Abstract
The urine of yaks (Bos grunniens) and Tibetan sheep (Pantholops hodgsoni) represents a vital source of nutrients in the alpine grasslands on the Qinghai-Xizang Plateau, and its crucial role in affecting soil properties and greenhouse gas (GHG) emissions cannot be underestimated. However, few in-situ experiments examining the impacts of yak and Tibetan sheep urine deposition on the alpine grassland nutrient dynamics and GHG fluxes, and the underlying mechanisms and influential factors associated with GHG emissions in urine deposited grasslands are still not well understood. This study conducted a 33-day simulated urine deposition experiment that include the treatments of yak urine (YU), Tibetan sheep urine (TSU), and a control (CK) without any application in an alpine steppe of northern Xizang Plateau. We collected soil samples at depths of 0–10 cm, 10–20 cm, and 20–30 cm after 11, 21, 33 days of experimental treatments to explore its physicochemical and microbial properties. The GHG samples were collected respectively at 1, 3, 5, 8, 13, 18, 27, and 33 d of the experiment by using the method of artificial static closed chamber and determined the nitrous oxide (N2O), carbon dioxide (CO2), and methane (CH4) concentration by using a gas chromatograph. The gene abundance of 0–10 cm soil ammonia-oxidizing archaea (AOA), ammonia-oxidizing bacteria (AOB), membrane-bound nitrate reductase (NarG), nitrite reductase encoding gene (NirS), nitric oxide reductase gene (NorB), and nitrous oxide reductase encoding gene (NosZ) were determined using real-time fluorescence quantitative PCR. The results showed that cumulative N2O, CO2, and CH4 emissions for YU treatment across the 33-day experimental period amounted to 49.6 g N ha− 1, 73.8 kg C ha− 1, and − 176 g C ha− 1, respectively, with the values significantly (P < 0.05) greater than the emissions for CK treatment (36.4 g N ha− 1, 23.5 kg C ha− 1, and − 232 g C ha− 1). The deposition of YU and TSU immediately increased soil nutrient availability, anaerobic conditions, and microbial activity, which led to increasing N2O, CO2, and CH4 emissions to vary degrees, and reduced CH4 uptake. Soil CO2 flux for YU treatment exhibited a significant (P < 0.05) negative correlation with NO3−-N concentration, while soil CH4 flux exhibited extremely significant (P < 0.01) negative correlation with SOC concentration. Soil N2O flux for TSU treatment was negatively correlated with soil NifH, AOB, AOA, and NirS gene abundances (P < 0.05). In contrast, the cumulative N2O, CO2, and CH4 emissions during the initial 13 days for YU and TSU treatments accounted for a significantly (P < 0.05) higher proportion (49.9% and 46.6%, 66.1% and 64.9%, 40.2% and 49.0%, respectively) of the total emissions compared to the CK treatment (43.8%, 40.7%, and 31.0%). Urine application resulted in 0–10 cm soil AOA, AOB, NirS, NarG, and NosZ gene abundances for YU treatment were significantly (P < 0.05) higher than that for CK treatment. The significant enhancement of 0–10 cm soil functional gene abundance (NirS, NorB, and NosZ), as well as increased levels of NH4+-N and NO3−-N concentrations for YU and TSU treatments during the early stage of the experiment (P < 0.05), proving the strengthen of denitrification activity and soil respiration and therefore resulted in a higher proportion of N2O and CO2 emissions. The conclusions would contribute to a better understanding of the short-term effects and functional mechanisms of urine deposition on alpine steppe GHG emissions, and provide scientific insights for optimising livestock urine management model and facilitating the greenhouse gas emission reduction strategies in pastoral areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nitrous oxide (N2O), carbon dioxide (CO2), and methane (CH4) are primary greenhouse gas (GHG) present in the atmosphere (Cristina et al. 2010). As of 2019, the average annual concentration of CO2 in the atmosphere had reached 410 ppm, while CH4 had reached 1.87 ppm, and N2O had reached 332 ppb (IPCC 2022). The GHG emitted by human activities are the predominant driver of global warming, accounting for a staggering 87% of the overall temperature rise (IPCC 2014). N2O is primarily generated through two processes include nitrification, which involves the oxidation of ammonium (NH4+) to nitrate (NO3−) under aerobic conditions, and denitrification, which reduces nitrate (NO3−) or nitrite (NO2−) to nitrogen in its reduced state (NO, N2O, N2) under anaerobic conditions (Di et al. 2009). Soil respiration plays a vital role in the carbon (C) cycle in terrestrial ecosystems and primarily involves plant root respiration and microbial respiration. The emitted CO2 is the second-largest C flux between the soil and the atmosphere (Guo et al. 2018). In contrast, CH4 is produced under anaerobic conditions through the decomposition of organic matter by anaerobic and facultative anaerobic bacteria, resulting in the formation of small molecular compounds. Subsequently, it undergoes a catalytic transformation by methanogens (such as acetoclastic methanogens) via the methyl-coenzyme M reductase (Mcr) pathway (Zhang et al. 2019). According to the report of Food and Agriculture Organization of the United Nations in 2006, livestock husbandry is responsible for 9%, 37%, and 65% of global CO2, CH4, and N2O emissions. Furthermore, livestock excreta contribute about 10% of the annual N2O emission from agricultural soils and 60% of the global N2O emission from cattle production systems (Oenema et al. 2005). Meanwhile, previous study has found that 20% of total CH4 emissions are caused by livestock and their wastes (Johnson et al. 1993). In recent years, the rapid socio-economic development has led to an increase in global livestock populations, resulting in a higher excretion of urine and dung, which subsequently become hotspots for the N2O, CO2, and CH4 emissions when deposited on grasslands (Cai et al. 2017a).
Grazed pastures are major contributor to GHG emissions, and urine deposition from grazing animals is the main source of the emissions (Luo et al. 2018; Cristina et al. 2010). Livestock urine, primarily in the form of nitrogen (N), plays a prominent role in influencing soil N transformation, GHG emissions, and ecosystem productivity (Wu et al. 2019; Stockdale 2005). Previous studies have revealed that majority of N in urine exists in the form of urea, which rapidly hydrolyses into NH4+ and subsequently oxidises to NO3−, and thereby undergoes reduction to N2O and N2 during denitrification process (Dixon et al. 2010). Additionally, urine application can lead to a short-term increase in soil organic carbon (SOC), total nitrogen (TN), ammonium nitrogen (NH4+-N), and nitrate nitrogen (NO3−-N) concentrations in the 0–10 cm soil layer of alpine steppe on the northern Tibet (Du et al. 2022). This increase is attributed to the enhanced nitrification under aerobic soil conditions and denitrification under anaerobic conditions, making it one of the significant factors contributing to soil N2O emissions (Hynšt et al. 2006; Nielsen et al. 1996; Du et al. 2022). This finding aligns partially with the research by Di and Cameron (2002), who observed that the N supply from cattle urine to patches exceeds the soil’s N demand, thereby promoting N loss through leaching and gaseous emissions. It has been reported that soil properties such as pH, TN, and C: N ratio were more important than the climatic factors in affecting soil N cycles and the application of organic fertilizer and balanced fertilization can promote soil N leaching and gas emission compared to the control (Elrys et al. 2023a; Elrys et al. 2023b). In addition, the returning of certain nutrients in the form of urine from livestock to the grassland alters soil nutrient availability and microbial activity, thereby promoting soil GHG emissions to varying degrees (Lombardi et al. 2022). Orwin et al. (2010) have revealed significant disparities in the community structure of NirS denitrifying bacteria between soil samples treated with cattle urine and the control. In contrast, research conducted on pastures in New Zealand has yielded contrasting findings (Morales et al. 2015). In the initial stages of the experiments, the application of urine led to a decrease of denitrifying bacteria, suggesting that the influence of urine on denitrifying microorganisms is inconsistent (Morales et al. 2015).
On the other hand, another study revealed that the application of livestock urine can accelerate the growth of plants in alpine grasslands, enhance the activity of SOC, and expedite C cycling, thereby ultimately contributing to increased CO2 emissions (Chang et al. 2014). Moreover, the application of cattle urine can stimulate CO2 emissions by enhancing microbial population size, dissolved organic carbon (DOC), and dissolved organic nitrogen (DON) (Clough et al. 2003). It can also facilitate CH4 emissions by increasing the availability of NH4+, possibly due to the inhibitory effect of NH4+ on CH4 oxidation (Boon et al. 2014). Flessa et al. (1996) reported that fresh cattle dung patches were identified as significant sources of CH4 for a certain period of time, while urine patches acted as CH4 sinks. Urine application resulted in an increase in soil moisture content, creating favourable anaerobic conditions that facilitated the decomposition of organic matter. This, in turn, promoted the production of CH4 through the catalytic activity of methanogens (Zhang et al. 2019). Generally, the nutrient and water content present in livestock urine collectively influence GHG emissions in grazed grasslands (Cai et al. 2017b). The application of urine increases soil TN content, consequently resulting in elevated emissions of N2O and CH4 (Jackson et al. 2015; Nichols et al. 2016). This finding is consistent with the research conducted by Uchida et al. (2011), who reported the application of cattle urine significantly increased cumulative CO2 and N2O emissions. In contrast, study conducted on typical brown soils in southwest England have indicated that the application of livestock urine had no discernible impact on soil respiration (Dixon et al. 2010). Furthermore, urine deposition has been found to augment CH4 emissions in peatland grassland ecosystems in the UK (Boon et al. 2014). Di et al. (2011) conducted field experiment in different regions of New Zealand and they found no significant effect of cattle urine application on soil CH4 emission.
Qinghai-Xizang Plateau (QXP) is the largest and highest plateau in the world, with an average elevation exceeding 4,000 m, and the main vegetation type is alpine grassland (Mao et al. 2015). The alpine grasslands cover an area of approximately 1.52 × 106 km2, accounting for approximately 60% of the total area of the QXP. Grazing serves as the principal form of grassland utilisation on the QXP, with yaks and Tibetan sheep being the predominant grazing livestock types. The population respectively exceeding 13 × 106 heads of yaks and 30 × 106 Tibetan sheep (Lin et al. 2009), and could generate significant impacts on both the region’s economic development and ecological environment. With the grazing period estimated to be 120 days per growing season, the annual excretion of TN from yaks and Tibetan sheep amounts to approximately 200,000 to 940,000 tonnes and 2,000 to 30,000 tonnes, respectively (van der Weerden et al. 2011). The considerable amount of N returned through urine deposition has a significant impact on soil N balance and GHG emissions should not be underestimated. Existing studies have primarily focused on the impact of urine application on soil C decomposition (Lambie et al. 2013, 2021), soil N dynamics (Singh et al. 2009; Wachendorf et al. 2008; Welten et al. 2013), and N2O emissions (Byrnes et al. 2016; Hamamoto et al. 2020; Luo et al. 2018) in tropical or temperate grasslands. In contrast, there is a greater lack of field research on the response of GHG emissions from alpine grassland on the QXP to livestock urine deposition. Thus, we hypothesize that (i) Urine deposition differently affect soil C and N availability and major transformation processes. (ii) Urine returning stimulates alpine steppe N2O, CO2 and CH4 emissions in the short-term and change gases source or sink properties. In order to validate the aforementioned hypotheses, this study conducted a 33-day field experiment to investigate the effects of livestock urine application on soil physicochemical properties, microbial communities, and GHG fluxes in alpine grasslands. The findings of the present study will provide valuable scientific evidence for optimising the management model of livestock urine and mitigating GHG emission in alpine pastoral regions.
2 Materials and Methods
2.1 Site Description
The field experiment was conducted at the Xainza Alpine Steppe and Wetland Ecosystem Observation Station of the Chinese Academy of Sciences, located at 30°57′N, 88°42′E, with an altitude of 4675 m. The study area is characterized by a semi-arid plateau monsoon climate, which is cold, dry, and windy. The annual average air temperature is around 0 °C, and the precipitation is about 300 mm (Du et al. 2021). Precipitation mainly occurs from May to September, the frost-free season is short, and the frost period lasts for up to 279 days (Du et al. 2017). The main plant species of alpine steppe in the study site dominated by Stipa purpurea and Carex moorcroftii, accompanied by Leontopodium alpinum, Artemisia nanschanica, Leontopodium alpinum, and Oxytropis glacialis (Hong et al. 2016). The alpine steppe and meadow soils are mostly equivalent to Cryic Aridisols and Gelic Cambisols according to the Chinese soil taxonomy, respectively (Cai et al. 2013). The upper soil layer consists of 91% sand, 7% silt and 2% clay and contains 0.88% organic C and 0.10% TN (Cai et al. 2014).
2.2 Experimental Methods
Field experiment was conducted from August 8, 2020 to September 9, 2020, with three treatments include: (i) Control (CK, no urine application), (ii) Soil amended with 692 kg N ha− 1 of yak urine (YU), and (iii) Soil amended with 153 kg N ha− 1 of Tibetan sheep urine (TSU). Each treatment had three replicates. The initial concentrations of NH4+-N and NO3−-N of YU were 5.05 and 0.31 g L− 1, respectively, while the initial NH4+-N concentration of TSU was 3.2 g L− 1 (Table 1). During the experiment, soil samples were collected from 9 plots (3 treatments×3 replicates) near the study area. Fresh yak and Tibetan sheep urine samples were manually collected from 8 randomly selected animals near the camping area. These grazing animals were confined within the campsite during the night, and the fresh urine was collected the next morning in plastic containers until the amount collected was enough for the field experiment. The yak and Tibetan sheep urine samples were mixed separately and stored at room temperature for approximately 6 h.
For the YU treatment, 1 L of fresh YU was evenly applied to each plot (1 × 1 m2), forming typical urine patches (40 cm in diameter). For the TSU treatment, 70 mL of fresh TSU was evenly applied to each plot, forming typical urine patches (16 × 20 cm2). These simulated urine patches were similar in size and volume to naturally deposited cattle and sheep urine in the field (Ma et al. 2006). To minimize the impact of soil sampling on its physical structure and microbial processes, three parallel plots were also set up for independent soil sampling at 11, 21, and 33 days after urine application. At each sampling event, soil samples covered by each urine patch were manually collected at depths of 0–10 cm, 10–20 cm, and 20–30 cm. For each soil layer, the soil samples were carefully mixed and sieved through a 2 mm mesh. Subsamples were then collected for further analysis, both as air-dried samples and fresh samples, and transported to the laboratory.
Soil GHG fluxes were measured and calculated using the static closed chamber technique (Cai et al. 2014; Zhang et al. 2022). The static chamber had dimensions of 50 × 50 × 50 cm and was equipped with a thermometer and a sampling port on its side. The outer shell of the chamber was covered with foamed plastic to ensure a constant temperature inside the chamber and improve observation quality. The whole chamber was made airtight by connecting the chamber top and base with a tight rubber belt (2.7 mm in thickness) (Chen et al. 2008). Gas sampling was conducted at 1, 3, 5, 8, 13, 18, 27, and 33 d after the start of the experiment. A medical syringe was used to extract 120 mL of gas, and the first gas sample was collected immediately after sealing the chamber and stored in a dedicated gas bag, along with recording the sampling time and internal chamber temperature. Subsequent gas samples were collected every 10 min, with gas collection performed at 0, 10, 20, and 30 min for each chamber, and the samples were promptly taken back to the laboratory for analysis after being sealed in pre-evacuated gas collecting bags. Concentrations of N2O, CO2, and CH4 were determined using a gas chromatograph (Agilent 7890 A, Santa Clara, USA) equipped with an electron capture detector for N2O analysis and a flame ionization detector for CO2 and CH4 analysis (Cai et al. 2017b).
2.3 Urine and Soil Samples Measurement
Soil moisture content was determined by drying the soil at 105 °C until a constant weight. Urine pH was measured using a glass electrode method. Soil pH measurement was conducted using a soil: water ratio of 1: 2.5. The concentrations of NH4+-N and NO3−-N were measured using a San++ continuous flow analyzer (Netherlands) (Liu et al. 2020). Soil TOC and TN concentrations were measured using the potassium dichromate external heating and wet micro-Kjeldahl methods, respectively (Carter 1993; Cai et al. 2013). The abundance of soil ammonia-oxidizing archaea (AOA), ammonia-oxidizing bacteria (AOB), membrane-bound nitrate reductase (NarG), nitrite reductase encoding gene (NirS), nitric oxide reductase gene (NorB), and nitrous oxide reductase encoding gene (NosZ) were determined using real-time fluorescence quantitative PCR, and the gene abundance was expressed as copies per gram of dry soil for each gene (Throbäck et al. 2004; Michotey et al. 2000). The total DNA of the lyophilized soil (0.5 g) was extracted using a Fast DNA Spin Kit for soil (MP Biomedicals, Illkirch, France) according to the manufacturer’s instructions. Functional genes AOA, AOB, NarG, norB, nirS, and nosZ were amplifed using unique forward primers on a thermal cycler (ABI QuantStudio3, Applied Biosystems, Foster City, USA). A plasmid DNA containing each target gene was used to prepare a standard curve. Each reaction mixture (total volume of 25 µL) contained a 20 ng DNA template, 2 µL of both primers, 2 µL dNTPs, 2.5 µL 10 × TransStart Bufer, 0.4 µL FastPfu Polymerase, 0.2 Μ BSA, and sterilized H2O (Tang et al. 2021).
2.4 Data Calculation and Statistical Analysis
The formula for calculating GHG flux is as follows:
Where F represents the GHG flux, measured in µg C (m2·h)−1 or mg C (m2·h)−1 or µg N (m2·h)−1; ρ is the density of GHG at standard conditions, measured in µg m− 3; V is the volume of the static chamber, in cm3; A is the soil surface area inside the sampling chamber base, in cm2; P is the gas pressure inside the sealed static chamber, P0 is the atmospheric pressure at standard conditions, which is 1.013 × 105 Pa; T is the temperature inside the static chamber, in ℃; dC/dt is the rate of change of greenhouse gas concentration in the static chamber per unit time, measured in 10− 9 min− 1.
Furthermore, the cumulative emission of gases can be calculated based on the gas flux using the following formula:
Where CE represents the cumulative emission of gases, F is the gas flux, Δt represents the number of days between the first and last sampling intervals, and n is the total number of measurements during the observation period.
All data were analyzed using the SPSS software package (version 18.0, SPSS Inc., Chicago, IL, USA) and plotted using Origin 8.0 (Origin Lab Corporation, Northampton, MA, USA). One-way analysis of variance (ANOVA) and the least significant difference (LSD) method were used to test the differences in soil physicochemical properties, microbial gene abundance, and cumulative soil GHG emissions among different sampling times for the same treatment or among different treatments at the same sampling time (P < 0.05). Pearson correlation analysis was used to evaluate the relationship between GHG flux and soil physicochemical indexes, and the relationship between N2O flux and microbial functional genes.
3 Results
3.1 Air Temperature and Precipitation Properties
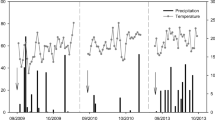
Air temperature showed a general trend of decreasing during the early stage (1–11 d) and the middle stage (12–21 d), and then increased during the later stage (22–33 d) across the whole experimental period (Fig. 1). The average air temperature was 10.8 °C during the whole 33 days, and the accumulated precipitation amounted to 54.2 mm. The precipitation occurred during the early, middle, and later stages accounted for 34.3%, 34.7% and 31.0% of the total precipitation through the whole experimental period, respectively (Fig. 1).
3.2 Changes of soil Physicochemical Parameters
Soil moisture content at 0–10 cm and 20–30 cm depth for YU and TSU treatments exhibited a decreasing trend during the field experiment (Fig. 2). After 21 days of urine application, the soil moisture content at 0–10 cm and 20–30 cm depth for YU and TSU treatments was significantly (P < 0.05) higher than that for CK treatment (Fig. 2(a) and 2(c)). However, YU and TSU treatment exhibited significantly (P < 0.05) higher 10–20 cm soil moisture content than the CK treatment (Fig. 2(b)). Soil pH for the three treatments ranged from 8.50 to 8.85. During the early stage of the experiment, the YU treatment exhibited a significantly (P < 0.05) higher 0–10 cm soil pH compared to the CK treatment (Fig. 2(d)), and the TSU and YU treatment simultaneously demonstrated a higher 0–10 cm and 10–20 cm soil pH than the CK treatment during the middle stage (Fig. 2(d)). Soil pH at 10–20 cm depth for CK treatment was significantly (P < 0.05) higher than that for YU and TSU treatments at the end of the experiment (Fig. 2(e)).
Temporal variation of soil moisture content at depth of (a) 0–10 cm, (b) 10–20 cm, and (c) 20–30 cm for the control (CK), yak urine (YU), and Tibetan sheep urine (TSU) treatments, and the soil pH at depths of (d) 0–10 cm, (e) 10–20 cm, and (f) 20–30 cm across the 33 days experimental period. Different lowercase letters at the same sampling time indicate the significant differences among the three treatments at P < 0.05, while different uppercase letters indicate the significant differences among the three sampling times at P < 0.05
The YU treatment always had statistically (P < 0.05) higher 0–10 cm and 10–20 cm soil NH4+-N concentration than the CK treatment (Fig. 3). At the end of the experiment, soil NH4+-N concentration for YU treatment significantly decreased in all three soil layers (P < 0.05), reaching values of 14.1, 1.95, and 1.21 mg kg− 1, respectively (Fig. 3(a), 3(b), and 3(c)). However, the corresponding soil NO3−-N concentration respectively significantly (P < 0.05) increased to 13.8, 4.83, and 3.96 mg kg− 1 (Fig. 3(d), 3(e), and 3(f)). In contrast, the 10–20 cm and 20–30 cm soil NH4+-N concentration for TSU treatment during the early and later stages was significantly (P < 0.05) higher than that for CK treatment (Fig. 3(b) and 3(c)). The TSU treatment consistently exhibited significantly (P < 0.05) higher 0–10 cm and 10–20 cm soil NO3−-N concentration than the CK treatment (Fig. 3(d), 3(e), and 3(f)). During the early and later stages, YU treatment demonstrated a significant (P < 0.05) increase of 0–10 cm SOC concentration compared to the CK and TSU treatments. However, CK treatment exhibited significantly (P < 0.05) higher SOC concentration during the middle stage of the experiment than the YU treatment (Table 2).
Variation of soil NH4+-N concentration at depth of (a) 0–10 cm, (b) 10–20 cm, and (c) 20–30 cm for the control (CK), yak urine (YU), and Tibetan sheep urine (TSU) treatments, and the soil NO3−-N concentration at depths of (d) 0–10 cm, (e) 10–20 cm, and (f) 20–30 cm across the 33 days experimental period. Different lowercase letters at the same sampling time indicate the significant differences among the three treatments at P < 0.05, while different uppercase letters indicate the significant differences among the three sampling times at P < 0.05
3.3 Variation of soil Microbial Characteristics
The YU treatment showed a significant (P < 0.05) increase in the abundance of AOB, NirS, and NosZ genes at 0–10 cm soil depth compared to the CK treatment during the early stage of the experiment, while the TSU treatment increased the abundance of the NorB gene, which was significantly (P < 0.05) higher than that for YU and CK treatments (Table 3). The YU treatment increased the abundance of AOB, NosZ, and NarG genes after 21 days of field experiment, with the values were significantly (P < 0.05) higher than those for TSU and CK treatments (Table 3). The TSU treatment increased the abundance of soil AOB and NorB genes (P < 0.05), displaying a pattern of TSU > YU > CK treatments at the end of the experiment (Table 3). Compared to the CK treatment, the YU treatment significantly (P < 0.05) increased the abundance of soil AOB, AOA, NarG, NirS, and NosZ genes at 0–10 cm depth by approximately 6,150%, 141%, 103%, 335%, and 256%, respectively. In contrast, the TSU treatment significantly (P < 0.05) increased the abundance of soil AOB, AOA, NirS, and NorB genes by approximately 21,209%, 88%, 270%, and 147%, respectively (Table 3).
3.4 Soil GHG Flux and Cumulative Emissions
Both YU and TSU treatments increased soil N2O flux, with peak values (15.3 and 8.40 µg N (m2·h)−1) occurring on the 5 d and 3 d after urine application, respectively (Fig. 4(a)). Simultaneously, CO2 emissions for YU and TSU treatments reached the peak value (39.4 and 17.3 mg C (m2·h)−1) on the 3 d after urine application, and CH4 emissions reached the maximum (-33.8 and − 39.8 µg C (m2·h)−1) on the 5 d, respectively (Fig. 4(c) and 4(e)). The N2O flux for the YU treatment increased during the first 5 days and then decreased gradually during the last 28 days (Fig. 4(a)). Cumulative N2O and CO2 emissions for YU treatment (49.6 g N ha− 1 and 73.8 kg C ha− 1) were significantly (P < 0.05) higher than those for CK treatment (36.4 g N ha− 1 and 23.5 kg C ha− 1) and the TSU treatment (41.4 g N ha− 1 and 39.3 kg C ha− 1) across the 33 days experiment (Fig. 4(b) and 4(d)). In contrast, for TSU treatment, it was only higher than the CK treatment during the first 13 days (Fig. 4(c)). Furthermore, the cumulative CH4 emissions for YU (-176 g C ha− 1) and TSU treatment (-206 g C ha− 1) were significantly (P < 0.05) higher than the CK treatment (-232 g C ha− 1) (Fig. 4(f)).
Changes in soil N2O flux (a) and cumulative emission (b), CO2 flux (c) and cumulative emission (d), CH4 flux (e) and cumulative emission (f) across the 33 days experimental period for the control (CK), yak urine (YU), and Tibetan sheep urine (TSU) treatments. Different lowercase letters indicate the significant differences among the three treatments for the same type of gas at P < 0.05
During the first 13 days, the cumulative N2O emissions for YU treatment accounted for 49.9% of the total emissions, which was significantly (P < 0.05) higher than the TSU (46.6%) and CK treatments (43.8%) (Fig. 5(a). Cumulative CO2 emissions for YU and TSU treatments during the first 13 days respectively accounted for 66.1% and 64.9% of the total emissions, with the proportion statistically (P < 0.05) greater than that for CK treatment (40.7%) (Fig. 5(b). In contrast, CH4 emissions during the first 13 days exhibited the proportion of TSU (49.0%) > YU (40.2%) > CK (31.0%) (Fig. 5(c). Besides, Over a 100-year time frame, the global warming potential (GWP) is 28 times higher for CH4 and 265 times higher for N2O than that of CO2 (IPCC 2014). The total GWP for YU and TSU (264.05 and 136.46 g CO2-equivalent kg− 1) treatments were significantly higher than CK (77.32 g CO2-equivalent kg− 1) treatment (P < 0.05) (Table 4). The GWP of N2O, CO2, and CH4 and total GWP for YU treatment were significantly higher than that for TSU and CK treatments (P < 0.05) (Table 4). Pearson correlation analysis showed that N2O flux for CK treatment was significantly positively (P < 0.05) correlated with 0–10 cm SOC and NH4+-N concentration (Fig. 6). For YU treatment, CO2 flux exhibited a significant (P < 0.05) negative correlation with NO3−-N concentration, while CH4 flux exhibited extremely significant (P < 0.01) negative correlation with SOC concentration. Soil CH4 flux for TSU treatment displayed a significant (P < 0.05) positive correlation with SOC and NO3−-N concentration (Fig. 6). Furthermore, soil N2O flux for CK treatment was significantly (P < 0.05) positively correlated with 0–10 cm soil AOA gene abundance, and extremely significantly (P < 0.01) positively correlated with AOB gene abundance. For YU treatment, N2O flux was negatively (P < 0.05) correlated with NirS and NosZ gene abundances, and extremely negatively (P < 0.01) correlated with AOA gene abundance. For TSU treatment, N2O was negatively (P < 0.05) correlated with soil NifH, AOB, AOA, and NirS gene abundance (Fig. 7).
Proportion of the cumulative soil emissions of N2O (a), CO2 (b) and CH4 (c) during the first 13 days and the last 20 days. Different lowercase letters indicate the significant difference in cumulative emission proportions between the first 13 days and last 20 days at P < 0.05 for the same treatment of each type of GHG, and different uppercase letters indicate the significant difference among the three treatments during the first 13 days or last 20 days at P < 0.05 for the same treatment of each type of GHG.
Pearson correlation analysis showed the relationship between greenhouse gas (GHG) flux and 0–10 cm soil physicochemical properties for the treatment of control (CK) (a), yak urine (YU) (b), and Tibetan sheep urine (TSU) (c). “*” indicates the significance level P < 0.05, “**” indicates the significance level P < 0.01. Note YU = Yak urine, TSU = Tibetan sheep urine, TN = Total nitrogen, TP = Total phosphorus, NH4+-N = Ammonium nitrogen, NO3−-N = Nitrate nitrogen. “/“ indicates not detected, “-” indicates not determined
4 Discussion
Urine deposition can alter soil nutrient availability, redox conditions, and microbial activity, thereby influencing soil GHGs emission to varying degrees (Du et al. 2022; Orwin et al. 2010; Wu et al. 2019). In the present study, the cumulative N2O emissions for YU and TSU treatments (49.6 and 41.4 g N ha− 1) were higher than the CK treatment (36.4 g N ha− 1) through the whole experimental period. This indicates that urine deposition resulted in increased soil N2O emissions. The observed increase in soil N2O flux following urine application may be attributed to the variations in soil conditions, including alterations in soil moisture, pH, nutrient content, and microbial activity. These changes promote nitrification and denitrification processes, ultimately leading to increased soil N2O emissions. This finding is consistent with the research conducted by Yamulki et al. (2000), who identified livestock urine patches as significant sources of N2O emissions, with YU treatment exhibiting significantly higher N2O emissions compared to other treatments. Urine deposition increased N concentration and stimulated N transformation process, thereby enhancing N2O flux. Sandoval et al. (2024) found that CO2 and N2O emissions increase with increasing N levels. In this study, we found YU application significantly increased 0–10 cm and 10–20 cm soil NH4+-N concentration compared to the CK treatment, which would provide sufficient substrates for nitrification and strengthen the potential of N2O emission. The gradual decrease of soil NH4+-N concentration and increase of NO3−-N concentration with the extension of experimental period could partly prove the nitrification and consistent with the variation of N2O flux. Moreover, soil NO3−-N concentration at 0–10 cm, 10–20 cm, and 20–30 cm soil depth was consistently higher in the YU and TSU treatments compared to the CK treatment during different stages of the experiment, indicating that YU and TSU deposition totally increased the soil NO3−-N concentration. It is partially aligns with the results of Carter (2007), who suggested that urine deposition can enhance denitrification rates in the short term by increasing soil moisture content, NO3−-N levels, and the availability of labile C. In this study, the soil NO3−-N concentration increased at 0–10 cm depth for YU and TSU treatments during the later stage, indicating that soil NO3−-N concentration increased gradually after urine deposition. The penetration of urine into the soil promptly augments the supply of available N and modifies the soil’s redox environment, subsequently intensifying denitrification processes and provoking notable N2O emission. This observation aligns with the findings of van Groenigen et al. (2005), who reported that urine application helps modify soil physicochemical properties, thereby enhancing soil denitrification and N2O emissions in the short-term. On the other hand, soil N2O emission for YU treatment was significantly negatively correlated with AOA and NirS, NosZ genes, and the increase of functional genes abundance and strengthen of denitrification would be important reasons that resulted in the increase of soil N2O flux. Furthermore, during the early stage of the experiment (1–11 days), YU treatment showed a significant increase in the abundance of AOB, NirS, and NosZ genes compared to the CK treatment. This is consistent with the finding of Di et al. (2014) who reported that urine stimulates AOB activity and significantly increases the abundance of NirK and NosZ genes. It has been reported that the high NH4+⁃N environment is more favorable for AOB gene growth (Verhamme et al. 2011). The 0–10 cm soil NH4+⁃N concentration for YU treatment was significantly higher than that for CK treatment, which was conducive to the growth of AOB gene. Correlation analysis showed that N2O emission was negatively correlated with AOB gene abundance for YU treatment, while the emission was significantly negatively correlated with AOA and AOB gene abundances for TSU treatment. This differs from the findings of Cai et al. (2017b), who suggested that urine deposition had little effect on the abundance of the denitrification functional gene NarG, but may have either no effect or increase the abundance of NirS, NirK, and NosZ genes. These disparities in findings may result from the differences and variations in soil texture and experimental conditions. The enhancement of gene abundance in this study facilitates the denitrification process and ultimately leads to soil N2O emission. Additionally, the N2O flux during the early stage of the experiment was higher than that during the later stage, with the cumulative emission for YU treatment during the first 13 days accounting for 49.9% of the total emissions. This proportion was significantly higher than those for CK (43.8%) and TSU (46.6%) treatments, indicating YU deposition largely contributed to the former period of N2O emission immediately contrast to other treatments in the alpine steppe.
In addition, following the application of YU and TSU, the cumulative CO2 emissions during the early stage of the experiment exhibited YU > TSU > CK treatment. This could be attributed to the impact of urine deposition on soil C content, resulting in increased SOC levels, which promotes soil microbial activity and CO2 emission. The YU treatment showed a significant increase of 0–10 cm SOC concentration during the early and later stages of the experiment. It could be speculated that the higher initial C content present in the urine during the early stage, and the increase in SOC concentration during the later stage could be ascribed to the high soil temperature, which promoted soil respiration and facilitated variations in C content. This finding is similar to the study conducted in India by Sakhare et al. (2022), who observed a significant increase (1.40%) in SOC after the application of bovine urine in red soil. On the other hand, soil CO2 flux for YU treatment exhibited a significant negative correlation with soil NO3−-N concentration, proving the decrease of CO2 emission with the extension of experimental period and the proceeding of nitrification increased soil NO3−-N concentration. Moreover, higher soil temperature during the early stage would differently contribute to increased soil respiration and subsequently enhanced CO2 emissions. This study revealed that soil CO2 emissions were higher during the first 3 days and the peak emissions for all three treatments occurred on 3 d following urine application, with the YU and TSU treatments exhibiting more pronounced changes. The result may be derived from the high moisture content present in the livestock urine as well as precipitation that occurred on 3 d. The increased soil moisture may facilitate the proliferation of microbial communities and provided abundant readily available C sources for CO2 production, thereby stimulated the release of soil CO2. These findings are consistent with the results of previous study conducted by Chimner and Cooper (2003), who demonstrated a significant positive correlation between CO2 emissions and soil moisture within a certain range. Furthermore, soil pH can directly influence soil physicochemical properties, leading to significant variations in the abundance of soil microorganisms and fauna, thereby indirectly affecting soil CO2 flux (Hanson et al. 2000). During the early stage of the experiment, the YU and TSU treatments exhibited higher soil pH values compared to the CK treatment, which probably indirectly promoting soil CO2 emissions to some extent. It was different from the report of Lambie et al. (2019) who found that soil pH did not exhibit significant changes following urine application.
The potential for soil CH4 production is closely related to soil environmental conditions, and elevated abundance of methanogens resulting from the application of livestock urine also serves as a vital factor influencing soil CH4 emission (Sheppard and Lloyd 2010). Previous study had indicated that CH4 uptake rates are higher during the growing season compared to the non-growing season (Zhao et al. 2006). The CH4 uptake rates are negatively correlated with soil moisture and positively correlated with soil temperature. In this study, CH4 flux for TSU treatment was detected positively correlated with soil temperature, SOC, and NO3−-N concentration, which may affect the trade-off between CH4 emission and uptake variously. In addition, our study found that soil CH4 uptake increased during the early stage following urine application compared to the CK treatment, but decreased during the later stage. This could be attributed to the increased soil moisture resulting from urine application, which, to some extent, hinders the entry of O2 from the atmosphere into the patches and the surrounding soil, thereby creating favourable anaerobic conditions for methanogen activities. Furthermore, this study revealed that soil CH4 uptake was the predominant process throughout the whole experimental period, with the cumulative uptake ultimately exhibiting a pattern of CK > TSU > YU. In the absence of fertilisation, grassland soils on the QXP typically act as sinks for CH4. Although urine application increases CH4 emissions, the amount is insufficient to offset the soil’s natural uptake, thereby resulting in a cumulative uptake. The reason is the application of YU and TSU promotes soil CH4 emission, but the emission rates were lower than the uptake rates, leading to a continuous net uptake. It has been reported that the application of urine increases the nitrification and enhances the emissions of soil N2O and CO2, while reducing the uptake of soil CH4 (Wu et al. 2019). This phenomenon was also observed in our study, where urine application led to an increase in soil moisture, pH, and C and N levels, thereby altering the soil physicochemical properties, nutrient transformation and migration, and microbial activity. Through the combined effects of nitrification and denitrification processes, urine application stimulated the emissions of soil N2O and CO2, while suppressing the soil CH4 uptake. These findings highlight the complicated influence of soil moisture, temperature, organic matter content, mineralisation rate, as well as the count and activity of microbial taxon on soil GHG emissions. In summary, further analysis and more efforts are needed to investigate the differential effects of key factors on GHG emissions from alpine grassland in future studies.
5 Conclusions
Yak and Tibetan sheep urine deposition generate varying effects on soil greenhouse gas emissions in the alpine grasslands of the northern Xizang Plateau. Compared to the control, the application of yak and Tibetan sheep urine promotes steppe soil N2O, CO2, and CH4 emissions. It was shown that yak and Tibetan sheep urine deposition had a short-term stimulating effect on greenhouse gas emission, and the cumulative emissions during the first 13 days were higher than those during the last 20 days. Urine deposition resulted in the increase of the NH4+-N and NO3−-N concentrations, abundance of NarG, NirS, NosZ, and NorB genes, and therefore obviously enhance denitrification and promoted N2O and CO2 emissions. Urine deposition result in the change of greenhouse gas source or sink properties, and decreased CH4 uptake compared to the natural alpine steppe without urine application. The conclusion of this study would helpful for understanding the short-term effects and functional mechanisms of livestock urine deposition on alpine grassland ecosystem, and providing theoretical references for optimizing livestock urine management mode in different seasons and greenhouse gas emission reduction strategies in pastoral areas.
References
Boon A, Robinson JS, Chadwick DR, Cardenas LM (2014) Effect of cattle urine addition on the surface emissions and subsurface concentrations of greenhouse gases in a UK peat grassland. Agr Ecosyst Environ 186:23–32. https://doi.org/10.1016/j.agee.2014.01.008
Byrnes RC, Nùñez J, Arenas L, Rao I, Trujillo C, Alvarez C, Arango J, Rasche F, Chirinda N (2016) Biological nitrification inhibition by Brachiaria grasses mitigates soil nitrous oxide emissions from bovine urine patches. Soil Biol Biochem 107:156–163. https://doi.org/10.1016/j.soilbio.2016.12.029
Cai YJ, Wang XD, Ding WX, Tian LL, Zhao H, Lu XY (2013) Potential short-term effects of yak and tibetan sheep dung on greenhouse gas emissions in two alpine grassland soils under laboratory conditions. Biol Fertil Soils 49:1215–1226. https://doi.org/10.1007/s00374-013-0821-7
Cai YJ, Wang XD, Tian LL, Zhao H, Lu XY, Yan Y (2014) The impact of excretal returns from yak and tibetan sheep dung on nitrous oxide emissions in an alpine steppe on the Qinghai-Tibetan Plateau. Soil Biol Biochem 76:90–99. https://doi.org/10.1016/j.soilbio.2014.05.008
Cai YJ, Chang SX, Cheng Y (2017a) Greenhouse gas emissions from excreta patches of grazing animals and their mitigation strategies. Earth-Sci Rev 171:44–57. https://doi.org/10.1016/j.earscirev.2017a.05.013
Cai YJ, Du ZY, Yan Y, Wang XD, Liu XP (2017b) Greater stimulation of greenhouse gas emissions by stored yak urine than urea in an alpine steppe soil from the Qinghai一Tibetan Plateau: a laboratory study. Grassl Sci 63:196–207. https://doi.org/10.1111/grs.12164
Carter MR (1993) Soil sampling and methods of analysis. Lewis, Boca Raton. https://doi.org/10.1201/9781420005271
Carter MS (2007) Contribution of nitrification and denitrification to N2O emissions from urine patches. Soil Biol Biochem 39:2091–2102. https://doi.org/10.1016/j.soilbio.2007.03.013
Chang XF, Zhu XX, Wang SX, Cui SJ, Luo CY, Zhang ZH, Wilkes A (2014) Impacts of management practices on soil organic carbon in degraded alpine meadows on the Tibetan Plateau. Biogeosciences 11:3495–3503. https://doi.org/10.5194/bg-11-3495-2014
Chen H, Yao SP, Wu N, Wang YF, Luo P, Tian JQ, Gao YH, Sun G (2008) Determinants influencing seasonal variations of methane emissions from alpine wetlands in Zoige Plateau and their implications. J Geophys Res Atmos 113:D12303. https://doi.org/10.1029/2006jd008072
Chimner RA, Cooper DJ (2003) Influence of water table levels on CO2 emissions in a Colorado subalpine Fen: an in situ microcosm study. Soil Biol Biochem 35:345–351. https://doi.org/10.1016/s0038-0717(02)00284-5
Clough TJ, Sherlock RR, Kelliher FM (2003) Can liming mitigate N2O fluxes from a urine-amended soil? Soil Res 41:439–457. https://doi.org/10.1071/sr02079
Cristina MVM, Leandro P, Carlos M, Manuel ZVE (2010) Greenhouse Gas (CO2 and N2O) emissions from soils: a review. Chil J Agric Res 70:485–497. https://doi.org/10.4067/S0718-58392010000300016
Di HJ, Cameron KC (2002) Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies. Nutr Cycl Agroecosyst 64:237–256. https://doi.org/10.1023/A:10214715311
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat Geosci 2:621–624. https://doi.org/10.1038/ngeo613
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2011) Methanotroph abundance not affected by applications of animal urine and a nitrification inhibitor, dicyandiamide, in six grazed grassland soils. J Soils Sediments 11:432–439. https://doi.org/10.1007/s11368-010-0318-z
Di HJ, Cameron KC, Podolyan A, Robinson A (2014) Effect of soil moisture status and a nitrification inhibitor, dicyandiamide, on ammonia oxidizer and denitrifier growth and nitrous oxide emissions in a grassland soil. Soil Biol Biochem 73:59–68. https://doi.org/10.1016/j.soilbio.2014.02.011
Dixon ER, Laughlin RJ, Watson CJ, Hatch DJ (2010) Evidence for the production of NO and N2O in two contrasting subsoils following the addition of synthetic cattle urine. Rapid Commun Mass Spectrom 24:519–528. https://doi.org/10.1002/rcm.4348
Du ZY, Cai YJ, Yan Y, Wang XD (2017) Embedded rock fragments affect alpine steppe plant growth, soil carbon and nitrogen in the northern Tibetan Plateau. Plant Soil 420:79–92. https://doi.org/10.1007/s11104-017-3376-9
Du ZY, Wang XD, Xiang J, Wu Y, Zhang B, Yan Y, Zhang XK, Cai YJ (2021) Yak dung pat fragmentation affects its carbon and nitrogen leaching in Northern Tibet, China. Agr Ecosyst Environ 310:107301. https://doi.org/10.1016/j.agee.2021.107301
Du ZY, Wang XD, Cai YJ (2022) Short-term effects of yak and tibetan sheep urine deposition on soil carbon and nitrogen concentrations in an alpine steppe of the northern Tibetan Plateau, China. J Mt Sci 19:1156–1167. https://doi.org/10.1007/s11629-021-7026-6
Elrys AS, Yves U, Zhang Y, Abdel-Fattah MK, Chen ZX, Zhang HM, Meng L, Wang J, Zhu TB, Cheng Y (2023a) Expanding agroforestry can increase nitrate retention and mitigate the global impact of a leaky nitrogen cycle in croplands. Nat Food 4:109–121. https://doi.org/10.1038/s43016-022-00657-x
Elrys AS, Wang J, Meng L, Zhu QL, El-Sawy MM, Chen ZX, Tu XS, El-Saadony MT, Zhang YH, Zhang JB, Cai ZC, Müller C, Cheng Y (2023b) Integrative knowledge-based nitrogen management practices can provide positive effects on ecosystem nitrogen retention. Nat Food 4:1075–1089. https://doi.org/10.1038/s43016-023-00888-6
Flessa H, Dörsch P, Beese F, König H, Bouwman AF (1996) Influence of cattle wastes on Nitrous Oxide and methane fluxes in pasture land. J Environ Qual 25:1366–1370. https://doi.org/10.2134/jeq1996.00472425002500060028x
Guo N, Wang AD, Degen AA, Deng B, Shang AH, Ding LM, Long RJ (2018) Grazing exclusion increases soil CO2 emission during the growing season in alpine meadows on the Tibetan Plateau. Atmospheric Environ 174:92–98. https://doi.org/10.1016/j.atmosenv.2017.11.053
Hamamoto T, Uchida Y, Rein IV, Mukumbuta I (2020) Effects of short-term freezing on nitrous oxide emissions and enzyme activities in a grazed pasture soil after bovine-urine application. Sci Total Environ 740:140006. https://doi.org/10.1016/j.scitotenv.2020.140006
Hanson PJ, Edwards NT, Garten CT, Andrews JA (2000) Separating Root and Soil Microbial contributions to Soil respiration: a review of methods and observations. Biogeochemistry 48:115–146. https://doi.org/10.1023/A:1006244819642
Hong JT, Ma XX, Wang XD (2016) Leaf meristems: an easily ignored component of the response to human disturbance in alpine grasslands. Ecol Evol 6:2325–2332. https://doi.org/10.1002/ece3.2059
Hynšt J, Šimek M, Brůček P, Petersen SO (2006) High fluxes but different patterns of nitrous oxide and carbon dioxide emissions from soil in a cattle overwintering area. Agr Ecosyst Environ 120:269–279. https://doi.org/10.1016/j.agee.2006.10.003
IPCC (2014) Climate Change 2014: impacts, adaptation and vulnerability: part B: Regional aspects: Working Group II Contribution to the IPCC Fifth Assessment Report: volume 2: Regional aspects. Cambridge University Press, Cambridge. https://www.ipcc.ch/report/ar5/wg2/
IPCC (2022) Climate change 2022: mitigation of climate change (summary for policymakers). Cambridge University Press, Cambridge. https://www.ipcc.ch/report/sixth-assessment-report-working-group-3/
Jackson RD, Oates LG, Schacht WH, Klopfenstein TJ, Undersander DJ, Greenquist MA, Bell MM, Gratton C (2015) Nitrous oxide emissions from cool-season pastures under managed grazing. Nutr Cycl Agroecosyst 101:365–376. https://doi.org/10.1007/s10705-015-9685-6
Johnson DE, Hill TM, Ward GM et al (1993) Ruminants andother animals/. In: Khalil MAK (ed) Atmospheric methane: sources, sinks, and Role in Global Change. Springer-, Berlin, pp 192–229. https://springerlink.bibliotecabuap.elogim.com/chapter/https://doi.org/10.1007/978-3-642-84605-2_11
Lambie SM, Schipper LA, Balks MR, Baisden WT (2013) Priming of soil decomposition leads to losses of carbon in soil treated with cow urine. Soil Res 51:513–520. https://doi.org/10.1071/SR13148
Lambie SM, Mason NWH, Mudge PL (2019) Bovine urine inhibits microbial function and increases urea turnover in dairy grazed soils. Soil Res 57:489–499. https://doi.org/10.1071/sr18257
Lambie SM, Mason NWH, Mudge PL (2021) Priming of carbon decomposition in 27 dairy grazed soils after bovine urine additions. Soil Res 60:124–136. https://doi.org/10.1071/sr20313/
Lin XW, Wang SP, Ma XZ, Xu GP, Luo CY, Jiang GM, Xie ZB (2009) Fluxes of CO2, CH4, and N2O in an alpine meadow affected by yak excreta on the Qinghai-Tibetan plateau during summer grazing periods. Soil Biol Biochem 41:718–725. https://doi.org/10.1016/j.soilbio.2009.01.007
Liu DY, Sun HY, Liao X, Lindsey S, Yuan JJ, He TH, Zaman M, Ding WX (2020) N2O and NO emissions as affected by the continuous combined application of Organic and Mineral N Fertilizer to a soil on the North China Plain. Agron J 10:1965–1983. https://doi.org/10.3390/agronomy10121965
Lombardi B, Alvarado PI, Ricci P, Buraschi LM, Viduzzi G, Palladino RA, Gonda HL, Juliarena MP (2022) Combination of cattle urine and dung patches synergically increased nitrous oxide emissions from a temperate grassland under wet conditions. Agr Ecosyst Environ 340:108147. https://doi.org/10.1016/j.agee.2022.108147
Luo J, Balvert SF, Wise B, Welten B, Ledgard SF, de Klein CAM, Lindsey S, Judge A (2018) Using alternative forage species to reduce emissions of the greenhouse gas nitrous oxide from cattle urine deposited onto soil. Sci Total Environ 610–611:1271–1280. https://doi.org/10.1016/j.scitotenv.2017.08.186
Ma XZ, Wang SP, Wang YF, Jiang GM, Nyren P (2006) Short-term effects of sheep excrement on carbon dioxide, nitrous oxide and methane fluxes in typical grassland of Inner Mongolia. N Z J Agric Res 49:285–297. https://doi.org/10.1080/00288233.2006.9513719
Mao DH, Luo L, Wang ZM, Zhang CH, Ren CY (2015) Variations in net primary productivity and its relationships with warming climate in the permafrost zone of the Tibetan Plateau. J Geogr Sci 25:967–977. https://doi.org/10.1007/s11442-015-1213-8
Michotey V, Méjean V, Bonin P (2000) Comparison of methods for quantification of cytochrome cd1-Denitrifying Bacteria in Environmental Marine samples. Appl Environ Microbiol 66:1564–1571. https://doi.org/10.1128/aem.66.4.1564-1571.2000
Morales SE, Jha N, Saggar S (2015) Impact of urine and the application of the nitrification inhibitor DCD on microbial communities in dairy-grazed pasture soils. Soil Biol Biochem 88:344–353. https://doi.org/10.1016/j.soilbio.2015.06.009
Nichols KL, Del Grosso SJ, Derner JD, Follett RF, Archibeque SL, Stewart CE, Paustian KH (2016) Nitrous oxide and methane fluxes from cattle excrement on C3 pasture and C4-dominated shortgrass steppe. Agr Ecosyst Environ 225:104–115. https://doi.org/10.1016/j.agee.2016.03.026
Nielsen TH, Nielsen LP, Revsbech NP (1996) Nitrification and coupled nitrification-denitrification Associated with a soil-manure interface. Soil Sci Soc Am J 60:1829–1840. https://doi.org/10.2136/sssaj1996.03615995006000060031x
Oenema O, Wrage N, Velthof GL, van Groenigen JW, Dolfing J, Kuikman PJ (2005) Trends in Global Nitrous Oxide emissions from Animal Production systems. Nutr Cycl Agroecosyst 72:51–65. https://doi.org/10.1007/s10705-004-7354-2
Orwin KH, Bertram JE, Clough TJ, Condron LM, Sherlock RR, Callaghan MO, Ray J, Baird DB (2010) Impact of bovine urine deposition on soil microbial activity, biomass, and community structure. Appl Soil Ecol 44:89–100. https://doi.org/10.1016/j.apsoil.2009.10.004
Sakhare VM, Jadhav AB, Patil GD, Patange DD (2022) Effect of cattle urine application on Soil properties of Lateritic soils under Spinach Cultivation. Int J Plant Soil Sci 34:117–124. https://doi.org/10.9734/ijpss/2022/v34i2031134
Sandoval MA, Nadine Loick N, Pinochet DE et al (2024) N losses from an Andisol via Gaseous N2O and N2 emissions increase with increasing ruminant Urinary-N deposition rate. Nitrogen 5(2):254–265. https://doi.org/10.3390/nitrogen5020017
Sheppard SK, Lloyd D (2010) Diurnal oscillations in gas production (O2, CO2, CH4, and N2) in soil monoliths. Biol Rhythm Res 33:577–591. https://doi.org/10.1076/brhm.33.5.577.13937
Singh J, Saggar S, Bolan NS (2009) Influence of dicyandiamide on nitrogen transformation and losses in cow-urine-amended soil cores from grazed pasture. Anim Prod Sci 49:253–261. https://doi.org/10.1071/ea08200
Stockdale EA (2005) Optimization of nutrient cycling and soil quality for sustainable grasslands. Grass Forage Sci 60:425. https://doi.org/10.1111/j.1365-2494.2005.00486.x
Tang RG, DU ZY, Zhu GD et al (2021) Yak dung pat fragmentation decreases yield–scaled growing–season nitrous oxide emissions in an alpine steppe on the Qinghai–Tibetan Plateau. Biol Fertil Soils 57:1103–1115. https://doi.org/10.1007/s00374-021-01601-0
Throbäck IN, Enwall K, Jarvis A, Hallin S (2004) Reassessing PCR primers targeting NirS, NirK and NosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol Ecol 49:401–417. https://doi.org/10.1016/j.femsec.2004.04.011
Uchida Y, Clough TJ, Kelliher FM, Hunt JE, Sherlock RR (2011) Effects of bovine urine, plants and temperature on N2O and CO2 emissions from a sub-tropical soil. Plant Soil 345:171–186. https://doi.org/10.1007/s11104-011-0769-z
van der Weerden TJ, Luo JF, de Klein CAM, Hoogendoorn CJ, Littlejohn RP, Rys GJ (2011) Disaggregating nitrous oxide emission factors for ruminant urine and dung deposited onto pastoral soils. Agr Ecosyst Environ 141:426–436. https://doi.org/10.1016/j.agee.2011.04.007
van Groenigen JW, Velthof GL, van der Bolt FJE, Vos A, Kuikman PJ (2005) Seasonal variation in N2O emissions from urine patches: effects of urine concentration, soil compaction and dung. Plant Soil 273:15–27. https://doi.org/10.1007/s11104-004-6261-2
Verhamme DT, Prosser Jl, Nicol GW (2011) Ammonia concentration determines diferential growth of ammonia-oxidising Archaea and bacteria in soil microcosms. ISMk Joumal 6:1067–1071. https://www.nature.com/articles/ismej2010191
Wachendorf C, Lampe C, Taube F, Dittert K (2008) Nitrous oxide emissions and dynamics of soil nitrogen under 15 N-labeled cow urine and dung patches on a sandy grassland soil. J Plant Nutr Soil Sci 171:171–180. https://doi.org/10.1002/jpln.200625217
Welten BG, Ledgard SF, Schipper LA, Judge AA (2013) Effect of amending cattle urine with dicyandiamide on soil nitrogen dynamics and leaching of urinary-nitrogen. Agr Ecosyst Environ 167:12–22. https://doi.org/10.1016/j.agee.2013.01.011
Wu Q, Kwak JH, Scott X, Chang SX, Han GD, Gong XQ (2019) Cattle urine and dung additions differently affect nitrification pathways and Greenhouse Gas Emission in a Grassland Soil. Biol Fertil Soils 56:235–247. https://doi.org/10.1007/s00374-019-01415-1
Yamulki S, Wolf I, Bol R, Grant B, Brumme R, Veldkamp E, Jarvis SC (2000) Effects of dung and urine amendments on the isotopic content of N2O released from grasslands. Rapid Commun Mass Spectrom 14:1356–1360. https://doi.org/10.1002/1097-0231(20000815)14:15%3C1356::aid-rcm30%3E3.0.co;2-c
Zhang YF, Ma AZ, Zhuang GQ, Zhuang XL (2019) The acetotrophic pathway dominates methane production in Zoige alpine wetland coexisting with hydrogenotrophic pathway. Sci Rep 9:1–8. https://doi.org/10.1038/s41598-019-45590-5
Zhang YR, Nie Y, Liu YL, Huang XC, Yang YH, Xiong H, Zhu HQ, Li Y (2022) Characteristics of Greenhouse Gas emissions from Yellow Paddy Soils under Long-Term Organic Fertilizer Application. Sustainability 14:12574. https://doi.org/10.3390/su141912574
Zhao L, Li YN, Xu SX, Zhou HK, Gu S, Yu GR, Zhao XQ (2006) Diurnal, seasonal and annual variation in net ecosystem CO2 exchange of an alpine shrubland on Qinghai-Tibetan plateau. Global Change Biol 12:1940–1953. https://doi.org/10.1111/j.1365-2486.2006.01197.x
Acknowledgements
This study was supported by the Second Tibetan Plateau Scientific Expedition and Research Program (Grant NO. 2019QZKK0404), National Natural Science Foundation of China (Grant NO. 41807109), Natural Science Foundation of Sichuan province in China (Grant NO. 2023NSFSC0205) and Innovation Team Funds of China West Normal University (Grant NO. KCXTD2023-6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Wang, X., Sun, J. et al. Simulated Yak and Tibetan Sheep Urine Deposition Fleetingly Promoted N2O, CO2 and CH4 Emissions in an Alpine Steppe of Northern Xizang Plateau. J Soil Sci Plant Nutr (2024). https://doi.org/10.1007/s42729-024-01876-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42729-024-01876-w