpose of functional grading. According to this gradient concept, the enthesis can be divided into four continuous regions (Fig. 2a) with different components [16, 19, 20]: Regions I, the tendon. This region has 90% (v/v) well-aligned collagen fibers (predominantly type I), small amounts of the proteoglycan decorin, and water; Regions II, the non-mineralized fibrocartilage. This area is mainly composed of collagen Types II and III. It also contains small amounts of types I, IX and X, and of the proteoglycans aggrecan and decorin; Regions III, the mineralized fibrocartilage. This area is composed of round and hypertrophic fibrochondrocytes. This area is also called “tidemark” as the mineralization front and a boundary between soft and hard tissue. In the final region, the mineralized fibrocartilage fuses into bone tissue containing osteoblasts, osteocytes, and osteoclasts. The bone zone contains ~ 40% (v/v) of type I collagen following the hierarchical structure of the tendon and contains ~ 50% (v/v) by volume of a stiff, carbonated apatite mineral.

Reproduced with permission from ref [41], Copyright 2021, Baishideng. b Traditional therapeutic strategies for TBJ repair; reproduced with permission from ref [27], Copyright 2020, Elsevier. c The three core factors of TBJ engineering technology are ideal scaffold, growth factor, and cultured cells
a Structure and composition of TBJ (fibrocartilaginous entheses); reproduced with permission from ref [16], Copyright 2015, Elsevier.
Treatment Strategy of TBJ Repair
TBJ injury is a kind of motor system trauma occurring at the tendon and bone junction, such as rotator cuff injury, anterior and posterior cruciate ligament injury, and Achilles tendon injury. According to statistics, about 30% of all clinical musculoskeletal cases are related to injuries of the TBJ. Globally, there are 4 million new cases of TBJ injuries each year, most of which require surgical intervention and long-term rehabilitation [17, 19, 21,22,23]. Clinically, there are many ways to repair and reconstruct TBJ injuries. Most acute tendon injuries can be treated by suturing the tendon to the tendon or re-inserting the tendon into the bone (Fig. 2b). However, according to clinical data statistics, the recurrent rate after rotator cuff injury is about 25%, and the recurrent rate after large rotator cuff defect is higher than that of 57% [24,25,26]. Tendon transplantation is considered a viable option for tendon reconstruction. However, the disadvantages are the need to kill healthy tissue and are prone to anchor failure, and lack of mechanical strength [19, 20]. Animal tissues from mammalian can also be used to replace human tissue to realize tendon transfer. Because it has good feasibility in theory, the related research develops rapidly [17, 19, 20]. Lu et al. [27] first reported a special gradient heterogeneous decellularized ECM scaffold by bone, fibrocartilage, and tendon tissue in 2020 (Fig. 2b). The complicated pretreatment and high cost are the disadvantages that cannot be ignored. Artificial synthetic and natural material scaffolds do not have this risk and have strong advantages in cost and preparation methods [15, 23, 28].
The three core factors of TBJ engineering technology are ideal scaffolds, growth factors, and cultured cells (Fig. 2c) [17]. The ideal scaffolds would provide mechanical strength, support cell adhesion and growth, and ultimately guide three-dimensional tissue formation. Therefore, the ideal scaffolds should be biocompatible, highly porous, and biodegradable [21, 28,29,30]. With the rapid development in recent years, researchers have proposed many methods for the preparation of artificial scaffolds. Sol–gel method is a commonly used method because many gel materials have very good cytocompatibility and biodegradability and have hydrophilic groups on the surface [31, 32]. The freeze-drying method has obvious controllability and maneuverability in endowing artificial scaffold materials with three-dimensional pores and anisotropic structures. Tellado et al. [33] designed a two-phase silk fibroin protein scaffold with anisotropic/isotropic pore arrangement through freeze-drying and salt-leaching treatment. Harley’s group demonstrated a collagen-GAG (CG) scaffold via freeze-drying [34]. This scaffold has a consistent mineralized gradient and geometric anisotropy at the primary TBJ. Braiding yarns into 3D scaffolds by machine or hand can control its 3D macrostructure well [35]. Besides, it is easy to fabricate layered or multiphase scaffolds with layered structures to simulate the multilayered structure, mechanical properties, and grading characteristics of TBJ [36]. Gomes’s group obtained Polycaprolactone (PCL)/gelatin and PCL/gelatin/hydroxyapatite nanoparticle (HAp) continuous microfiber via wet spinning [37]. By adjusting the extrusion rate, microfibers with composition gradients were obtained to simulate soft (tendon) and hard (bone) tissues to form the TBJ scaffold. The coating method has the best operability and simplicity in constructing gradients of three-dimensional materials’ composition or properties. Xia’s group developed a biomimetic approach to design hierarchical scaffolds for tendon-to-bone repair [38]. The length scale of the mineral gradients of this scaffold (a tunable distance of 25–50 μm) matches the length of the enthesis.
But for now, these methods more or less have some disadvantages that cannot be ignored. In terms of the sol–gel method and the freeze-drying method, they have poor control over morphology and cannot design materials at the micro-scale. Moreover, the obtained scaffolds often have a certain thickness and rigidity, which is not conducive to the repair of TBJ in the human body. The limitations of braiding and wet spinning also regulate the material at the micro-scale and complex preparation steps. In addition, it is challenging to fabricate large-scale scaffolds efficiently by using these two methods, which are not cost-effective and time-consuming. In the end, the coating method cannot be used as an individual fabrication method for scaffolds.
At present, electrospinning is the most widely used method in the research of artificial scaffolds for TBJ repair. The advantages of electrospinning technology in this field can be summarized as follows [6, 19, 21]: (1) Good controllability. The pore size, diameter and orientation morphology of electrospun nanofibers as well as the physical properties (thickness, number of layers, mechanical strength and area) and chemical properties (wettability, conductivity, biocompatibility, etc.) of electrospun membranes can be determined or controlled artificially [39, 40]. This advantage is also beneficial to the construction of structural gradient and component gradient of electrospinning scaffold. (2) There is a wide range of polymers (natural and synthetic) with benign biocompatibility and biodegradability, as well as composites containing inorganic materials that can be used for electrospinning [17, 22]. This can give the electrospinning scaffolds more functionality. (3) Electrospinning membrane usually has high porosity and large surface area to load and wrap active biological species, such as ECM protein, enzyme, nucleic acid, and growth factor. This is very conducive to cell differentiation and proliferation [19, 20, 41]. (4) Electrospinning materials can have a variety of different macro-morphology in three-dimensional space, including one-dimensional yarns, two-dimensional membranes, or three-dimensional scaffolds. Therefore, different combinations can be stacked, arranged, and composed. Because of these advantages, electrospinning technology has made great contributions in this field and obtained a series of significant research achievements [6, 22, 28, 39].
Principle and Development of Electrospinning Technology
Nanofibers have attracted great interest from materials scientists for their unique features of large specific surface area and high porosity to apply them in advanced application fields. Methods to fabricate nanofibers have been summarized and discussed elsewhere [42]. Current nanofiber fabrication strategies include electrospinning, polymerization, self-assembly, CO2 laser supersonic drawing, solution blow spinning, plasma-induced synthesis, electrohydrodynamic direct writing, centrifugal jet spinning, etc. Electrospinning is the most established and widely adopted nanofiber fabrication strategy for its easy manipulability. The electrospinning strategy and its application in the biomedical field will be discussed in this section.
Principle and Equipment of Electrospinning
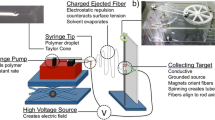
The advantages of artificial electrospun scaffolds for TBJ repair are due to the chemical and physical properties of the scaffolds. These properties are largely determined by the essence and principle of electrospinning. The modernization of electrospinning, historically, has been traced to Anton Formhals’ first patent on electrospinning in 1934 [43, 44]. After nearly 100 years of development, the electrospinning mechanism is constantly improving, and technology is constantly developing. At present, it has developed into a convenient, efficient and controllable nanomaterial preparation technology. As to laboratory research, the basic electrospinning equipment mainly includes a power supply, conductive needle, injection pump and collector (Fig. 3a) [43, 45]. The electrospinning research can be popularized in the common laboratory because the easy construction of the equipment. As to the electrospinning process, from the high voltage applied to the needle to the formation of the nanofiber membranes, there are four successive stages [46]: In the first stage, the syringe pushes the polymer liquid out of the needle to form small droplets. It generates an induced charge under the action of a high-voltage electric field, resulting in a repulsive force in the opposite direction to the surface tension. This leads to the formation of Taylor’s cone. As the voltage rises, the electrostatic repulsion overtakes the surface tension, and the charged liquid shoots out of the tip of Taylor’s cone as a fiber. In the second stage, the fibers appear straight in the near field and travel in a stable state for some distance (Fig. 3b). In the third stage, after entering the far-field region, the viscoelastic properties of the liquid itself could no longer restrain Rayleigh instability, resulting in bending disturbance and beginning to transform into a spiral state [47]. In the final stage, the helical jets solidify on the receiver to form solid fibers. The repetition of these stages leads to the continuous superposition of a large number of fibers and the formation of porous electrospinning nanofiber membranes [48,49,50].
It can be inferred from the whole electrospinning process that many factors have a decisive influence on the morphology of electrospinning fibers. The first important factor is the spinning solution parameters, which include viscosity, concentration, surface tension, and conductivity of polymer precursor [43, 44]. For example, when the viscosity is too low, the liquid cannot form continuous and stable nanofiber but form independent micro/nanodroplets; the second one is the processing parameters, which include collecting distance, voltage and receiver state. If the receiving distance is too small, the stabilized nanofibers have not been formed. When the distance is too large, the fiber may not reach the receiver; the third one is the environmental parameters, including temperature and humidity, which are extremely for quality control. Both of these factors affect the conductivity, viscosity and evaporation rate of the solution [51]. Therefore, the expected electrospun nanofiber membrane is the result of the synergy of many factors. Researchers can also control some factors and design electrospinning nanofiber membranes to achieve specific functions. This is very important for artificial electrospun scaffolds for TBJ repair design [45, 51,52,53,54].
With the development of technology and the change in demand, basic electrospinning equipment has been unable to meet scientific research needs, especially in industrial production. In recent years, electrospinning technology has undergone many iterations and upgrades, mainly in the following two aspects: One is the design of needles with complex structures (Fig. 3c) [55,56,57,58], and another is the breakthrough of needle-free electrospinning technology [59,60,61]. For example, co-axial, triaxial, or eccentric needles can produce nanofibers with cores, hollow structures, or Janus structures [58]; the multi-nozzle needle design enables simultaneous spinning of many different solutions, and the electrospinning core-spun yarn can be generated on a single collector using a dual-system conjugated electrospinning technique [43]. The multi-needle system can improve electrospinning efficiency to a certain extent, but it is still out of reach for industrial production. By exploiting single, co-axial, multi-axial, eccentric, and side-by-side needles, nanofibers with different morphologies could be produced, such as single-component nanofibers, multi-component nanofibers, core–shell nanofibers and Janus fibers. These nanofibers have been applied in tissue engineering with unique functions. The multi-component (especially the blending of natural polymer and synthetic polymer) nanofibers combine biocompatibility and high mechanical properties to make the scaffolds. In contrast, the core–shell fiber could protect the drugs or bioactive components in the core and achieve a controlled release effect. The Janus fibers possess two individual polymers with different properties and could load two separate bioactive components to achieve multi-function. Combining different types of nanofibers could achieve better treatment effects for TBJ. Therefore, needle-free electrospinning driven by industrialization technology emerged as required. Development so far, a variety of needle-free electrospinning technology, has been very mature, and many related enterprises have been established [60, 62]. By mechanism, they first attach polymer solutions to one-dimensional lines or two-dimensional surfaces. A high voltage is then applied to the solid, resulting in a large number of filamentous sites on the surface. Sometimes, however, the polymer solution solidifies on a solid surface, making it unconducive and unable to spin. Therefore, the technology has high requirements for environmental parameters and physical parameters of the solution.
Electrospinning Materials
As mentioned earlier, one of the great advantages of electrospinning technology is the wide selection of raw materials, which can be divided into synthetic polymers and natural polymers. The synthetic polymer shows favorable spinnability, high tolerance and good extensibility. Synthetic polymer (like PVDF and PU) electrospun nanofiber membranes possess benign mechanical strength that could be used for protective clothing, separation membrane, etc. [22, 43, 63]. However, these synthetic polymer materials are often non-biodegradable, so their prospects for biomedical applications are limited. Apart from them, some synthetic materials possess good biocompatibility and biodegradation properties, such as PGA, PLGA and PCL [21, 29]. But in general, natural polymers generally show better biocompatibility and lower immunogenicity. In this way, natural polymers are usually co-spun with synthetic polymers to acquire scaffolds with biocompatibility and robust mechanical properties. In recent years, electrospinning with collagen, gelatin, elastin and silk fibroin blended with synthetic polymers has been reported, and such scaffolds showed favorable clinical function [54, 64, 65].
Here are a few representative examples. Collagen is a major component of human tissues such as skin. It is highly conserved and relatively non-immunogenic that has been used in a variety of tissue-engineering applications, particularly in wound dressings. The critical problem of collagen electrospinning lies in the control of the concentration. Collagen can be dissolved properly at a sufficient concentration and become the original electrospinning solution. Gelatin is a natural polymer hydrolyzed from collagen, which shows biodegradability and biocompatibility in physiological environments. Electrospun gelatin fibers are easily fabricated due to the favorable spinnability of the gelatin solution. This electrospinning system is also environmentally friendly and non-toxic since the gelatin is dissolved in acidic or high-temperature aqueous solutions. However, its poor mechanical performance hinters at the application in tissue engineering alone. Silk fibroin protein extracted from silk is also a commonly used natural materials in biomedical scaffolds. Its advantages lie in the minimal inflammatory response, good mechanical properties and optional wettability. Solvent selection is the most important problem in silk fibroin electrospinning [54, 66]. Chitosan, which can be extracted from shrimp shells and crab shells, is an economically safe and biodegradable natural polymer. In earlier studies, chitosan needed to be mixed with other polymers to be electrospun. It was not until 2004 that pure chitosan electrostatic fabrics were successfully prepared. Direct electrospinning of pure chitosan was carried out by using tetrahydrofuran (THF) and acetic acid as a mixed solvent. For the electrospinning scaffolds for TBJ repair, it is extremely important to deeply understand natural polymer materials and their selection. However, it should be emphasized that natural polymer materials also have some limitations, such as easy degeneration, uncontrollable degradation time, poor chemical stability and the weak mechanical stability mentioned before [52, 53, 67].
Electrospun Textiles for Biomedical Application
After the rapid development in recent years, electrospinning technology has been gradually applied in various research fields [43, 44, 49, 55, 68,69,70]. For example, in the fields of environment, energy, and especially in the textile, the contribution of electrospinning technology is of epoch-making significance [71,72,73]. Innovative technologies such as waterproof and moisture permeability, radiation refrigeration, thermal cloth and intelligent fabrics are inseparable from the contribution of electrospinning technology [74,75,76,77,78,79,80,81,82]. Electrospinning technology is also applied in biomedical applications, including TBJ repair. In this respect, due to the advantages of natural polymer materials and the advantages of electrospinning technology, some applications of great significance to human health have been gradually developed (Fig. 4).
Novel medical dressings for wound healing need to have a number of specific properties, such as promoting wound healing, appropriate interaction with wound exudates, good mechanical properties, and comfort. Electrospun nanomaterials have a higher surface area to volume ratio, which makes efficient packaging and controlled release kinetics of drugs possible. Therefore, it can meet the special requirements mentioned above [64, 83]. In addition, the potential for nanoscale fibrous materials to interact with the wound area is increased, improving contact with the wound site and thereby influencing cell behavior at the wound site. Meanwhile, the physical and chemical properties of electrospun nanomaterials are easily modified and can be designed to mimic ECM or other cellular components, making them more immune system-friendly [52, 67]. Recently, Qi’s Group reported on an electrospinning wound dressing with great methicillin-resistant Staphylococcus aureus (MRSA) inhibition ability [84]. Tang’s group proposed a new personalized electrospinning nanofiber dressing with in-situ deposition [85].
Drug delivery and wound dressing have certain similarities in their modes of action. Electrospinning nanofibers show advantages in material selection, preparation method, drug packaging, and drug release controllability [86, 87]. Parin et al. proposed a synchronous method of electrospinning and electrospraying to prepare electrospun nanofiber membranes containing folic acid [88]. Celebioglu and Uyar developed the electrospinning of polymer-free and free-standing acyclovir/cyclodextrin nanofibers for the first time [86]. Although significant progress has been made in vitro and in vivo research on on-demand nanofiber drug delivery systems, most studies are still limited to laboratory validation. Meanwhile, clinical transformation of such technologies remains to be further developed. The main challenges are the chemical complexity, biological reactivity and predictability of electrospinning carriers used in an in vivo physiological environment.
Electrospun nanofibers play an important role in the construction of diagnostic sensing platforms due to their large surface area to volume ratio, high porosity, and versatility in structure and composition [89, 90]. Crapnell and coworkers have fabricated nylon 6,6 fibers incorporating polypyrrole (PPy) molecular-imprinted polymers (MIPs) for the selective detection of D-glucose by electrospinning [91]. This research has a very important contribution to the health of human beings, especially diabetics. Andrade’s group has designed hierarchal composite polyaniline-(electrospun nanofiber) hydrogel mat (ENM) [92], which can realize simultaneous enrichment and simultaneous detection of Zika virus particles.
TBJ, the focus of this review, is an important part of the field of tissue engineering. In fact, electrospinning has more than one application in tissue engineering. Similarly, bone tissue regeneration, artificial blood vessels, and the nerve can be achieved by electrospinning biological scaffolds [12, 18, 66, 67, 93]. They all take advantage of the fact that the structure of electrospun biological scaffolds is highly similar to the ECM structure of natural tissues. Also, the advantages of high porosity and surface area volume promote the cell migration and differentiation. For another example, Electrospun materials for skin repair is a new frontier field. Water-soluble electrospun nanofibers were used to achieve antimicrobial, anti-UV, and oxidation resistance. Integrating of these applications in tissue engineering will be very enlightening for TBJ repair research [20, 40, 53, 65, 94, 95].
Macroscopic Structure of Electrospun Scaffolds in Tendon–Bone Junction Treatment
Electrospun Nanofiber Membrane Scaffold
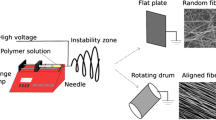
Electrospun nanofiber membranes are fabricated by collecting nanofibers using a metal plate or roller, which is the most traditional method and most convenient method to fabricate nanofiber membranes with high porosity. Fibers inside the membranes could be separated inside the membranes randomly or aligned. Regarding the specific hierarchical structure of the TBJ, scaffolds for the treatment of this site are required to possess a gradient fiber structure from random to aligned, as well as a gradient mineralization content. Highly aligned fibers and non-mineralized components lead to the proliferation of tenocytes, while random matrix and highly mineralized content facilitate osteoblast formation. The early fabrication method of gradient mineralization structure on electrospun scaffolds was reported by Li et al. in 2009 (Fig. 5a). Highly aligned nanofiber membranes are mechanical anisotropic and perform well in the force load direction. In the regeneration of tissues like tendons, ligaments, muscles, of which the extracellular matrix is highly aligned in the longitudinal direction for superior mechanical performance, scaffolds are in need to be made with high alignment for the guidance of cell proliferation and migration. Methods to prepare highly aligned electrospun fiber membranes required some modification of the collector, such as high rotational roller, gap assisted metal, magnetic, centrifugal electrospinning apparatus, near field, and the post drawing of samples could also achieve the alignment [96]. In order to improve the mechanical properties of electrospun scaffolds, some researchers used traditional textiles as the substrate for enhancement (Fig. 5b).
Schematic illustration of the application of electrospun nanofiber membrane scaffolds. a Fabrication process of gradient calcification of electrospun membrane; reproduced with permission from Ref. [11], Copyright 2009, American Chemical Society. b The cross-section of electrospun nanofiber membrane on woven fabric substrate; reproduced with permission from Ref. [104], Copyright 2015, Elsevier. c Bipolar eletctrospun nanofibrous membrane with PLLA layer and nHA-PLLA layer to mimic the normal fibrocartilage enthesis; reproduced with permission from Ref. [10], Copyright 2018, PubMed Central. d Bi-layered eletrocspun membrane scaffolds with aligned and random layers; reproduced with permission from ref [103], Copyright 2017, Elsevier
To achieve better treatment, the structure of the fibers could be varied to acquire other functions. These novel fiber structures could provide scaffolds with advanced functions in the treatment of enthesis. For example, the core–shell nanofibers are suitable for loading and protecting the drug or active bio factors from the extra environment during processing. Side-by-side nanofibers are of great significance in loading two drugs separately. The blend electrospinning with two or more components could exploit the advantage and mediate their performance to meet the practical need.
Regarding the characteristic of the enthesis, electrospun fibrous membranes have been designed and applied in the TBJ for evaluation. Zhao, etc. [97] exploited the stagger-electrospinning technology to produce a hierarchical and stretchable fibrous membrane containing PCL and chitosan. Results exhibited that the combination of chitosan significantly enhanced new bone formation. This kind of method is relatively simple, but it is difficult for TBJ regeneration. By mineralization, the strength and toughness of the TBJ scaffold were both enhanced as well as the treatment performance [98]. Further, in Wang’s work, they used a solvent-post-treated method to treat the PU/gelatin electrospun scaffold to make crimped nanofibers and welded joints to mimic the intricate natural TBJ site. This method stabilized the fiber network and facilitated the proliferation and differentiation of stem cells [99]. Different cytokines and bioactive materials can be incorporated into the nanofibers to stimulate the differentiation of MSCs. Kartogenin could stimulate chondrocyte differentiation of MSCs and it has been embedded in the PCL aligned nanofibers fibrous membranes. It showed that the incorporation of KGN has significantly stimulated the chondrogenic and tenogenic differentiation [100]. The gradient structure could also be constructed into single nanofiber membranes via post-treatment. In Li’s work, they achieved the gradient alignment structure using the solvent vapor-induced welding method. In this work, PLGA electrospun nanofibers were first fabricated with high alignment and followed by immersing part of the membranes in ethanol and exposed to its vapor. The post-treated part had a welding effect on the fibers so as to change the porosity and fiber direction. This scaffold mimicked the natural connective tissue and promised the integrity of newly generated tissues. Yu et al.. reported a similar work with the method of photothermal-triggering on the aligned membranes, which is a novel and smart method to modulate the arrangement of the fiber alignment [9].
In order to recapitulate the structure and component of the native TBJ tissue, biphasic, triphasic and multiphasic biomimetic scaffolds have been fabricated and shown great potential in practical surgery uses. Zhu et al.. [38] fabricated a hierarchically structured scaffold with a channel array and gradient mineralized inverse opal region. The channel is suitable for cells to infiltrate and proliferate, and the stem cells differentiate into tenocytes within one direction and into osteoblasts along the mineral gradient direction. Based on the same mechanism, dual-layered [10, 101] and multi-layered [102] electrospun membranes with hierarchical and gradient structures are designed and fabricated to facilitate the regeneration of TBJ with desired functions. According to the natural structure of enthesis tissue, the fibers are highly aligned in the tendon side but randomly dispersed in the bone side.
Regarding this characteristic, multi-layered membranes should be produced with high alignment to guide the cell migration in the tendon side. Cai et al. [10] designed and fabricated the dual-layer nanofibrous scaffolds for improving the healing of the TBJ in a rabbit model. In this scaffold, one layer is highly aligned for tendon regeneration while the other layer is random for the healing of bone (Fig. 5c). Histological assessment showed that the dual-layered scaffold decreased the interface width and enhanced the gradient structure. Based on the structure gradient, the gradient of mechanical properties could also be created to meet the real application need. In the meantime, the nanofillers and bioactive factors are also graded and distributed in the scaffolds from the tendon to the bone side in order to induce different cell proliferation. Li et al. [103] designed the flexible bipolar nanofiber membranes to improve the formation of gradient microstructure in enthesis healing. Briefly, two layers of the PLLA nanofiber membranes were deposited on the collector successively, and apatite nanoparticles grew in situ on the lower layer while the upper layer kept its original properties for the purpose of inducing tendon regeneration. Implanting the bipolar membrane induced bone formation and fibrillogenesis (Fig. 5d). Although many research works have testified to the feasibility of electrospun nanofiber membranes for TBJ healing, the 2D dimension and poor mechanical properties still hamper the practical application. In this way, textile technology stands out to be an excellent method for fabricating 3D and robust nanofiber scaffolds for biomedical applications.
Electrospun Nanofibrous Textiles SCAFFOLD
Textile is a traditional and mature manufacturing technique. Textile is an excellent technology for constructing hierarchical scaffold structures that mimic the native tissue and facilitate the reconstruction of the injured tissue. Apart from electrospun nanofibers and nanoyarns, researchers have been processing the yarns into textiles via mature manufacturing in order to improve the mechanical properties and other performance. Textile technology is superior in changing the 2D electrospun nanofiber matrix into 3D scaffolds to mimic the natural tissue better and improve mechanical performance. These kinds of textiles could be categorized into woven fabric, knitted fabric, and braided fabrics according to their structure, and these will be discussed in detail in this part.
Electrospun Nanofiber Yarns Scaffolds
Yarn is the secondary structure of the textiles, which presents a slim and continuous strip composed of fibers or filaments. Compared with nonwovens, the textile format without yarn structure, yarn structure makes it possible to construct textiles with advancing biomimetic hierarchical 3D structure and better mechanical properties. The nanofiber yarns are microscale (size) composed of fibers in the nanoscale (size), making it possible to produce textiles with higher specific area and porosity that are beneficial in the biomedical scaffold application. In the early research, electrospun nanofiber yarns were produced by cutting the electrospun films into slim strips and then twisting them into the so-called yarns [105, 106]. This method produced yarns with limited length and the yarn diameters and evenness were hard to control. Later, the electrospinning apparatus is updated and modified to produce continuous nanofiber yarns, as presented in Fig. 6. They are mainly achieved by water bath method or conjugated spinning [107,108,109,110]. The water bath method is achieved by collecting the nanofibers that first being stretched by the high voltage from the water bath and then drawn by the rotation of the collecting roller (Fig. 6a). Jiang et al., has used this method to prepare PCL nanofiber yarns and then fabricated into textile scaffolds for vascular [107] and skin tissue engineering [108]. In Smit’s work [109], they used a similar method to produce the nanofiber yarns with the assistance of a water reservoir collector. However, bead structure could be clearly observed on the nanofiber yarns, which may result in poor mechanical performance. Tian etc. [111] also used this wet-electrospun method to produce nanofiber yarns. They modified the mechanical properties by post-treating them with plying and twisting. Besides, an innovative and clever method to fabricate nanofiber yarns is using of the water vortex to collect and twist the as spun nanofibers into yarns (Fig. 6b) [112]. The authors have testified the oriented nanoyarns, and larger pore size was positive for cell growth.
Schematic illustration of fabrication process and SEM images of electrospun nanofiber yarns. a Wet-electrospinning; reproduced with permission from ref [107], Copyright 2021, American Chemical Society. b Water vortex assisted method; reproduced with permission from ref [112], Copyright 2021, Elsevier. c Conjugated electrospinning; reproduced with permission from ref [114], Copyright 2009, John Wiley and Sons. d Using funnel collector, scale bar 200 μm; reproduced with permission from ref [115], Copyright 2019, Elsevier
Conjugated electrospinning is another method to produce nanofiber yarns [113, 114]. Li [114] used the conjugate electrospinning method to produce PLLA/zein nanofiber. Two high electrical voltage suppliers were applied to the two spinnerets and fibers were merged at the center and then collected (Fig. 6c). The yarns produced by this method are relatively incompact and show poor mechanical properties. Later, conjugated electrospinning is updated with a funnel-shaped metal collector. Funnel-shaped metal collector-assisted electrospinning apparatus has been reported in many references to produce continuous nanofiber yarns with adjustable fiber alignment and yarn twist. In Wu’s work [115], PLGA/PLA nanofibers/microfibers hybrid yarns were fabricated by the funnel-shaped collector assisted apparatus (Fig. 6d). The yarns were loaded with thymosin Beta-4 loaded and the yarns could promote tendon tissue engineering effect by improving tenogenesis of adult stem cells.
In the application of biomedical use, electrospun yarns are very suitable for degradable sutures. However, in other tissue engineering scaffolds, including the TBJ treatment, yarns are limited by their dimension and mechanical performance and should be further processed into textiles for better application.
Woven Scaffolds
Weaving is an ancient textile processing method to create clothing. The most convenient and traditional fabricating method is to interlace two systems of yarns (warp yarn and weft yarn) perpendicular to each other by hand. By exploiting the weaving loom, fabrics with more complicated structures could be fabricated effectively and uniformly. Woven fabrics have been used as the tissue engineering scaffold by utilizing excellent mechanical properties and biomimetic bulk construction. Compared with knitting, weaving is the better method of producing fabrics with anisotropic mechanical properties. Hence, weaving is more suitable for making scaffolds for anisotropic tissue regeneration in tendons [104, 116], cartilage [117], vascular conduit [118], etc. Woven fabrics exhibit better stable dimensional structure and higher stiffness when compared with knitted fabrics, which makes them the better candidate for the treatment of TBJ. By changing the inserted weft yarns, woven scaffolds with gradient structure could be easily acquired to facilitate the formation of graded tissue. Recently, it has been found that the combination of weaving and electrospinning in the fabrication of biomedical scaffolds significantly improves the scaffold performance in accelerating the regeneration of repaired tissue.
There are two methods to combine electrospinning and weaving to fabricate tissue engineering scaffolds. One of them is to use traditional woven fabric as the substrate for mechanical support, while the electrospun membranes provide an environment for cell adhesion. However, such a combination of woven fabric and electrospun membranes is easy to delamination when treated with water or mechanical stress. The other one is to fabricate the woven fabric directly using the nanofiber yarns produced by electrospinning. This method provides the fabric structure with more integrity and avoids delamination. Micropores in the yarns and fabrics provide a path for cells to migrate and nutrition exchange, leading to a better regeneration effect on tissue repair. Because electrospun nanofiber yarns do not possess comparative mechanical properties with the traditional yarns; they are usually applied in the weft system to guarantee the structure and properties. Besides, due to the ECM-like structure, these fabrics are also suitable for cells to adhere, migrate and penetrate. Drug-loaded electrospun nanofiber yarn-woven fabrics have been made by Padmakumar et al. [119] for drug delivery systems in peritoneum. They showed that this nanotextile is mechanically strong and exhibited therapeutic efficacy.
In the healing of TBJ, weaving could be exploited to easily manipulate the hierarchical scaffold structure for the gradient tissue structure formation. Xie et al. [120] have reported a novel woven scaffold made from electrospun aligned nanofiber yarns. The woven scaffold had a gradient HAp component from tendon part to bone part in order to induce the differentiation of tenocytes and osteocytes and the different mineralization of tendon and bone. They achieved the gradient structure by gradually changing the inserted weft yarns that contain different ratios of HAp. The in vivo study revealed that the mineralized segment promoted the osteogenic differentiation of rBMSCs, while the non-calcified part improved the tenocyte differentiation of rBMSCs. Figure 7 presents the fabrication and structure of the hierarchical and gradient structure of the woven scaffold.
a Schematic illustration of electrospun yarns. b The nanofiber yarns with different contents of HA. c Textile weaving process to make scaffolds with HA gradient. d rBMSCs differentiation on the different nanofiber yarns. e Schematic illustration of the insertion of the HA gradient scaffolds. f Bone-like ECM deposition of rBMSCs on scaffold segments. g Quantitative analyses of calcium deposition by ARS staining; reproduced with permission from Ref. [120], Copyright 2021, Elsevier
Weaving is a facile fabrication method to construct scaffolds with anisotropic structure and tunable mechanical properties to mimic the properties of native TBJ tissue and enable the regeneration of junctions with gradient composition and structure. Cell behavior could be modulated and controlled by regulating the weaving pattern. Meanwhile, the toughness and structural stability of woven scaffolds are better than knitted scaffolds.
Knitted Scaffolds
Knitting is a mature textile manufacturing method for fabricating fabrics with 2D or 3D structures. Knitted fabrics are constructed by interlooping yarns to form a sheet-like cloth with a connected and uniform porous structure with yarn loops. According to the direction of yarn loops, the knitting process could be classified into two major categories, warp knitting and weft knitting. The yarn loops are arranged vertically in warp-knitted fabrics and horizontally in weft-knitted fabrics. Due to the existence of yarn loops, knitted fabrics are more stretchable and ductile than woven fabrics. Knitting has been extended to many fields besides apparel, including the biomedical field as scaffolds for tissue engineering. Knitted fabrics possess high flexibility, and the pore size could modulate by yarn loops structure to meet the properties of scaffolds for vascular, tendon and bone et al. According to the references we have found, in the 1980s, knitted Dacron fabrics have been explored to be arterial graft in artificial circulation, and the thrombogenicity of fabrics with different structures have been studied [121]. Although Dacron is a kind of traditional and representative textile fiber that shows favorable mechanical properties, it is nonbiodegradable and the poor biocompatibility hinters at its application in biomaterials. Thus, coating the knitted scaffolds with biocompatible materials, such as collagen, is a facile method to improve the scaffolds’ biocompatibility [122]. The better solution is fabricating the scaffold by using biodegradable and biocompatible yarns. In 2003, Ouyang etc. [123] studied and evaluated the morphology and biomechanical function of regenerated tendons using a PLGA knitted scaffold. Results showed that the knitted scaffold effectively promoted structure and function restoration. More recently, knitted silk-collagen fabrics have been explored to be scaffolds for ACL reconstruction [124]. Apart from these, knitting could be used as biomaterials in combination with electrospinning technologies to utilize both advantages of them. One of the methods is to exploit knitted fabric as the substrate to provide excellent mechanical support. It was reported that the knitted silk and collagen sponge scaffolds improve functional ligament regeneration by regulating collagen fibril assembly [125]. Another method is to fabricate the knitted fabrics with electrospun nanofiber yarns. The knitted fabrics could be used as the template for guiding the regeneration of the new tissue. Cai et al. [126] reported work on using electrospun core–shell yarns to fabricate knitted fabrics for tendon tissue engineering (Fig. 8). Both the core micro yarns and the shell nano yarns were made of biodegradable polymers, and the electrospun shell part was blended with silk fibroin to improve the biocompatibility. This weft-knitted scaffold showed good mechanical performance and could promote cell adhesion and proliferation and enhance the tenogenic differentiation of rBMSCs. The regenerated tendon tissue performed well in the failure load and stiffness after 6 months.
a Schematic illustration electrospun nanofiber yarns producing process. b SEM images of the PCL microfibers, PCL–PCL and PCL-SF/PLCL yarns, knitted scaffolds and the photographs. c Schematic diagram of the PCL-SF/PLCL knitted scaffolds for tendon regeneration; reproduced with permission from Ref. [126], Copyright 2020, Royal Society of Chemistry.
Electrospun knitted fabrics have been studied extensively in tissue engineering, but this method is still difficult to adjust properties in different directions. Especially in the TBJ repair, the isotropic mechanical property is of great significance. Thus, in the combination of knitting and electrospinning for the application of bone to tendon tissue engineering, structure design should be modified. These methods include using highly aligned electrospun nanofiber yarns for knitting, depositing highly aligned nanofiber membranes onto the knitted substrate, etc.
Braided Scaffolds
Braiding is a versatile way of producing strip-like textiles by interlacing three or more sets of yarns in one direction. 2D and 3D textile structures could be produced on the braiding machine. Braiding is an easy method to produce an anisotropic structure to meet the mechanical requirement of anisotropic tissues like vascular stents, tendons, ligaments, etc. The braided fabrics are stable in dimension and possess favorable mechanical properties, especially in the axial direction. Yarns in the braided textile are arranged along the axial direction, making it an anisotropic structure with favorable mechanical performance in the axial direction. By exploiting 3D circular braiding technology, a 3D circular braided chitosan scaffold has been fabricated for tendon augmentation. The 3D scaffold well mimicked the native tendon tissue and possessed better mechanical performance than the native tendon tissue [127]. The research work proposed a facile method for designing tendon analogs, which maintained important flexibility in scaffold design and improved the mechanical properties of the resulting scaffold. It has been reported that cell adhesion and proliferation could be enhanced by increasing the specific surface area. Thus, electrospinning is a simple way to achieve this. In the meantime, by using aligned electrospun nanofiber yarns for braiding, scaffolds with higher fiber alignment could be produced to guide the formation of regenerated anisotropic tissue. Hence, braiding is the most widely studied textile technology for constructing scaffolds for tendons, bones, and TBJ repair.
Porosity and yarn alignment could be tuned by adjusting the braiding angle. Research reported an electrospun aligned nanoyarns braiding scaffold for tenogenic differentiation from MSCs. It has testified that the braiding angle is significant for the tissue-specific commitment of HiPSC-MSCs [128]. Microfibers produced by wet-spinning have been made into the braided textile with a gradient structure for TBJ treatment [37]. In this report, PCL/gelatin microfibers with different content HAp were fabricated by wet spinning. Then these fibers were braided into the textile with a gradient structure by gradually changing the fibers with higher HAp content (Fig. 9a). This gradient scaffold could mimic the characteristics of TBJ and facilitate the regeneration. Another method to achieve the transition of tendon/ligament to bone by braiding is shown in Fig. 9b, which is fabricated by destructing the yarn structure at the bone segment to make the fiber randomly align.
a Braided scaffold with HA gradient content; reproduced with permission from Ref. [37], Copyright 2019, John Wiley and Sons. b Braided ligament scaffolds with structure of the bone segment and intra-articular zone; reproduced with permission from Ref. [131], Copyright 2020, Springer Nature. c Photographs and SEM of PLLA braided scaffolds with different braiding angles, Scale bar of the SEM image, 500 μm; reproduced with permission from Ref. [128], Copyright 2014, Elsevier
However, compared with nanofibers, microfibers have fewer pores for cells to penetrate and migrate. By reducing the diameter of the fibers to the nanoscale, the higher specific surface area will benefit the adhesion of the cells. Thus, electrospun nanofiber bundles and yarns are exploited in braided scaffolds. By varying the braiding angle, nanofiber braiding scaffolds change their yarn alignment to satisfy different tissue regeneration (Fig. 9c). Continuous aligned-nanofiber threads from PCL/CHT/CNT have been spun and braided into a scaffold for artificial tendon construction [129]. Rothrauff, etc. [130] fabricated the braided scaffold from electrospun nanofibers by cutting the nanofiber membranes into strips and stacking them and then braiding. The mechanical performance and differentiation of MSCs could be enhanced by the braided structure. However, although such scaffolds were produced by braiding, they are not the real braided textile due to the lack of yarn structure. Compared with knitted and woven textiles, the braiding structure is more anisotropic and possesses better mechanical tensile stress in the axial direction, making it suitable for scaffolds for TBJ.
Functional Electrospinning Scaffolds in Tendon–Bone Junction Repair
Piezoelectrical Electrospinning Nanofiber Scaffold
As mentioned above, the three major elements of interfacial tissue engineering are scaffold materials, growth factors, and cells [17, 19, 28]. Although this review focuses on scaffold materials, the effects of materials on growth factors and, ultimately, cell growth are essential factors that researchers must consider. In tissues like TBJ, signaling pathways are often dynamically transmitted through electrical synapses or chemical protrusions to better reshape their function [132, 133]. The transmission of these signaling pathways is controlled by the cell and extracellular matrix. However, when we introduce artificial tendons and bone repair scaffold materials, it is easy to adversely affect these natural signaling pathways. So, researchers began exploring intelligent artificial scaffolds to solve this challenge.
When stimulated by external stimuli, intelligent materials will output at least one stable, reproducible and significant signal of at least one characteristic. Electrical stimulation is closely related to tissue repair among many signal stimulations. Therefore, electroactive intelligent artificial scaffold plays a crucial role in TBJ repair research. Piezoelectric materials, in particular, can generate electrical signals in response to pressure. In the absence of an external power supply, piezoelectric artificial scaffolds can be forced to produce specific electrical stimulation and stimulate tissue regeneration through certain pathways [134,135,136,137,138].
In electrospinning materials, many polymers, such as PVDF and nylon, show good piezoelectric properties [43, 139]. The structure of piezoelectric polymers requires permanent molecular dipoles and the ability to align or orient. In addition, these polymers need to be able to withstand periodic and repeated strains under mechanical stress. Notably, many components in tissues, such as tendons and bones, also have piezoelectric properties. Collagen, for example, the main component of bone and cartilage, is piezoelectric, generating electrical signals in response to internal forces. Tissue can, therefore, interact with the material, allowing these signals to be transmitted via ECM to voltage-gated channels in the cell membrane. In general, electrospinning is an excellent strategy for constructing a piezoelectric TBJ scaffold [135].
However, in the field of tissue engineering, there is much research on bone repair and cartilage repair using piezoelectric materials, but little research on TBJ repair. The lack of research on this topic may be due to the complexity of TBJ’s structure and composition. It is a significant problem to stimulate the electrical signals generated by piezoelectric supports. In 2021, Biggs and Coworkers proposed an electrospun nanofiber scaffold made from a ferroelectric material poly (vinylidene fluoro-co-trifluoroethylene) [133]. The results showed that motion-driven electromechanical stimulation of the tendon tissue with piezoelectric electromechanical devices could regulate ion channels and specific tissue regeneration signaling pathways in vitro. Using a rat model of acute Achilles tendon injury, the authors demonstrated the potential of piezoelectric bioelectronic devices in regulating the tendinopathy-related processes in vivo (Fig. 10). This work showed that electromechanical stimulation can regulate mechanosensitive ion channel sensitivity and can promote tendon-specific over non-tenogenic tissue repair processes. From the perspective of future development, it is a promising and significant new research field that is advantageous to repair TBJ by piezoelectric scaffolds.
a This mechanical stress is borne by a highly anisotropic extracellular matrix composed of collagen type I, a high-tensile, piezoelectric material which undergoes electrical polarization in response to mechanical loading. b Direct comparison of piezoresponse values between samples. c The progression of calcification is observed in the Injury group; reproduced with permission from Ref. [133], Copyright 2021, John Wiley and Sons
Metal Ion-Stimulated Electrospinning Nanofiber Scaffolds
In addition to electrical signals, some metal ions also play an essential role in maintaining human biological functions and promoting tissue repair [140, 141]. These metal ions are essential trace elements in the body and play a key role. Iron, for example, is involved in oxygen transport and blood cell production; Copper ions and zinc ions can promote the formation of bone, skin, and connective tissue [142,143,144]. Magnesium ion (Mg) can stabilize the transition state of high negative charge during ATP hydrolysis [101]. Therefore, it can be inferred that trace metal elements can act as bioactive structural factors and therapeutic elements for tissue repair during TBJ repair.
In an earlier study (2015), calcium and zinc have been shown to play an important role in the normal growth, mineralization, and repair of the fibrochondral region (TBJ) at the bone–tendon junction [145]. More importantly, calcium and zinc showed a special gradient distribution pattern in PPTC fibrous cartilage region. For example, zinc was enriched in the transition zone between uncalcified fibrocartilage and calcified fibrocartilage, with the highest zinc content at the fibrocartilage band TM, 3.17 times that of the patellar tendon. Inspired by this gradient morphology of the tendon–bone interface, Yang et al. proposed a bipolar metal flexible electrospinning fiber film based on a metal–organic framework (MOF) [101]. MOF was integrated into flexible biomaterials using electrospinning technology. First, the coated MOF particles will be released gradually with the degradation of electrospinning nanofibers. In turn, metal ions bound to the spatial structure of the MOF particles were released from the MOF particles and can maintain biological effects (Fig. 11). The experimental results showed that the bipolar membrane possessed good mechanical properties and continuous release of metal ions, which can promote biological mineralization and angiogenesis. Meanwhile, the side containing PLA-H showed good tendon formation ability in vitro. In general, in vitro and in vivo experiments have verified the ability of tendon–bone interface integration regeneration of bipolar metal flexible electrostatic spinning fiber. Metal ion-stimulated electrospinning nanofiber scaffold of TBJ repair is a novel strategy with promising clinical application. In future studies, the selection of metal ions, the distribution of gradients, the release ability and the stability of materials are all factors that can be studied.
The schematic illustration of the fabrication and implantation method of the bimetallic bioinspired bicomponent integral nanofibrous membrane and the effect of regulating the synchronous regeneration of the bone–tendon interface by metal ions released from the nanofiber in situ; Reproduced with permission from Ref. [101], Copyright 2022, John Wiley and Sons
External Field-Assisted Electrospun Nanofiber Scaffolds
The morphology, structure, and composition of artificial scaffolds have a decisive influence on the structure of TBJ repair [21, 29, 146]. As discussed in Sect. 3, the structure and morphology of electrospun nanofibers can be regulated by many factors, and the external environment is a crucial factor in regulating the morphology of the macroscopic nanofiber membranes [147]. For example, the needle and receiver are modified to obtain electrospun films with Janus asymmetry or gradient morphology [43, 60, 148]. Another more controllable and convenient way is to construct nanofiber scaffolds with orientation or gradient structure with the help of the external field. The number of studies in this area is large and has achieved outstanding therapeutic effects [149,150,151].
To achieve precise spatial control of fiber alignment to better simulate structural gradients in interfacial tissues such as the tendon–bone interface, a modularized magnetic field-assisted nanofiber scaffold with gradient orientation was prepared by Tindell et al. [152]. If the magnet is fixed to the receiver, it is found that the fibers near the magnet are highly oriented, while the fibers farther away are more disordered. This is because the magnetic field strength increases with decreased distance away from its surface. The obtained gradient nanofibrous scaffold can be divided into three regions: magnetic (M) region, transition (T) region, and nonmagnetic (NM) region (Fig. 12a). This is similar to the natural structural gradients present in many interfacial tissues, such as TBJ. The strength of these three regions was tested along the scaffold direction to correspond to the fiber alignment direction in the magnetic region. The results showed that the aligned fibers had a statistically higher Young’s modulus in the magnetic region than those of the randomly aligned fibers without a magnetic region. Cell experiments demonstrated that cells implanted with gradient fiber scaffolds were elongated and aligned on aligned fibers, but showed no preference for randomly aligned fibers. This gradient structure benefited stem cells differentiated towards a tenogenic lineage and an osteogenic lineage on separate aligned and randomly aligned nanofibers, respectively. Although the researchers did not conduct further human or animal studies, this method is expected to lead to a regenerative TBJ tissue-engineered scaffold.
a Spatially controlling fiber alignment using magnetically assisted electrospinning and the anisotropic mechanical properties of gradient fibrous scaffolds; reproduced with permission from Ref. [152], Copyright, 2022, ChemRxiv. b Schematic illustration of the fabrication process. The photographs and SEM images of the corresponding nanofiber scaffolds with gradient morphologies and mineralization; reproduced with permission from Ref. [9], Copyright, 2022, Springer Nature
In addition to magnetic fields, thermal fields are also an effective method for constructing gradient structures. Yu and coworkers introduced indocyanine green (ICG), a near-infrared (NIR) dye, into electrospinning nanofibers [9]. The ICG is excited by laser irradiation, which eventually leads to welding and even fusion of the nanofibers at the intersection. The degree of welding can be controlled by adjusting the power and duration of the NIR laser in different regions, thus generating gradient structural characteristics from alignment to randomness. By using the method of gradual soaking time, the authors proposed a graded mineralization strategy, which successfully realized the gradient increase of hydroxyapatite content in the direction of the structural gradient (Fig. 12b). Finally, the researchers constructed an electrospun nanofiber scaffold with double gradients. This scaffold could simultaneously realize the tendinous and osteogenic differentiation of tendon-derived stem cells. This dual-gradient nanofiber scaffold has been demonstrated to have broad potential in clinical TBJ healing. However, the author also pointed out that the two-dimensional electrospun scaffold material still has some limitations in clinical experiments. The design of a three-dimensional artificial scaffold with spatial gradients in fiber alignment, mineral contents, and cell types will be of great clinical significance [40, 147].
Magnetic and photothermal fields not only play a positive role in modulating the morphology of the nanofiber scaffolds but could also accelerate tissue regeneration. It is reported that inorganic nanomaterials such as Mexne [153], black phosphorous (BP) [154, 155], titanium sheet [156] and tellurium [157] show the photothermal therapeutic effects for pathological tissue by killing the cancer cells in a mild and non-contact way. BP is biocompatible and biodegradable, which is suitable for biomedical applications, while the toxicity and biocompatibility of Mexne remain to be deeply investigated. By incorporating these photothermal nanomaterials into nanofibers, smart scaffolds, which are sensitive to external stimulation, could be fabricated and structure mediation could be achieved even after scaffold implantation. To our knowledge, some research is now using thermal therapy for bone regeneration. However, there is little report on the thermal therapeutical for TBJ, which shows tremendous potential in facilitating the repair of TBJ.
Mechanical Stimulation
Mechanical stimulation is another crucial factor in facilitating the regeneration and maturation of the TBJ [158]. Although a great number of researchers reported that the as-made electrospun scaffolds showed a positive effect on the regeneration, they are based on the static culture of the cells. However, in the real application, scaffolds are always undergoing mechanical stress, including compression and stretch. Mechanical stress during the TBJ maturation has been shown partially influence cell differentiation and cell morphology changes. In general, high tensile stress positively affects tenogenesis, while low tensile stress is associated with osteogenesis [159]. In the investigation of mechanical stimulation on the effect of MSCs proliferation and collagen secretion and alignment, a bioreactor with a stretching session is needed to provide stretching on the scaffold during cell culturing. Researchers have testified that the external force that generates mechanical stimulation will induce the proliferation of the MSCs. In Wu’s work [160], they cultured the constructs in a dynamic environment and demonstrated that dynamic stretching positively promoted total collagen secretion and tenogenic differentiation. In their work, PCL nanofiber yarns were prepared and made into woven fabrics with better mechanical properties and fiber alignment. Fibroblast morphology changed under the incubation of static and dynamic conditions [161].
Thus, shape memory polymers could be introduced into the scaffolds for TBJ regeneration to provide dynamic mechanical stimulation. Shape memory polymers could respond to external changes, including water, temperature, and other signals, and change the bulk shape. For example, when the thermal sensitive polymers were applied onto the scaffolds, the shape of the scaffold could be dynamically changed under the external temperature variation. In this way, the MSCs on the implanted site will better proliferate into tenocytes and benefit the regeneration. Tseng et al. [162] prepared an electrospun scaffold using shape memory polymer and testified that the shape-memory stimulated change in scaffold fiber alignment showed the effect on controlling the behavior of attached and viable cells. The programming of the electrospun scaffold was conducted under strain to induce alignment. After incubation at a physiological temperature (37 ℃) for 24 h, the fibers inside the scaffold returned to the original random state. They found that before shape memory actuation (with fibers highly aligned), cells preferentially aligned along with the fiber direction. After the shape memory actuation (with fibers returned to the random state), cells remained attached and viable but lost preferential alignment. The results demonstrated that the shape memory effect could be used to manipulate cell attachment and cell alignment and thus develop functional tissue engineering. Therefore, shape memory polymers are suitable for fabricating functionalized scaffolds to imitate the hierarchical structure of native TBJ tissue.
Growth Factor Delivery
Pure polymeric scaffolds, including electrospun nanofiber scaffolds, usually cause severe inflammatory cell infiltration and limit tissue regenerative capability, which hinders the formation of functional native collagen tissue at the insertion. To facilitate tissue regeneration, bio factors are expected to be embedded in nanofibers to improve the proliferation of MSCs and enhance the regeneration of the enthesis. A variety of growth factors have been exploited to stimulate and promote stem cell proliferation and differentiation in musculoskeletal tissue engineering. Based on the gradient nature of native TBJ tissue, growth factors should be loaded onto scaffolds with gradients to provide cell proliferation and differentiation into different cells, such as tenocytes and osteoblasts. After being implanted at the insertion, growth factors will slowly release from the scaffolds and work on the cells. In the fabrication process of electrospinning, growth factors are usually embedded inside the nanofibers to avoid destruction from the electrical field. The main growth factors for bone regeneration include BMP and VEGF. GDFs, TGF-β3, bFGF, PDGF, and Scx are growth factors for tendon regeneration. Regarding the regeneration of the interface, BMPs, TGF-β1, and PDGF-BB have been testified to facilitate the healing at the TBJ. Wu etc. fabricated electrospun microfiber yarns to construct tendon tissue grafts, which were loaded with thymosin Beta-4 (Tβ4) [115]. Tβ4 presented a sustained release behavior for 28 days and effectively improved on migration, proliferation, and tenogenic differentiation. TGF-β3 has been embedded in an electrospun PCL nanofibers scaffold and used for rotator cuff tear repair. The effect of the TGF-β3 was also testified in vivo study on rats [163]. TGF-β3 could alleviate inflammation and reduce scar formation, leading to a more robust tissue reconstruction. Melatonin is an endogenous molecule widely applied in regenerative medicine [164]. It is reported to induce chondrogenesis of BSCs in an inflammatory environment due to its immunomodulatory, anti-inflammatory, and anti-oxidative effects [165]. In Song’s work [166], they fabricated a kind of melatonin-loaded aligned PCL electrospun nanofiber membrane for TBJ healing (Fig. 13). They found that at the early healing stage after membrane implantation, melatonin-loaded PCL nanofiber membranes inhibited macrophage infiltration at the TBJ interface. In the meantime, the membranes increased the chondroid zone formation and promoted collagen maturation and finally enhanced the biomechanical strength of the regenerated enthesis. Growth factors are important in the healing of TBJ to promote cell proliferation and differentiation, reduce scar tissue, and facilitate the formation of robust insertion tissue that resembles the native one.
Fabrication process of pro-chondrogenic and immunomodulatory melatonin-loaded aligned PCL electrospun membranes and the application in a rat rotator cuff tear model; reproduced with permission from Ref. [166], Copyright 2012, Royal Society of Chemistry.
Conclusion and Perspective
The regeneration of tendon to bone junction by electrospun scaffolds to provide functional grafts integration after injury surgery to meet the mechanical need and for cell adhesion and proliferation, which might result in better regeneration of the gradient structure of the enthesis. However, the repair of the TBJ remains a big challenge because of the complicated structure, components, and cell types. Via electrospinning, 2D membranes with gradient structure could be produced to mimic the native tissue, with fibers highly aligning at the tendon side and gradually transiting to randomly disperse at the bone side. MSCs will adhere to the fibers and proliferate along the direction of fiber alignment, resulting in the reconstruction of a structure that resembles the native one. The 3D electrospun scaffolds for TBJ repair usually involve more complex methods by first fabricating nanofiber yarns and then processing them into textiles with different structures, including braiding, knitting, and weaving. The textiles possess a hierarchical structure that resembles the native tissue, which could facilitate the generation of hierarchical structure in the tissue. After being integrated into textiles, the mechanical properties will be enhanced to resemble the native tissue.
However, Designing and fabricating scaffolds with biomimetic structures is not enough to achieve the regeneration of TBJ tissue and restore functionality. Growth factors and mechanical stimulation are also indispensable factors for facilitating the repair. Growth factors also need to be embedded in the scaffolds in a gradient manner to facilitate cell proliferation into different phenotypes and exhibit their function. Mechanical stimulation shows a significant positive effect on cell proliferation. So, the functionalization of electrospun scaffolds could promote tissue regeneration. More stimulations could be added, such as heat, magnetic and electrical. In summary, using electrospinning technology to prepare scaffolds for TBJ repair is a simple and suitable method that could construct micro- and nano-structures that mimic the native tissue. And the repair effect could be significantly improved by functionalization.
Challenges still exist in the fabrication, experiment, and practical application of this research field, as illustrated in Fig. 14. Most importantly, the mechanical properties of electrospinning technology remain the pain points in the application of tissue reconstruction, especially in the repair of robust tissue like TBJ. In this case, textile structure is crucial in improving mechanical performance. In functionalization, the first existing challenge is the scale issue. It is reported that the length of the TBJ is at the millimeter scale, and many of the current research reports on constructing hierarchical scaffolds are actually magnified to the centimeter scale. Thus, it is still difficult to precisely fabricate the gradient structure with 1 mm. The second one is the in vivo experiment. In vitro experiments have been conducted, and most of the electrospun scaffolds showed a positive effect on inducing MSCs adhesion, proliferation, and differentiation. Meanwhile, dynamic culture of the cells in vitro has also been achieved. Nevertheless, the in vivo experiments are still only conducted in animal rather than human. Thirdly, the in-body tissue is growing under multi-stimulation, and this complicated environment is difficult to replicate in in vitro experiments. To mimic the growth environment of native tissue, scaffolds could be designed to be field sensitive, such as electrical sensitive, mechanical sensitive, and thermal sensitive. These signals could stimulate the cell growth and differentiation into tenocytes and osteoblasts, facilitating the formation of the gradient components in TBJ. Another difficulty is the monitoring of the tissue regeneration process. Currently, for observing the regeneration process of tissue after scaffold implantation in animals, the closure will be reopened and the tissue will be taken out, observed, and analyzed after preparing the tissue section, which is not applicable in the practical treatment in the human body. Regarding this problem, conductive elements could be incorporated into the scaffold, a remote monitoring device could be designed and connected with the scaffolds via Bluetooth, and the regeneration progress could be displayed for monitoring and observation. Hence, multidiscipline will help solve the challenges mentioned above. The fabrication and application of gradient and hierarchical electrospun nanofiber scaffolds with multifunction for TBJ treatment are significant and still need to be explored.
References
Lim WL, Liau LL, Ng MH, Chowdhury SR, Law JX. Current progress in tendon and ligament tissue engineering. Tissue Eng Regen Med 2019;16:549.
Stannard J, Fortington L. Musculoskeletal injury in military special operations forces: a systematic review. BMJ Mil Health 2021;167:255.
Derwin KA, Galatz LM, Ratcliffe A, Thomopoulos S. Enthesis repair: challenges and opportunities for effective tendon-to-bone healing. J Bone Joint Surg Am 2018;100:e109.
McCarron JA, Derwin KA, Bey MJ, Polster JM, Schils JP, Ricchetti ET, Iannotti JP. Failure with continuity in rotator cuff repair “healing.” Am J Sports Med 2013;41:134.
Thomopoulos S. The role of mechanobiology in the attachment of tendon to bone. IBMS BoneKey 2011;8:271.
Agarwal S, Wendorff JH, Greiner A. Use of electrospinning technique for biomedical applications. Polymer 2008;49:5603.
Huang Y, Du Z, Li K, Jing W, Wei P, Zhao B, Yu Y, Cai Q, Yang X. ROS-scavenging electroactive polyphosphazene-based core-shell nanofibers for bone regeneration. Adv Fiber Mater 2022;4:894.
Xue Y, Kim HJ, Lee J, Liu Y, Hoffman T, Chen Y, Zhou X, Sun W, Zhang S, Cho HJ, Lee J, Kang H, Ryu W, Lee CM, Ahadian S, Dokmeci MR, Lei B, Lee K, Khademhosseini A. Co-electrospun silk fibroin and gelatin methacryloyl sheet seeded with mesenchymal stem cells for tendon regeneration. Small 2022;18:e2107714.
Yu C, Wang T, Diao H, Liu N, Zhang Y, Jiang H, Zhao P, Shan Z, Sun Z, Wu T, Mo X, Yu T. Photothermal-triggered structural change of nanofiber scaffold integrating with graded mineralization to promote tendon-bone healing. Adv Fiber Mater 2022;2022:5.
Cai J, Wang J, Ye K, Li D, Ai C, Sheng D, Jin W, Liu X, Zhi Y, Jiang J, Chen J, Mo X, Chen S. Dual-layer aligned-random nanofibrous scaffolds for improving gradient microstructure of tendon-to-bone healing in a rabbit extra-articular model. Int J Nanomed 2018;13:3481.
Li X, Xie J, Lipner J, Yuan X, Thomopoulos S, Xia Y. Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano Lett 2009;9:7.
Chen Y, Dong X, Shafiq M, Myles G, Radacsi N, Mo X. Recent advancements on three-dimensional electrospun nanofiber scaffolds for tissue engineering. Adv Fiber Mater 2022;2022:55.
Jiao Y, Li C, Liu L, Wang F, Liu X, Mao J, Wang L. Construction and application of textile-based tissue engineering scaffolds: a review. Biomater Sci 2020;8:3574.
Saveh-Shemshaki N, Nair LS, Laurencin CT. Nanofiber-based matrices for rotator cuff regenerative engineering. Acta Biomater 2019;94:64.
Stace ET, Nagra NS, Tiberwel S, Khan W, Carr AJ. The Use of Electrospun Scaffolds in Musculoskeletal Tissue Engineering: A Focus on Tendon and the Rotator Cuff. Curr Stem Cell Res T 2018;13:619.
Font Tellado S, Balmayor ER, Van Griensven M. Strategies to engineer tendon/ligament-to-bone interface: Biomaterials, cells and growth factors. Adv Drug Deliver Rev 2015;94:126.
Lei T, Zhang T, Ju W, Chen X, Heng BC, Shen W, Yin Z. Biomimetic strategies for tendon/ligament-to-bone interface regeneration. Bioact Mater 2021;6:2491.
Wang D, Zhang X, Huang S, Liu Y, Fu BS, Mak KK, Blocki AM, Yung PS, Tuan RS, Ker DFE. Engineering multi-tissue units for regenerative medicine: bone-tendon-muscle units of the rotator cuff. Biomaterials 2021;272:120789.
Baldino L, Maffulli N, Reverchon E. Regenerative enginnering of musculoskeletal tissue and interfaces. London: Woodhead Publishing; 2015. p. 345–61.
Baldino L, Cardea S, Maffulli N, Reverchon E. Regeneration techniques for bone-to-tendon and muscle-to-tendon interfaces reconstruction. Brit Med Bull 2016;117:25.
Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliver Rev 2007;59:1413.
Peng S, Jin G, Li L, Li K, Srinivasan M, Ramakrishna S, Chen J. Multi-functional electrospun nanofibres for advances in tissue regeneration, energy conversion & storage, and water treatment. Chem Soc Rev 2016;45:1225.
Jiang C, Wang K, Liu Y, Zhang C, Wang B. Application of textile technology in tissue engineering: a review. Acta Biomater 2021;128:60.
Furtado M, Chen L, Chen Z, Chen A, Cui W. Development of fish collagen in tissue regeneration and drug delivery. Eng Regener 2022;3:217.
Li C, Cui W. 3D bioprinting of cell-laden constructs for regenerative medicine. Eng Regener 2021;2:195.
Liu C, Xu X, Cui W, Zhang H. Metal-organic framework (MOF)-based biomaterials in bone tissue engineering. Eng Regener 2021;2:105.
Tang Y, Chen C, Liu F, Xie S, Qu J, Li M, Li Z, Li X, Shi Q, Li S, Li X, Hu J, Lu H. Structure and ingredient-based biomimetic scaffolds combining with autologous bone marrow-derived mesenchymal stem cell sheets for bone-tendon healing. Biomaterials 2020;241:119837.
Kirillova A, Yeazel TR, Asheghali D, Petersen SR, Dort S, Gall K, Becker ML. Fabrication of biomedical Scaffolds using biodegradable polymers. Chem Rev 2021;121:11238.
Place ES, George JH, Williams CK, Stevens MM. Synthetic polymer scaffolds for tissue engineering. Chem Soc Rev 2009;38:1139.
Akbari M, Tamayol A, Bagherifard S, Serex L, Mostafalu P, Faramarzi N, Mohammadi MH, Khademhosseini A. Textile technologies and tissue engineering: a path toward organ weaving. Adv Healthc Mater 2016;5:751.
Echave MC, Domingues RMA, Gomez-Florit M, Pedraz JL, Reis RL, Orive G, Gomes ME. Biphasic hydrogels integrating mineralized and anisotropic features for interfacial tissue engineering. ACS Appl Mater Inter 2019;11:47771.
Huang K, Du J, Xu J, Wu C, Chen C, Chen S, Zhu T, Jiang J, Zhao J. Tendon-bone junction healing by injectable bioactive thermo-sensitive hydrogel based on inspiration of tendon-derived stem cells. Mater Today Chem 2022;23:100720.
Font Tellado S, Bonani W, Balmayor ER, Foehr P, Motta A, Migliaresi C, van Griensven M. Fabrication and characterization of biphasic silk fibroin scaffolds for tendon/ligament-to-bone tissue engineering. Tissue Eng PT A 2017;23:859.
Caliari SR, Weisgerber DW, Grier WK, Mahmassani Z, Boppart MD, Harley BA. Collagen scaffolds incorporating coincident gradations of instructive structural and biochemical cues for osteotendinous junction engineering. Adv Healthc Mater 2015;4:831.
Ramakrishna H, Li T, He T, Temple J, King MW, Spagnoli A. Tissue engineering a tendon-bone junction with biodegradable braided Scaffolds. Biomater Res 2019;23:11.
Li H, Fan J, Sun L, Liu X, Cheng P, Fan H. Functional regeneration of ligament-bone interface using a triphasic silk-based graft. Biomaterials 2016;106:180.
Calejo I, Costa-Almeida R, Reis RL, Gomes ME. A textile platform using continuous aligned and textured composite microfibers to engineer tendon-to-bone interface gradient Scaffolds. Adv Healthc Mater 2019;8:e1900200.
Zhu C, Pongkitwitoon S, Qiu J, Thomopoulos S, Xia Y. Design and fabrication of a hierarchically structured scaffold for tendon-to-bone repair. Adv Mater 2018;30:e1707306.
Brennan DA, Conte AA, Kanski G, Turkula S, Hu X, Kleiner MT, Beachley V. Mechanical considerations for electrospun nanofibers in tendon and ligament repair. Adv Healthc Mater 2018;7:e1701277.
Lim TK, Dorthe E, Williams A, D’Lima DD. Nanofiber Scaffolds by electrospinning for rotator cuff tissue engineering. Chonnam Med J 2021;57:13.
Xu Y, Zhang WX, Wang LN, Ming YQ, Li YL, Ni GX. Stem cell therapies in tendon-bone healing. World J Stem Cells 2021;13:753.
Kenry LCT. Nanofiber technology: current status and emerging developments. Prog Polym Sci 2017;70:1.
Shi S, Si Y, Han Y, Wu T, Iqbal MI, Fei B, Li RKY, Hu J, Qu J. Recent progress in protective membranes fabricated via electrospinning: advanced materials, biomimetic structures, and functional applications. Adv Mater 2022;34:e2107938.
Xue J, Wu T, Dai Y, Xia Y. Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem Rev 2019;119:5298.
Park J-S. Electrospinning and its applications. Adv Nat Sci-Nanosci 2011;1:043002.
Zhang X, Xie L, Wang X, Shao Z, Kong B. Electrospinning super-assembly of ultrathin fibers from single- to multi-Taylor cone sites. Appl Mater Today 2022;26:101272.
SalehHudin HS, Mohamad EN, Mahadi WNL, Muhammad AA. Multiple-jet electrospinning methods for nanofiber processing: a review. Mater Manuf Process 2017;33:479.
Li Y, Zhu J, Cheng H, Li G, Cho H, Jiang M, Gao Q, Zhang X. Developments of advanced electrospinning techniques: a critical review. Adv Mater Technol 2021;6:2100410.
Huang C, Thomas NL. Fabrication of porous fibers via electrospinning: strategies and applications. Polym Rev 2019;60:595.
Hou L, Wang N, Wu J, Cui Z, Jiang L, Zhao Y. Bioinspired superwettability electrospun micro/nanofibers and their applications. Adv Funct Mater 2018;28:1801114.
Wen X, Xiong J, Lei S, Wang L, Qin X. Diameter refinement of electrospun nanofibers: from mechanism, strategies to applications. Adv Fiber Mater 2021;4:145.
Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv 2010;28:325.
Kchaou M, Alquraish M, Abuhasel K, Abdullah A, Ali AA. Electrospun nanofibrous Scaffolds: review of current progress in the properties and manufacturing process, and possible applications for COVID-19. Polymers (Basel) 2021;2021:13.
Ogueri KS, Laurencin CT. Nanofiber technology for regenerative engineering. ACS Nano 2020;14:9347.
Nie G, Zhang Z, Wang T, Wang C, Kou Z. Electrospun one-dimensional electrocatalysts for oxygen reduction reaction: insights into structure-activity relationship. ACS Appl Mater Inter 2021;13:37961.
Yoon J, Yang HS, Lee BS, Yu WR. Recent progress in coaxial electrospinning: new parameters, various structures, and wide applications. Adv Mater 2018;30:e1704765.
Bi F, Dong X, Wang J, Liu G. Flexible Janus nanofiber to acquire tuned and enhanced simultaneous magnetism-luminescence bifunctionality. J Mater Sci 2014;49:7244.
Zhan L, Deng J, Ke Q, Li X, Ouyang Y, Huang C, Liu X, Qian Y. Grooved fibers: preparation principles through electrospinning and potential applications. Adv Fiber Mater 2021;4:203.
Forward KM, Rutledge GC. Free surface electrospinning from a wire electrode. Chem Eng J 2012;183:492.
Vass P, Szabo E, Domokos A, Hirsch E, Galata D, Farkas B, Demuth B, Andersen SK, Vigh T, Verreck G, Marosi G, Nagy ZK. Scale-up of electrospinning technology: Applications in the pharmaceutical industry. Wires Nanomed Nanobi 2020;12:e1611.
Wang Z, Tian Y, Zhang C, Wang Y, Deng W. Massively multiplexed electrohydrodynamic tip streaming from a thin disc. Phys Rev Lett 2021;126:064502.
Liu SL, Huang YY, Zhang HD, Sun B, Zhang JC, Long YZ. Needleless electrospinning for large scale production of ultrathin polymer fibres. Mater Res Innov 2014;18:S4.
Zhang CL, Yu SH. Nanoparticles meet electrospinning: recent advances and future prospects. Chem Soc Rev 2014;43:4423.
Juncos Bombin AD, Dunne NJ, McCarthy HO. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mat Sci Eng C-Mater 2020;114:110994.
Dziemidowicz K, Sang Q, Wu J, Zhang Z, Zhou F, Lagaron JM, Mo X, Parker GJM, Yu DG, Zhu LM, Williams GR. Electrospinning for healthcare: recent advancements. J Mater Chem B 2021;9:939.
Zhang X, Li L, Ouyang J, Zhang L, Xue J, Zhang H, Tao W. Electroactive electrospun nanofibers for tissue engineering. Nano Today 2021;39:101196.
Zare M, Davoodi P, Ramakrishna S. Electrospun shape memory polymer micro-/nanofibers and tailoring their roles for biomedical applications. Nanomater (Basel) 2021;2021:11.
Dou Y, Zhang W, Kaiser A. Electrospinning of metal-organic frameworks for energy and environmental applications. Adv Sci 2020;7:1902590.
Tebyetekerwa M, Ramakrishna S. What is next for electrospinning? Matter 2020;2:279.
Hao Y, Hu F, Chen Y, Wang Y, Xue J, Yang S, Peng S. Recent progress of electrospun nanofibers for zinc-air batteries. Adv Fiber Mater 2021;4:185.
Agarwal S, Greiner A, Wendorff JH. Functional materials by electrospinning of polymers. Prog Polym Sci 2013;38:963.
Suja PS, Reshmi CR, Sagitha P, Sujith A. Electrospun nanofibrous membranes for water purification. Polym Rev 2017;57:467.
Zhang F, Chen J, Yang J. Fiber materials for electrocatalysis applications. Adv Fiber Mater. 2022;2022:558.
Zhao J, Zhu W, Wang X, Liu L, Yu J, Ding B. Fluorine-free waterborne coating for environmentally friendly, robustly water-resistant, and highly breathable fibrous textiles. ACS Nano 2019;14:1045.
Zhou W, Yu X, Cao L, Yang M, Li Y, Si Y, Yu J, Ding B. Green and ethanol-resistant polyurethane nanofibrous membranes based on an ethanol solvent for waterproof and breathable textiles. Adv Sustain Syst 2020;4:2000105.
Zhou W, Yu X, Li Y, Jiao W, Si Y, Yu J, Ding B. Green-solvent-processed fibrous membranes with water/oil/dust-resistant and breathable performances for protective textiles. ACS Appl Mater Inter 2021;13:2081.
Zhou W, Gong X, Li Y, Si Y, Zhang S, Yu J, Ding B. Environmentally friendly waterborne polyurethane nanofibrous membranes by emulsion electrospinning for waterproof and breathable textiles. Chem Eng J 2022;427:130925.
Peng Y, Cui Y. Advanced textiles for personal thermal management and energy. Joule 2020;4:724.
Li D, Liu X, Li W, Lin Z, Zhu B, Li Z, Li J, Li B, Fan S, Xie J, Zhu J. Scalable and hierarchically designed polymer film as a selective thermal emitter for high-performance all-day radiative cooling. Nat Nanotechnol 2021;16:153.
Zhong H, Li Y, Zhang P, Gao S, Liu B, Wang Y, Meng T, Zhou Y, Hou H, Xue C, Zhao Y, Wang Z. Hierarchically hollow microfibers as a scalable and effective thermal insulating cooler for buildings. ACS Nano 2021;15:10076.
Liu L, Xu W, Ding Y, Agarwal S, Greiner A, Duan G. A review of smart electrospun fibers toward textiles. Compos Commun 2020;2020:22.
Iqbal MI, Shi S, Kumar GMS, Hu JL. Evaporative/radiative electrospun membrane for personal cooling. Nano Res 2020;2020:698.
Gao C, Zhang L, Wang J, Jin M, Tang Q, Chen Z, Cheng Y, Yang R, Zhao G. Electrospun nanofibers promote wound healing: theories, techniques, and perspectives. J Mater Chem B 2021;9:3106.
Zhang X, Lv R, Chen L, Sun R, Zhang Y, Sheng R, Du T, Li Y, Qi Y. A multifunctional Janus electrospun nanofiber dressing with biofluid draining, monitoring, and antibacterial properties for wound healing. ACS Appl Mater Inter 2022;14:12984.
Dong R, Li Y, Chen M, Xiao P, Wu Y, Zhou K, Zhao Z, Tang BZ. In situ electrospinning of aggregation-induced emission nanofibrous dressing for wound healing. Small Methods 2022;6:e2101247.
Celebioglu A, Uyar T. Electrospun formulation of acyclovir/cyclodextrin nanofibers for fast-dissolving antiviral drug delivery. Mat Sci Eng C-Mater 2021;118:111514.
Luraghi A, Peri F, Moroni L. Electrospinning for drug delivery applications: a review. J Control Release 2021;334:463.
Parın FN, Aydemir Çİ, Taner G, Yıldırım K. Co-electrospun-electrosprayed PVA/folic acid nanofibers for transdermal drug delivery: Preparation, characterization, and in vitro cytocompatibility. J Ind Text 2021;2021:1528.
Ahmed J. Electrospinning for the manufacture of biosensor components: a mini-review. Med Dev Sensors. 2020;2020:4.
Liu Y, Hao M, Chen Z, Liu L, Liu Y, Yang W, Ramakrishna S. A review on recent advances in application of electrospun nanofiber materials as biosensors. Curr Opin Biomed Eng 2020;13:174.
Crapnell RD, Street RJ, Ferreira-Silva V, Down MP, Peeters M, Banks CE. Electrospun nylon fibers with integrated polypyrrole molecularly imprinted polymers for the detection of glucose. Anal Chem 2021;93:13235.
Frias IAM, Vega-Gonzales-Gil LH, Cordeiro MT, Oliveira MDL, Andrade CAS. Self-enriching electrospun biosensors for impedimetric sensing of zika virus. ACS Appl Mater Inter 2022;14:41.
Wang Z, Wang Y, Yan J, Zhang K, Lin F, Xiang L, Deng L, Guan Z, Cui W, Zhang H. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv Drug Deliver Rev 2021;174:504.
Rahmati M, Mills DK, Urbanska AM, Saeb MR, Venugopal JR, Ramakrishna S, Mozafari M. Electrospinning for tissue engineering applications. Prog Mater Sci 2021;117:100721.
Si Y, Guo C, Xu X, Zhang K, Tan R, Lau K-T, Hu J. Bioinspired Janus all-natural electrospinning membranes with directional water transport as ecofriendly dry facial masks. ACS Sustain Chem Eng 2022;2022:3659.
Robinson AJ, Pérez-Nava A, Ali SC, González-Campos JB, Holloway JL, Cosgriff-Hernandez EM. Comparative analysis of fiber alignment methods in electrospinning. Matter 2021;4:821.
Zhao S, Zhao X, Dong S, Yu J, Pan G, Zhang Y, Zhao J, Cui W. A hierarchical, stretchable and stiff fibrous biotemplate engineered using stagger-electrospinning for augmentation of rotator cuff tendon-healing. J Mater Chem B 2015;3:990.
Kolluru PV, Lipner J, Liu W, Xia Y, Thomopoulos S, Genin GM, Chasiotis I. Strong and tough mineralized PLGA nanofibers for tendon-to-bone scaffolds. Acta Biomater 2013;9:9442.
Wang L, Zhu T, Kang Y, Zhang J, Du J, Gao H, Chen S, Jiang J, Zhao J. Crimped nanofiber scaffold mimicking tendon-to-bone interface for fatty-infiltrated massive rotator cuff repair. Bioact Mater 2022;16:149.
Zhu Q, Ma Z, Li H, Wang H, He Y. Enhancement of rotator cuff tendon-bone healing using combined aligned electrospun fibrous membranes and kartogenin. RSC Adv 2019;9:15582.
Yang R, Zheng Y, Zhang Y, Li G, Xu Y, Zhang Y, Xu Y, Zhuang C, Yu P, Deng L, Cui W, Chen Y, Wang L. Bipolar metal flexible electrospun fibrous membrane based on metal-organic framework for gradient healing of tendon-to-bone interface regeneration. Adv. Healthc. Mater. 2022;2022:e2200072.
Cong S, Sun Y, Lin J, Liu S, Chen J. A synthetic graft with multilayered co-electrospinning nanoscaffolds for bridging massive rotator cuff tear in a rat model. Am J Sports Med 1826;2020:48.
Li X, Cheng R, Sun Z, Su W, Pan G, Zhao S, Zhao J, Cui W. Flexible bipolar nanofibrous membranes for improving gradient microstructure in tendon-to-bone healing. Acta Biomater 2017;61:204.
Hakimi O, Mouthuy PA, Zargar N, Lostis E, Morrey M, Carr A. A layered electrospun and woven surgical scaffold to enhance endogenous tendon repair. Acta Biomater 2015;26:124.
Guan X, Xia H, Ni QQ. Shape memory polyurethane-based electrospun yarns for thermo-responsive actuation. J Appl Polym Sci 2021;2021:138.
Zhou Y, Fang J, Wang X, Lin T. Strip twisted electrospun nanofiber yarns: Structural effects on tensile properties. J Mater Res 2011;27:537.
Jiang C, Wang K, Liu Y, Zhang C, Wang B. Using wet electrospun PCL/Gelatin/CNT yarns to fabricate textile-based scaffolds for vascular tissue engineering. ACS Biomater Sci Eng 2021;7:2627.
Jiang C, Wang K, Liu Y, Zhang C, Wang B. Textile-based sandwich scaffold using wet electrospun yarns for skin tissue engineering. J Mech Behav Biomed Mater 2021;119:104499.
Smit E, Bűttner U, Sanderson RD. Continuous yarns from electrospun fibers. Polymer 2005;46:2419.
Wei L, Qin X. Nanofiber bundles and nanofiber yarn device and their mechanical properties: a review. Text Res J 1885;2016:86.
Tian L, Yan T, Pan Z. Fabrication of continuous electrospun nanofiber yarns with direct 3D processability by plying and twisting. J Mater Sci 2015;50:7137.
Chen S, Wang J, Chen Y, Mo X, Fan C. Tenogenic adipose-derived stem cell sheets with nanoyarn scaffolds for tendon regeneration. Mat Sci Eng C-Mater 2021;119:111506.
Li X, Yao C, Sun F, Song T, Li Y, Pu Y. Conjugate electrospinning of continuous nanofiber yarn of poly(L-lactide)/nanotricalcium phosphate nanocomposite. J Appl Polym Sci 2008;107:3756.
Yao C, Li X, Song T. Preparation and characterization of zein and zein/poly-L-lactide nanofiber yarns. J Appl Polym Sci 2009;114:2079.
Wu S, Zhou R, Zhou F, Streubel PN, Chen S, Duan B. Electrospun thymosin Beta-4 loaded PLGA/PLA nanofiber/ microfiber hybrid yarns for tendon tissue engineering application. Mat Sci Eng C-Mater 2020;106:110268.
Learn GD, McClellan PE, Knapik DM, Cumsky JL, Webster-Wood V, Anderson JM, Gillespie RJ, Akkus O. Woven collagen biotextiles enable mechanically functional rotator cuff tendon regeneration during repair of segmental tendon defects in vivo. J Biomed Mater Res B Appl Biomater 2019;107:1864.
Moutos FT, Freed LE, Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat Mater 2007;6:162.
Krishnan AG, Joseph J, Chandra RR, Nair SV, Nair M, Menon D. Silk-based bilayered small diameter woven vascular conduits for improved mechanical and cellular characteristics. Int J Polym Mater 2021;2021:1.
Padmakumar S, Paul-Prasanth B, Pavithran K, Vijaykumar DK, Rajanbabu A, Sivanarayanan TB, Kadakia E, Amiji MM, Nair SV, Menon D. Long-term drug delivery using implantable electrospun woven polymeric nanotextiles. Nanomedicine 2019;15:274.
Xie X, Cai J, Yao Y, Chen Y, Khan AR, Wu J, Mo X. A woven scaffold with continuous mineral gradients for tendon-to-bone tissue engineering. Compos Part B-Eng 2021;2021:212.
Deacon F, Crow M, Rajah S, Kester R. Comparison of the thrombogenicity of four types of knitted Dacron arterial graft in an artificial circulation. Biomaterials 1985;6:64–7.
Guo D, Wang S, Yin Y, Luo J, Meng C, Liu Q, Ni H, Feng J. Preparation of three-dimensional multilayer ECM-simulated woven/knitted fabric composite scaffolds for potential tissue engineering applications. Text Res J 2019;90:925.
Ouyang HW, Goh JCH, Thambyah A, Teoh SH, Lee EH. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit achilles tendon. Tissue Eng 2003;9:431.
Ruan D, Zhu T, Huang J, Le H, Hu Y, Zheng Z, Tang C, Chen Y, Ran J, Chen X, Yin Z, Qian S, Pioletti D, Heng BC, Chen W, Shen W, Ouyang HW. Knitted silk-collagen scaffold incorporated with ligament stem/progenitor cells sheet for anterior cruciate ligament reconstruction and osteoarthritis prevention. ACS Biomater Sci Eng 2019;5:5412.
Chen X, Qi YY, Wang LL, Yin Z, Yin GL, Zou XH, Ouyang HW. Ligament regeneration using a knitted silk scaffold combined with collagen matrix. Biomaterials 2008;29:3683.
Cai J, Xie X, Li D, Wang L, Jiang J, Mo X, Zhao J. A novel knitted scaffold made of microfiber/nanofiber core-sheath yarns for tendon tissue engineering. Biomater Sci 2020;8:4413.
Nowotny J, Aibibu D, Farack J, Nimtschke U, Hild M, Gelinsky M, Kasten P, Cherif C. Novel fiber-based pure chitosan scaffold for tendon augmentation: biomechanical and cell biological evaluation. J Biomat Sci-Polym E 2016;27:917.
Czaplewski SK, Tsai TL, Duenwald-Kuehl SE, Vanderby R Jr, Li WJ. Tenogenic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells dictated by properties of braided submicron fibrous scaffolds. Biomaterials 2014;35:6907.
Laranjeira M, Domingues RMA, Costa-Almeida R, Reis RL, Gomes ME. A 3D mimicry of native-tissue-fiber architecture guides tendon-derived cells and adipose stem cells into artificial tendon constructs. Small 2017;2017:13.
Rothrauff BB, Lauro BB, Yang G, Debski RE, Musahl V, Tuan RS. Braided and stacked electrospun nanofibrous Scaffolds for tendon and ligament tissue engineering. Tissue Eng Pt A 2017;23:378.
Silva M, Ferreira FN, Alves NM, Paiva MC. Biodegradable polymer nanocomposites for ligament/tendon tissue engineering. J Nanobiotechnol 2020;18:23.
Akbari N, Khorshidi S, Karkhaneh A. Effect of piezoelectricity of nanocomposite electrospun scaffold on cell behavior in bone tissue engineering Iran. Polym J 2022;31:919–30.
Fernandez-Yague MA, Trotier A, Demir S, Abbah SA, Larranaga A, Thirumaran A, Stapleton A, Tofail SAM, Palma M, Kilcoyne M, Pandit A, Biggs MJ. A self-powered piezo-bioelectric device regulates tendon repair-associated signaling pathways through modulation of mechanosensitive ion channels. Adv Mater 2021;33:e2008788.
Wang L, Zhang F, Liu Y, Leng J. Shape memory polymer fibers: materials, structures, and applications. Adv Fiber Mater 2021;4:5.
Jacob J, More N, Kalia K, Kapusetti G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm Regen 2018;38:2.
Barbosa F, Ferreira FC, Silva JC. Piezoelectric electrospun fibrous Scaffolds for bone, articular cartilage and osteochondral tissue engineering. Int J Mol Sci 2022;23:1.
Khare D, Basu B, Dubey AK. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials 2020;258:120280.
More N, Kapusetti G. Piezoelectric material—a promising approach for bone and cartilage regeneration. Med Hypotheses 2017;108:10.
Azimi B, Milazzo M, Lazzeri A, Berrettini S, Uddin MJ, Qin Z, Buehler MJ, Danti S. Electrospinning piezoelectric fibers for biocompatible devices. Adv Healthc Mater 2020;9:e1901287.
Gritsch L, Maqbool M, Mourino V, Ciraldo FE, Cresswell M, Jackson PR, Lovell C, Boccaccini AR. Chitosan/hydroxyapatite composite bone tissue engineering scaffolds with dual and decoupled therapeutic ion delivery: copper and strontium. J Mater Chem B 2019;7:6109.
Zhang X, Li Z, Yang P, Duan G, Liu X, Gu Z, Li Y. Polyphenol scaffolds in tissue engineering. Mater Horiz 2021;8:145.
Chen W, Zheng D, Chen Y, Ruan H, Zhang Y, Chen X, Shen H, Deng L, Cui W, Chen H. Electrospun fibers improving cellular respiration via mitochondrial protection. Small 2021;17:2104012.
Wang Y, Zhang W, Yao Q. Copper-based biomaterials for bone and cartilage tissue engineering. J Orthop Translat 2021;29:60.
Dong Y, Zhang T, Lin X, Feng J, Luo F, Gao H, Wu Y, Deng R, He Q. Graphene/aptamer probes for small molecule detection: from in vitro test to in situ imaging. Mikrochim Acta 2020;187:179.
Lu H, Chen C, Wang Z, Qu J, Xu D, Wu T, Cao Y, Zhou J, Zheng C, Hu J. Characterization of calcium and zinc spatial distributions at the fibrocartilage zone of bone–tendon junction by synchrotron radiation-based micro X-ray fluorescence analysis combined with backscattered electron imaging. Spectrochim Acta B 2015;111:15.
Lake SP, Liu Q, Xing M, Iannucci LE, Wang Z, Zhao C. Tendon and ligament tissue engineering. Principl Tissue Eng 2020;2020:989–1005.
Xie X, Chen Y, Wang X, Xu X, Shen Y, Khan AR, Aldalbahi A, Fetz AE, Bowlin GL, El-Newehy M, Mo X. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. J Mater Sci Technol 2020;59:243.
Zhong H, Huang J, Wu J, Du J. Electrospinning nanofibers to 1D, 2D, and 3D scaffolds and their biomedical applications. Nano Res 2021;15:787.
Mertz D, Harlepp S, Goetz J, Bégin D, Schlatter G, Bégin-Colin S, Hébraud A. Nanocomposite polymer scaffolds responding under external stimuli for drug delivery and tissue engineering applications. Adv Therapeut 2019;3:1900143.
Reina G, González-Domínguez JM, Criado A, Vázquez E, Bianco A, Prato M. Promises, facts and challenges for graphene in biomedical applications. Chem Soc Rev 2017;46:4400.
Kuo WS, Chang CN, Chang YT, Yang MH, Chien YH, Chen SJ, Yeh CS. Gold nanorods in photodynamic therapy, as hyperthermia agents, and in near-infrared optical imaging. Angew Chem Int Edit 2010;49:2711.
Tindell RK, Busselle L, Holloway J. Magnetic fields enable precise spatial control of electrospun fiber alignment for fabricating complex gradient materials. Polym Sci 2022;2022:55.
Xing C, Chen S, Liang X, Liu Q, Qu M, Zou Q, Li J, Tan H, Liu L, Fan D, Zhang H. Two-dimensional MXene (Ti3C2)-integrated cellulose hydrogels: toward smart three-dimensional network nanoplatforms exhibiting light-induced Swelling and bimodal photothermal/chemotherapy anticancer activity. ACS Appl Mater Inter 2018;10:27631.
Xie Z, Peng M, Lu R, Meng X, Liang W, Li Z, Qiu M, Zhang B, Nie G, Xie N, Zhang H, Prasad PN. Black phosphorus-based photothermal therapy with aCD47-mediated immune checkpoint blockade for enhanced cancer immunotherapy. Light Sci Appl 2020;9:161.
Yin F, Hu K, Chen S, Wang D, Zhang J, Xie M, Yang D, Qiu M, Zhang H, Li ZG. Black phosphorus quantum dot based novel siRNA delivery systems in human pluripotent teratoma PA-1 cells. J Mater Chem B 2017;5:5433.
Xie Z, Chen S, Duo Y, Zhu Y, Fan T, Zou Q, Qu M, Lin Z, Zhao J, Li Y, Liu L, Bao S, Chen H, Fan D, Zhang H. Biocompatible two-dimensional titanium nanosheets for multimodal imaging-guided cancer theranostics. ACS Appl Mater Inter 2019;11:22129.
Chen S, Xing C, Huang D, Zhou C, Ding B, Guo Z, Cao Y, et al. Eradication of tumor growth by delivering novel photothermal selenium-coated tellurium nanoheterojunctions. Sci Adv 2020;6:15.
Lee WC, Maul TM, Vorp DA, Rubin JP, Marra KG. Effects of uniaxial cyclic strain on adipose-derived stem cell morphology, proliferation, and differentiation. Biomech Model Mechan 2007;6:265.
Altman GH, Horan RL, Martin I, Farhadi J, Stark PRH, Volloch V, Richmond JC, Vunjak-Novakovic G, Kaplan DL. Cell differentiation by mechanical stress. FASEB J 2001;16:2.
Wu S, Wang Y, Streubel PN, Duan B. Living nanofiber yarn-based woven biotextiles for tendon tissue engineering using cell tri-culture and mechanical stimulation. Acta Biomater 2017;62:102.
Sensini A, Cristofolini L, Zucchelli A, Focarete ML, Gualandi C, Dem A, Kao AP, Roldo M, Blunn G, Tozzi G. Hierarchical electrospun tendon-ligament bioinspired scaffolds induce changes in fibroblasts morphology under static and dynamic conditions. J Microsc 2020;277:3.
Tseng LF, Mather PT, Henderson JH. Shape-memory-actuated change in scaffold fiber alignment directs stem cell morphology. Acta Biomater 2013;9:8790.
Reifenrath J, Wellmann M, Kempfert M, Angrisani N, Welke B, Gniesmer S, Kampmann A, Menzel H, Willbold E. TGF-beta3 loaded electrospun polycaprolacton fibre scaffolds for rotator cuff tear repair: an in vivo study in rats. Int J Mol Sci 2020;2020:21.
Majidinia M, Reiter RJ, Shakouri SK, Mohebbi I, Rastegar M, Kaviani M, Darband SG, Jahanban-Esfahlan R, Nabavi SM, Yousefi B. The multiple functions of melatonin in regenerative medicine. Ageing Res Rev 2018;45:33.
Gao W, Lin M, Liang A, Zhang L, Chen C, Liang G, Xu C, Peng Y, Chen C, Huang D, Su P. Melatonin enhances chondrogenic differentiation of human mesenchymal stem cells. J Pineal Res 2014;56:62.
Song W, Ma Z, Wang C, Li H, He Y. Pro-chondrogenic and immunomodulatory melatonin-loaded electrospun membranes for tendon-to-bone healing. J Mater Chem B 2019;7:6564.
Acknowledgements
The authors gratefully acknowledge the financial support from the Startup Grant of CityU (“Laboratory of Wearable Materials for Healthcare”, Grant 9380116), National Natural Science Foundation of China (“Study of high performance fiber to be achieved by mimicking the hierarchical structure of spider-silk”, Grant 52073241; “Study of Multi-Responsive Shape Memory Polyurethane Nanocomposites Inspired by Natural Fibers”, Grant 51673162; “Developing Spider-Silk-Model Artificial Fibers by A Chemical Synthetic Approach”, Grant 15201719), and the Contract Research (“Development of Breathable Fabrics with Nano-Electrospun Membrane”, CityU ref.: 9231419).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, X., Si, Y., Zhao, Y. et al. Electrospun Textile Strategies in Tendon to Bone Junction Reconstruction. Adv. Fiber Mater. 5, 764–790 (2023). https://doi.org/10.1007/s42765-022-00233-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-022-00233-9