Abstract
Soil nematodes are ideal indicators for soil food webs, ecosystem conditions, and soil health. However, current research focuses on how plant removal affects soil nematodes while ignoring the importance of the trophic cascading effects. The study aims to elucidate the direct and indirect effects of long-term plant community removal on soil nematode communities, especially through soil physicochemical properties and trophic cascading effects. A 6-year field all-aboveground plant community removal experiment was conducted to evaluate the effects of plant removal on soil nematode communities and use piecewise structural equation modeling to better understand the direct and indirect effects of plant removal on different trophic group nematodes. The removal of plants did not significantly influence the total abundance, richness, or trophic group richness of soil nematodes, but it did considerably reduce the number of herbivorous and fungivorous nematodes. Our results revealed that the removal of plants significantly altered the nematode community composition mainly by changing the relative abundance of the genera Helicotylenchus, Tylenchus, Tylopharynx, and Aphelenchoides. The abundance of predatory-omnivorous nematodes was dramatically and directly enhanced by the removal of plants, but it was also indirectly changed by a decrease in the abundance of fungivorous and herbivorous nematodes. The most significant mechanism for plant removal to impact predatory-omnivorous nematodes might be through the fungal channels, which are mainly mediated by fungivorous nematodes. These results indicated that plant removal affects predatory-omnivorous nematodes primarily through fungal channels and elucidated the importance of trophic cascading in mediating the effects of plant communities on soil nematode communities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, the topic of the ecological connection between aboveground and below-ground biota has received extensive attention from scholars (van der Putten et al. 2013; Zhang et al. 2018). Nematodes are the most ubiquitous invertebrates in soil habitats (van den Hoogen et al. 2019), and they play an important role in decomposition (Taylor et al. 2020), nutrient transformation and cycling (Lazarova et al. 2021; Melakeberhan et al. 2021), and energy transformation (Zuo et al. 2020). According to trophic groups and morphological traits, free-living soil nematodes are broadly classified into five taxonomic/functional types, including herbivores, bacterivores, fungivores, omnivores, and predators (Yeates et al. 1993). Additionally, nematode trophic groups as well as their reactions to environmental changes (tolerance vs. susceptibility) give crucial information for detecting changes in soil properties (Cesarz et al. 2015).
Trophic cascading effects play an important role in regulating the soil nematode community (Rasmann et al. 2012). Predatory-omnivorous nematodes are generally considered K-strategists, which have a longer generation time, lower fecundity, and higher c-p value, and they are more sensitive to disturbance (Bongers and Ferris 1999). In addition, previous studies showed that herbivores, bacterivores, and fungivores significantly affect predatory-omnivorous nematodes (Kitagami and Matsuda 2020; Wang et al. 2022). For example, Dasiphora fruticosa can reduce the richness of bacterivorous nematodes, which then affects the richness of predatory-omnivorous nematodes (Wang et al. 2019), and increased biomass of bacterivore nematodes leads to increased biomass of predatory-omnivorous nematodes (Laliberte et al. 2017). Changes in nematode caused by cascading effects can alter the nematode community composition and reflect soil ecological processes (Yeates 1999).
According to recent research, soil nematode can be influenced by plant communities either directly or indirectly through the soil physicochemical properties (van den Hoogen et al. 2020) and the trophic cascading of the soil biota (Wang et al. 2019). Soil nematode communities are strongly dependent on soil pH (Nielsen et al. 2011) and soil organic matter (Moens et al. 2002), and removing plant induced an increase in soil pH in a semi-arid grassland (Chen et al. 2018) and a decrease in soil organic matter content in an alpine meadow (Yang et al. 2021). Additionally, forb biomass increased soil water content, which consequently had an impact on the number of soil nematodes (Wang et al. 2018). And Dasiphora fruticosa increased soil ammonium and then affect fungivorous nematode biomass (Wang et al. 2019). In summary, removing plants can influence soil nematode communities through soil physicochemical properties.
Plant communities have an impact on soil nematode communities both directly and indirectly (Dietrich et al. 2021; Lu et al. 2023; Shao et al. 2015). Inputs from plant litter, root characteristics, and exudates are the key factors by which plants directly affect soil nematodes (Wardle 2002). Through inputs of litter, plants considerably enhanced the abundance of several trophic group nematode and total soil nematodes (Dietrich et al. 2021). Plant roots produce a variety of organic compounds (Nguyen 2003), which they subsequently use to either encourage or suppress some trophic groups of nematodes (Niu et al. 2019). In the meantime, it was also discovered that root characteristics, such as root length and the C:N ratio, had a direct impact on soil nematode communities (Zhang et al. 2020a, 2022). So, soil nematode will be greatly affected by plant removal; the effect of both the plant litters and the root systems on soil nematode will disappear when the plants are removed.
Terrestrial ecosystems worldwide are under multiple stresses, such as global climate change, land use change, and inappropriate human activities (like overgrazing) (Lyu et al. 2020; Wang et al. 2020). One of the most important consequences of these stressors is the rapid loss of plant number and diversity in terrestrial ecosystems (Zhang et al. 2020b). Plant removal is currently an important experimental method for studying the effects and mechanisms of plant communities or species on soil biomes, mainly by controlling the presence or absence of aboveground plant communities (Chen et al. 2016; Fanin et al. 2019). Although many studies have explored the effects of plant removal on soil nematode communities, few have considered the direct and indirect effects of long-term all-aboveground plant community removal on nematode communities, especially through cascading effects on nematode communities. In the present study, we carried out a 6-year field all-aboveground plant community removal experiment to investigate the effect of plants on soil nematode communities, and we used Piecewise SEM to elucidate the direct and indirect effects of plant removal on soil nematode communities through soil physicochemical properties and nematode trophic cascading in an alpine meadow on the Tibetan plateau. We hypothesized that (1) plant removal will significantly decrease herbivorous nematode abundance; (2) plant removal will significantly change nematode community composition; moreover, trophic groups will have different responses to plant removal; and (3) plant removal will directly and indirectly affect soil nematodes through soil physicochemical properties and trophic cascading within nematode communities.
2 Materials and Methods
2.1 Research Site
This study was conducted at the Gansu Gannan Grassland Ecosystem National Field Scientific Observation and Research Station (33°40′ N, 101°51′ E), which is located in Gannan Tibetan Autonomous Prefecture on the eastern edge of the Tibetan Plateau, southern of Gansu Province, China. The experimental site was located in a relatively flat alpine meadow at 3500 m above sea level. The annual precipitation is 620 mm, and the rain falls mainly during the short, cool summer, with approximately 2580 h of cloud-free solar radiation annually. The mean annual temperature is 1.2 °C, with approximately 270 frost days per year. And according to the US Department of Agriculture’s (USDA) Soil Taxonomy, the soil type is a sub-alpine meadow soil, which is similar to cryrendoll. The vegetation is dominated by Kobresia capillifolia (Cyperaceae), Elymus nutans (Poaceae), Agrostis spp. (Poaceae), Festuca ovina (Poaceae), and Poa pachyantha (Poaceae).
2.2 Experimental Design
The experiment was conducted on a completely randomized design in long-term field trials (100 m × 100 m), which were established in early May of 2014, and there were no significant differences in plant communities. Ten 5 m × 5 m plots were randomly arranged with a 3-m buffer strip between plots to prevent species interactions across neighboring plots. Two treatments included in this study were the complete removal of all-aboveground plant communities (with removal) and all plant communities (without removal). Each treatment had 5 replicates. Plant removal treatment was conducted every 2 weeks during the whole growing season (early May to September) and performed by mowing plants along the soil surface, and all plants were killed and removed from the sample plot in plant removal treatment. In without removal treatment, the plant community was retained throughout the experimental period.
2.3 Soil Sampling and Preservation
In late August of 2019, three soil cores (5 cm diameter, 20 cm depth) were collected from the diagonal of each plot (n = 5) using a soil auger and combined to form one composite soil sample per plot. Roots and stones were removed by hand; each soil sample was separated into two parts. The first part was maintained fresh for measuring soil water content and soil nematodes and preserved at 4℃; the second part was air-dried naturally, sieved through 60 mesh (0.25 mm), and then stored in self-sealed bags for measuring soil physicochemical properties at room temperature.
2.4 Measurements
We used a beaker to measure approximately 50 mL (and weigh approximately 30 g) of fresh soil stored at 4 °C and extracted the nematodes using the modified Baermann wet funnel technique with the modifications outlined in Hu et al. (2015). Although some studies used 30 g or less of soil for nematode extraction (Jagdale and Grewal 2002; Kitagami et al. 2021; Xue et al. 2022), we admitted that a large quantity of soil samples should be used for nematode extraction more rigorously. According to their morphological characteristics, soil nematodes were assigned to different genera, the number of all observed nematodes was counted, and their abundances were converted to individuals per 100 g of dry soil. Furthermore, nematodes were classified according to their tropic groups following Yeates et al. (1993), i.e., bacterivore, fungivore, herbivore, and predator-omnivore.
Soil water content was measured by fresh soil: 30 g of soil was weighed and dried at 105 ℃ for 48 h. The remaining soil was air-dried, avoiding direct sunlight, following sieving through a 60 mesh (0.25 mm). Air-dried soil was analyzed for pH using a pH meter (PHSJ-3F, Shanghai INESA Scientific Instrument Co. Ltd. China) in a 1:2.5 soil:deionized water slurry. Soil organic carbon was measured based on dichromate digestion (Kalembasa and Jenkinson 1973). Soil total nitrogen and phosphorus were both digested by concentrated H2SO4 at 375 ℃ for 3 h and 45 min, respectively, followed by semi micro-Kjeldahl and Mo-Sb antispectrophotography (Cui et al. 2022) using an auto-chemistry analyzer (SmartChem 200, AMS Alliance, Italy). In addition, soil ammonium (NH4+-N) and nitrate (NO3−-N) concentrations were measured by colorimetric assay following mixing 10 g sieved soil in 100 mL of 2 mol/L KCl using an auto-chemistry analyzer (Cui et al. 2022).
2.5 Data Analysis
We used the Shapiro–Wilk test to test the normality of variance and Levene’s test to test the homogeneity of variance prior to analyses, and if necessary, log transformations are performed to ensure that the data conform to normality and homogeneity of variance. In our data, only soil water content was log-transformed. A one-way ANOVA was used to evaluate the effect of plant removal on soil physicochemical properties and soil nematodes. A Tukey HSD post hoc test was used for pairwise comparison. Spearman’s correlation analysis was used to investigate the correlation between soil physicochemical properties and soil nematode the dominant genus.
Non-metric multidimensional scaling (NMDS) was used to assess differences between the nematode assemblages with and without plants. Furthermore, non-parametric multivariate analysis of variance (NPMANOVA) based on the Bray–Curtis dissimilarity coefficient with 9999 permutations was used to assess differences in soil nematode community composition between treatments. The Mantel test was used to investigate the correlation between soil physicochemical properties and soil nematode community composition.
We acknowledged that our limited sample size may prevent us from fitting the structural equation models, and we therefore carried out piecewise structural equation modeling (piecewise SEM) to explore the direct and indirect effects of all-aboveground plant community removal on soil nematode by soil physicochemical properties and trophic cascading. The goodness of piecewise SEM was evaluated by Shipley’s test of d-separation through Fisher’s C statistic (Shipley 2009). We divided the soil physicochemical properties into microhabitat and soil nutrition. Microhabitats are habitats that have a direct impact on the life cycle of biota, which mainly include soil pH and soil water content (SWC), and the remaining factors all belong to soil nutrition (including soil organic carbon (SOC), total nitrogen (TN), total phosphorus (TP), soil ammonium (NH4+-N), and soil nitrate (NO3−-N)). And principal component analysis (PCA) was used to construct feature space to extract important information from microhabitat and soil nutrition data.
We conducted piecewise SEM according to an a priori model (Fig. S1) with the following premises: (1) plant community directly changes soil nematode community; (2) plant community changes soil physicochemical properties (both microhabitat and soil nutrient); (3) both microhabitat and soil nutrient influence soil nematode community; and (4) trophic cascading within the nematode community plays an important role in influencing soil nematode community.
All statistical analyses were conducted in R 4.0.4. The variance homogeneity was checked using the “car” package (Fox et al. 2012); the post hoc test was conducted with the “emmeans” package (Lenth et al. 2019). Spearman’s correlation analysis was conducted with the “psych” package (Revelle and Revelle 2015). NMDS and PCAs were performed with the “vegan” package (Oksanen et al. 2013). The Mantel test was completed using the “linkET” package (Chi et al. 2022). Piecewise SEM was performed using the “piecewiseSEM” package (Lefcheck et al. 2016). And the figures were plotted using the “ggplot2” package (Wickham et al. 2016).
3 Results
3.1 Soil Physicochemical Properties
Soil water content (SWC, P < 0.05) and nitrate (NO3−-N, P < 0.05) were significantly decreased by the aboveground plant community removal (Table 1). In the plant removal treatment, soil water content and soil nitrate significantly decreased by 20.37% and 53.13%, respectively. The PCA of soil nutrition showed that soil organic carbon, soil total nitrogen, and soil phosphorus were mostly related to axis 1, and soil ammonium and nitrate were mostly related to axis 2 (Fig S2). And the PCA results for microhabitat showed that both soil pH and soil water content were mostly related to axis 1 (Fig. S3).
3.2 Soil Nematode Abundance and Community
A total of 35 nematode genera were collected. The most common nematode genera included: Helicotylenchus, Tylenchus, Tylopharynx, Acrobeloides, Rotylenchus, and Aphelenchoides (Table 2). Herbivores were the most abundant trophic group, followed by predator-omnivores and bacterivores (Fig. 1). There was no significant effect of plant removal on the abundance of total nematodes (Fig. 1a), bacterivores (Fig. 1c), and predator-omnivores (Fig. 1e). However, there were significant negative effects of plant removal on the abundance of herbivores (Fig. 1b, P < 0.05) and fungivores (Fig. 1d, P < 0.05). Various nematode genera correlate differently with soil physicochemical properties (Table 3). A positive correlation was found between soil water content and herbivorous Helicotylenchus and Tylenchus. Also, soil nitrate and nitrogen were positively correlated with fungivorous Aphelenchoides. Plant removal had no significant effects on the richness of total nematodes and different trophic groups (Fig. S4).
Response of soil nematode abundance with removal and without removal treatment. Data are means ± SE. Different lower case letters above the columns indicate statistical differences at P < 0.05 according to ANOVA and Tukey HSD post hoc test. And the same lower case letters above the columns indicate no statistical differences at P > 0.05. *: P < 0.05
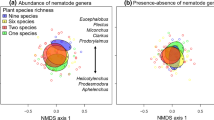
NMDS and NPMANOVA results showed that nematode community composition had a significant difference between with and without plant treatment (P < 0.01, Fig. 2). We found the abundance of Helicotylenchus (P < 0.05) and Tylenchus (P < 0.01), which belong to herbivores, was significantly decreased in plant removal treatment (Table 2). Meanwhile, the abundance of Aphelenchoides (P < 0.05) and Tylopharynx (P < 0.1), which belong to fungivorous nematodes, was significantly and marginally decreased in plant removal treatment, respectively (Table 2). Soil nematode communities varied with soil total phosphorus content (Fig. 3).
Community species composition of soil nematodes with removal and without removal treatment based on non-metric multidimensional scaling (NMDS) using Bray–Curtis similarity index. Significant results of non-parametric multivariate analysis of variance (NPMANOVA) are indicated on the left top part of graph. The circles surrounding the clusters represent 95% confidence intervals. **: P < 0.01
Mantel test of soil physicochemical properties and soil nematode community composition. Color gradient denotes Pearson’s correlation coefficients. Edge color denotes statistical significance. Edge width corresponds to Mantel’s r statistic for corresponding distance correlations. And the line types indicate the positive and negative effects
3.3 Piecewise Structural Equation Modeling
We used piecewise SEM to explore the effects of plant removal on the abundance of nematode tropic groups (Fig. 4a). The piecewise SEM assessing nematode abundance explained 88% of the variation in the predatory-omnivorous nematode and explained 12%, 55%, and 67% of the variation in abundance of the bacterivorous nematode, the fungivorous nematode, and the herbivorous nematodes, respectively. The piecewise SEM explained 88%, 12%, 55%, and 67% of the variation in the abundance of predatory-omnivorous, bacterivorous, fungivorous, and herbivorous nematode, respectively. Piecewise SEM showed that the abundance of fungivores and herbivores was directly and negatively affected by the removal of plant communities (Fig. 4), which means plant removal significantly decreased the abundance of fungivorous and herbivorous nematodes. Predatory-omnivorous nematodes were directly and indirectly affected by plant removal (Fig. 4). The piecewise SEM showed not only a directly and positive effect of plants on the abundance of predator-omnivore, but also positively and negatively and indirect effects on predatory-omnivorous nematode abundance through the abundance of fungivorous nematode and herbivorous nematodes, respectively (Fig. 4). And we found that although microhabitat and bacterivorous nematode abundance had a significant relationship with predatory-omnivorous nematode abundance, there was no significant relationship between plant removal and microhabitat and bacterivorous nematode abundance (Fig. 4).
Piecewise structural equation modeling is shown the direct and indirect effects of plant removal on soil nematode abundance (Fisher’s C = 3.809, P-value = 0.432) and total effects of plant community removal (direct way), microhabitat, soil nutrient, bacterivore abundance, fungivore abundance, and herbivore abundance on predator-omnivore nematode abundance. The red and black solid arrows indicate significant negative and positive effects, respectively, and the dashed arrows indicate marginally significant effects (***, P < 0.001; **, P < 0.01; *, P < 0.05; (*), P < 0.1), while the gray arrows indicate non-significant effect. The arrow width shows the standardized path coefficient which is associated with arrows. R2 values indicate the proportion of explained variation, and the values associated with arrows indicate standardized path coefficients
Herbivores showed total positive effects on predatory-omnivorous nematode abundance, while fungivores, bacterivores, and soil physicochemical properties (soil nutrients and microhabitat) showed total negative effects on predatory-omnivorous nematode abundance (Fig. 4). Moreover, plant removal showed a direct and positive effect on predatory-omnivorous nematode abundance (Fig. 4). The indirect effect of plant removal on soil predatory-omnivorous nematodes was mainly mediated by fungivores, although it is negative (standardized total effect: − 3.318, accounting for 42.34% of the standardized total effect). The direct pathway is next (standardized total effect: 2.2618, accounting for 28.86% of the standardized total effect). Herbivores and bacterivores account for 24.92% and 3.01% of the standardized total effect, respectively. Soil physicochemical properties accounted for 0.85% of the standardized total effect. We, therefore, conclude that the fungal channels associated with fungivorous nematodes are the most important channels affecting predatory-omnivorous nematodes in plant removal.
We also explored the effects of plant removal on the richness of nematode trophic groups using piecewise SEM (Fig. S5). The piecewise SEM model explained 7% of the variation in predatory-omnivorous nematode richness and 45%, 45%, and 11% of the variation in richness of bacterivorous nematodes, fungivorous nematodes, and herbivorous nematodes, respectively. Soil nematode richness was not significantly affected by plant removal (Fig. S5).
4 Discussion
Our 6-year study by manipulation of whole aboveground plant community removal showed that variation in abundance of individual nematode trophic groups was associated with changes in plant removal. Plant removal significantly reduced fungivore and herbivore abundance and affected predatory-omnivorous nematode abundance through direct and indirect pathways, mainly through the fungal channel. Our study provides evidence that the soil nematode community is influenced by the aboveground plant, while nematode trophic groups respond differently to plant removal.
The insignificant change in nematode taxonomic richness but significant change in nematode taxonomic abundance observed in this study suggests that richness may be relatively insensitive to plant removal compared to abundance, and the relative insensitivity of richness compared to abundance has been reported previously (Clements 2004). Consistent with our hypothesis 1, we found that plant removal reduced the abundance of herbivores. Plant removal reduced the nutrient inputs into the soil food web, resulting in fewer trophic resources (Ayal 2007). Plant roots serve as the food source for soil herbivorous nematodes (Wilschut and Geisen 2021). In addition, we found herbivorous Helicotylenchus and Tylenchus positively correlated with soil water content, and plant removal reduced soil water content by increasing ground irradiance and evaporation (Diabate et al. 2018; Ning et al. 2022), which may have led to a reduction in herbivorous nematode abundance.
Meanwhile, fungivorous nematode abundance was reduced by plant removal. Plant removal reduces litter inputs and sediment, leading to a reduction in fungal abundance (Li et al. 2020; Zubek et al. 2016). Fungivorous nematodes are the majority of the fungal-feeding microfauna (Kitagami and Matsuda 2022), and the abundance, diversity, species composition, and structure of fungal communities are influenced by plants (Bollmann-Giolai et al. 2022), as plants can selectively attract mycorrhizal fungi, pathogenic fungi, and saprophytic fungi with specific abilities (Heinen et al. 2020). And the fungivorous Aphelenchoides was positively correlated with soil nitrate, plant removal reduced soil nitrate in our study, and reduced nitrate nitrogen may have led to a reduction in fungus-feeding nematodes. In short, plant removal had no significant effects on soil total nematode abundance and richness but reduced the abundance of herbivorous and fungivorous nematodes, possibly due to altered soil physicochemical properties, plant nutrient resource inputs, and root deposits.
However, our study showed plant removal does not significantly alter soil bacterivore abundance. This result was different from Li et al. (2021b), who found that plant removal increased the relative abundance of bacterivores. This insignificant change may be attributable to location differences. We suspect that bacterivores as opportunistic nematodes have a greater tendency to enrich in conditions of more resources (Ferris et al. 2004; Shaw et al. 2019). And in our study, with the exception of soil nitrate, soil nutrients were not significantly altered by plant removal. There is a possible reason to drive the insignificant changes in soil nutrient that plant may result in increases (Yang et al. 2021), decreases (Ade et al. 2021), or no difference in any of the soil nutrient variables (Wang et al. 2019), and the offsetting of positive and negative effects contributes to non-significant net plant effects on soil physicochemical properties. And our ANOVA results showed that there were no significant effects of plant removal on predatory-omnivorous nematodes, which may be caused by the offset of positive and negative effects.
More importantly, our study found that plant removal significantly modified the community composition of soil nematodes (Fig. 2), which agrees with our hypothesis 2. Herbivores and fungivores were the main taxa causing significant changes in soil nematode community composition. Removing plant communities leads to changes in herbivorous nematodes as changes in food resources and herbivorous nematodes may be host-specific (Yin et al. 2022). Previous studies have found that the relative abundance of pathogenic fungi increased with the increase in grass coverage (Heinen et al. 2020), fungivorous nematodes are susceptible to fungi as a food resource, and the plant removal may lead to a change in fungivorous nematodes. In addition, soil nematodes also selectively choose food resources, and many studies have shown feeding preferences in nematodes (Liu et al. 2018; Manwaring et al. 2020). For instance, the fungivorous Aphelenchoides sp. and Aphelenchus avenae selectively graze fungal food resources (Hasna et al. 2007; Ruess et al. 2000). Meanwhile, the bacterivorous Caenorhabditis elegans chooses between bacterial foods (Katzen et al. 2023). In summary, plant removal changes nematode community composition by altering nematode food resources.
Our Piecewise SEM suggested both direct and indirect effects of plant removal on soil predatory-omnivorous nematodes, but indirect effects only through the trophic cascade within the nematode and not through soil physicochemical properties, which is inconsistent with hypothesis 3. Different effects of plant removal on predator-omnivore abundance could be explained by differences in food sources (Niu et al. 2019). The significant direct effect of plant removal on predatory-omnivorous nematodes may result from higher trophic levels where nematodes (predator-omnivores) are more sensitive to plant leachates, volatiles, the secretion of root-derived compounds (root exudation), and defensive secondary metabolites (Hu et al. 2018; Timonen et al. 2004; Wilschut and Geisen 2021), because predatory-omnivorous nematodes have a higher c-p value and imply greater sensitivity to the change of soil environment (Ferris et al. 2001). For example, Brassica spp. release volatile compounds (like glucosinolates) and reduce soil nematodes (Farooq et al. 2011). When the plant (Tagetes spp.) removal reduced the secondary metabolites produced by plants entering the soil detritus food web, they directly and positively affected the nematode community (Karakas and Bolukbasi 2019). Compared to predatory-omnivorous nematodes, herbivorous and fungivorous nematodes have lower c-p values and are more tolerant of adverse conditions and more susceptible to food resources, whereas predatory-omnivorous nematodes are more sensitive to environmental changes (Ferris et al. 2001). The different trophic groups and c-p values of nematodes result in their different responses to plant removal.
We found similar bacterial-based, fungal-based, and root-based energy pathways in the soil detritus food web (Laliberte et al. 2017), although plant removal was not significantly associated with bacterivorous nematodes. The negative effect of the abundance of herbivorous and bacterivorous nematodes on predatory-omnivorous nematode may be caused by herbivorous and bacterivorous overgrazing (Wang et al. 2019). And fungivorous nematodes significantly and positively affect soil predatory-omnivorous nematodes, which indicates a bottom-up regulation in the soil food web (Liu et al. 2022). In addition, plant removal can also indirectly affect soil predatory-omnivorous nematodes by fungivores and herbivores, and the fungal channel mediated by fungivorous nematodes was the most important pathway. Important fungivorous pathways for the impact of plant removal on soil predatory-omnivorous nematodes may be caused by the following reasons: as mentioned above, plants attract specialist mycorrhizal fungi and putative fungal pathogens, and plant removal may lead to a reduction in fungal abundance (Semchenko et al. 2018), which then leads to a reduction in the abundance of fungivorous nematodes. Moreover, since soil organisms in the fungal channel are thought to be more resistant to low water content conditions than those in the bacterial channel (Andrés et al. 2016) and plant removal significantly reduced soil water content, this may have contributed to the effect of plant removal on nematodes primarily through the fungal channel (Ullah et al. 2023). And in addition, temperature is one of the most important factors affecting the decomposition of plant litter (Li et al. 2021a). In alpine meadows, low temperatures are not conducive to organic matter decomposition, which may lead to fungal decomposition pathways that degrade recalcitrant litter (Osono 2019). In short, plant removal alters the soil nematode community mainly through a fungal channel mediated by fungivorous nematodes, which may be related to the impact of plant removal on the soil environment and plant litter inputs.
5 Conclusion
Our results showed that long-term plant removal had no significant effect on nematode total abundance and richness but significantly reduced the abundance of herbivorous and fungivorous nematodes. Plant removal altered soil nematode community composition, and soil total phosphorus content was an important influence factor. Plant removal had positive and direct effects but negative and indirect effects, resulting in a non-significant effect on nematode abundance. Moreover, plant removal negatively altered the soil nematode community, mainly through the trophic cascading effects of fungal channel. Our research provides a better understanding of how 6-year plant removal affects soil nematode trophic groups in alpine meadows and emphasizes the importance of trophic cascades in regulating the effects of plant communities on soil nematode communities.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ade L, Millner JP, Hou F (2021) The dominance of Ligularia spp related to significant changes in soil microenvironment. Ecol Indic 131. https://doi.org/10.1016/j.ecolind.2021.108183
Andrés P, Moore JC, Simpson RT, Selby G, Cotrufo F, Denef K, Haddix ML, Shaw EA, de Tomasel CM, Molowny-Horas R, Wall DH (2016) Soil food web stability in response to grazing in a semi-arid prairie: the importance of soil textural heterogeneity. Soil Biol Biochem 97:131–143. https://doi.org/10.1016/j.soilbio.2016.02.014
Ayal Y (2007) Trophic structure and the role of predation in shaping hot desert communities. J Arid Environ 68:171–187. https://doi.org/10.1016/j.jaridenv.2006.05.013
Bollmann-Giolai A, Malone JG, Arora S (2022) Diversity, detection and exploitation: linking soil fungi and plant disease. Curr Opin Microbiol 70:102199. https://doi.org/10.1016/j.mib.2022.102199
Bongers T, Ferris H (1999) Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol Evol 14:224–228. https://doi.org/10.1016/S0169-5347(98)01583-3
Cesarz S, Reich PB, Scheu S, Ruess L, Schaefer M, Eisenhauer N (2015) Nematode functional guilds, not trophic groups, reflect shifts in soil food webs and processes in response to interacting global change factors. Pedobiologia 58:23–32. https://doi.org/10.1016/j.pedobi.2015.01.001
Chen D, Pan Q, Bai Y, Hu S, Huang J, Wang Q, Naeem S, Elser JJ, Wu J, Han X, McCulley R (2016) Effects of plant functional group loss on soil biota and net ecosystem exchange: a plant removal experiment in the Mongolian grassland. J Ecol 104:734–743. https://doi.org/10.1111/1365-2745.12541
Chen D, Xing W, Lan Z, Saleem M, Wu Y, Hu S, Bai Y, Wang F (2018) Direct and indirect effects of nitrogen enrichment on soil organisms and carbon and nitrogen mineralization in a semi-arid grassland. Funct Ecol 33:175–187. https://doi.org/10.1111/1365-2435.13226
Chi J, Xu G, Yang Q, Sun J (2022) Water quality mediated community variations and niche differentiation of macroinvertebrates in Qingyijiang River Basin. China. Ecol Indic 138. https://doi.org/10.1016/j.ecolind.2022.108830
Clements WH (2004) Small-scale experiments support causal relationships between metal contamination and macroinvertebrate community responses. Ecol Appl 14:954–967
Cui H, Wagg C, Wang X, Liu Z, Liu K, Chen S, Chen J, Song H, Meng L, Wang J, Yang X, Kou X, Wang Y, Wang Y, Jin M, Xiao S (2022) The loss of above- and belowground biodiversity in degraded grasslands drives the decline of ecosystem multifunctionality. Appl Soil Ecol 172:104370. https://doi.org/10.1016/j.apsoil.2021.104370
Diabate B, Wang X, Gao Y, Yu P, Wu Z, Zhou D, Yang H (2018) Tillage and haymaking practices speed up belowground net productivity restoration in the degraded Songnen grassland. Soil Tillage Res 175:62–70. https://doi.org/10.1016/j.still.2017.08.003
Dietrich P, Cesarz S, Liu T, Roscher C, Eisenhauer N (2021) Effects of plant species diversity on nematode community composition and diversity in a long-term biodiversity experiment. Oecologia 197:297–311. https://doi.org/10.1007/s00442-021-04956-1
Fanin N, Kardol P, Farrell M, Kempel A, Ciobanu M, Nilsson MC, Gundale MJ, Wardle DA (2019) Effects of plant functional group removal on structure and function of soil communities across contrasting ecosystems. Ecol Lett 22:1095–1103. https://doi.org/10.1111/ele.13266
Farooq M, Jabran K, Cheema ZA, Wahid A, Siddique KH (2011) The role of allelopathy in agricultural pest management. Pest Manag Sci 67:493–506. https://doi.org/10.1002/ps.2091
Ferris H, Bongers T, de Goede RGM (2001) A framework for soil food web diagnostics: extension of the nematode faunal analysis concept. Appl Soil Ecol 18:13–29. https://doi.org/10.1016/S0929-1393(01)00152-4
Ferris H, Venette RC, Scow KM (2004) Soil management to enhance bacterivore and fungivore nematode populations and their nitrogen mineralisation function. Appl Soil Ecol 25:19–35. https://doi.org/10.1016/j.apsoil.2003.07.001
Fox J, Weisberg S, Adler D, Bates D, Baud-Bovy G, Ellison S, Firth D, Friendly M, Gorjanc G, Graves S (2012) Package ‘car’. Vienna: R Foundation for Statistical Computing 16
Hasna MK, Insunza V, Lagerlöf J, Rämert B (2007) Food attraction and population growth of fungivorous nematodes with different fungi. Ann Appl Biol 151:175–182. https://doi.org/10.1111/j.1744-7348.2007.00163.x
Heinen R, Hannula SE, De Long JR, Huberty M, Jongen R, Kielak A, Steinauer K, Zhu F, Bezemer TM (2020) Plant community composition steers grassland vegetation via soil legacy effects. Ecol Lett 23:973–982. https://doi.org/10.1111/ele.13497
Hu J, Wu J, Ma M, Nielsen UN, Wang J, Du G (2015) Nematode communities response to long-term grazing disturbance on Tibetan plateau. Eur J Soil Biol 69:24–32. https://doi.org/10.1016/j.ejsobi.2015.04.003
Hu L, Robert CAM, Cadot S, Zhang X, Ye M, Li B, Manzo D, Chervet N, Steinger T, van der Heijden MGA, Schlaeppi K, Erb M (2018) Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat Commun 9:2738. https://doi.org/10.1038/s41467-018-05122-7
Jagdale GB, Grewal PS (2002) Identification of alternatives for the management of foliar nematodes in floriculture. Pest Manag Sci 58:451–458. https://doi.org/10.1002/ps.472
Kalembasa SJ, Jenkinson DS (1973) A comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. J Sci Food Agric 24:1085–1090
Karakas M, Bolukbasi E (2019) A review using marigolds (Tagetes spp.) as an alternative to chemical nematicides for nematode management. Int J Adv Eng Manage Sci 5:556–560. https://doi.org/10.22161/ijaems.59.3
Katzen A, Chung H, Harbaugh WT, Della Iacono C, Jackson N, Glater EE, Taylor CJ, Yu SK, Flavell SW, Glimcher PW, Andreoni J, Lockery SR (2023) The nematode worm C. elegans chooses between bacterial foods as if maximizing economic utility. Elife 12:e69779. https://doi.org/10.7554/eLife.69779
Kitagami Y, Kawai K, Ekino T (2021) Soil physicochemical properties shape distinct nematode communities in serpentine ecosystems. Pedobiologia 85–86. https://doi.org/10.1016/j.pedobi.2021.150725
Kitagami Y, Matsuda Y (2020) Temperature changes affect multi-trophic interactions among pines, mycorrhizal fungi, and soil nematodes in a microcosm experiment. Pedobiologia 78:150595. https://doi.org/10.1016/j.pedobi.2019.150595
Kitagami Y, Matsuda Y (2022) Effect of ectomycorrhizal fungal species on population growth and food preference of a fungivorous nematode. Mycorrhiza 32:95–104. https://doi.org/10.1007/s00572-021-01063-0
Laliberte E, Kardol P, Didham RK, Teste FP, Turner BL, Wardle DA (2017) Soil fertility shapes belowground food webs across a regional climate gradient. Ecol Lett 20:1273–1284. https://doi.org/10.1111/ele.12823
Lazarova S, Coyne D, G. Rodríguez MG, Peteira B, Ciancio A, (2021) Functional diversity of soil nematodes in relation to the impact of agriculture—a review. Diversity 13:64. https://doi.org/10.3390/d13020064
Lefcheck J, Byrnes J, Grace J (2016) Package ‘piecewiseSEM’. R package version 1
Lenth R, Singmann H, Love J, Buerkner P, Herve M (2019) Package ‘emmeans’
Li H, Yang S, Semenov MV, Yao F, Ye J, Bu R, Ma R, Lin J, Kurganova I, Wang X, Deng Y, Kravchenko I, Jiang Y, Kuzyakov Y (2021a) Temperature sensitivity of SOM decomposition is linked with a K-selected microbial community. Glob Chang Biol 27:2763–2779. https://doi.org/10.1111/gcb.15593
Li S, Huang X, Shen J, Xu F, Su J (2020) Effects of plant diversity and soil properties on soil fungal community structure with secondary succession in the Pinus yunnanensis forest. Geoderma 379. https://doi.org/10.1016/j.geoderma.2020.114646
Li S, Song M, Jing S (2021b) Effects of different carbon inputs on soil nematode abundance and community composition. Appl Soil Ecol 163:103915. https://doi.org/10.1016/j.apsoil.2021.103915
Liu H, Zhang X, Zhang G, Kou X, Liang W (2022) Partial organic substitution weakens the negative effect of chemical fertilizer on soil micro-food webs. J Integr Agric 21:3037–3050. https://doi.org/10.1016/j.jia.2022.07.043
Liu T, Yu L, Li M, Wu J, Li H, Whalen JK, Hu F (2018) Food familiarity does not change nematode feeding behavior. Soil Biol Biochem 125:136–143. https://doi.org/10.1016/j.soilbio.2018.07.011
Lu Q, Wang K, Dou Z, Zhong L, Yao Y, Zuo Y (2023) Vanillin in resistant tomato plant root exudate suppresses Meloidogyne incognita parasitism. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.3c00661
Lyu Y, Shi P, Han G, Liu L, Guo L, Hu X, Zhang G (2020) Desertification control practices in China. Sustainability 12. https://doi.org/10.3390/su12083258
Manwaring M, Nahrung HF, Wallace H (2020) Attack rate and prey preference of Lasioseius subterraneous and Protogamasellus mica on four nematode species. Exp Appl Acarol 80:29–41. https://doi.org/10.1007/s10493-019-00456-3
Melakeberhan H, Bonito G, Kravchenko AN (2021) Application of nematode community analyses-based models towards identifying sustainable soil health management outcomes: A review of the concepts. Soil Systems 5:32. https://doi.org/10.3390/soilsystems5020032
Moens T, Luyten C, Middelburg JJ, Herman PMJ, Vincx M (2002) Tracing organic matter sources of estuarine tidal flat nematodes with stable carbon isotopes. Mar Ecol Prog Ser 234:127–137
Nguyen C (2003) Rhizodeposition of organic C by plants: mechanisms and controls. Agronomie 23:375–396. https://doi.org/10.1051/agro:2003011
Nielsen UN, Wall DH, Li G, Toro M, Adams BJ, Virginia RA (2011) Nematode communities of Byers Peninsula, Livingston Island, maritime Antarctica. Antarct Sci 23:349–357. https://doi.org/10.1017/S0954102011000174
Ning Q, Jiang L, Niu G, Yu Q, Liu J, Wang R, Liao S, Huang J, Han X, Yang J (2022) Mowing increased plant diversity but not soil microbial biomass under N-enriched environment in a temperate grassland. Plant Soil. https://doi.org/10.1007/s11104-022-05332-5
Niu X, Zhai P, Zhang W, Gu Y (2019) Effects of earthworms and agricultural plant species on the soil nematode community in a microcosm experiment. Sci Rep 9:11660. https://doi.org/10.1038/s41598-019-48230-0
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara R, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) Package ‘vegan.’ Community Ecol Packag Version 2:1–295
Osono T (2019) Functional diversity of ligninolytic fungi associated with leaf litter decomposition. Ecol Res 35:30–43. https://doi.org/10.1111/1440-1703.12063
Rasmann S, Ali JG, Helder J, van der Putten WH (2012) Ecology and evolution of soil nematode chemotaxis. J Chem Ecol 38:615–628. https://doi.org/10.1007/s10886-012-0118-6
Revelle W, Revelle MW (2015) Package ‘psych’. The comprehensive R archive network 337
Ruess L, Zapata EJG, Dighton J (2000) Food preferences of a fungal-feeding Aphelenchoides species. Nematology 2:223–230. https://doi.org/10.1163/156854100508962
Semchenko M, Leff JW, Lozano YM, Saar S, Davison J, Wilkinson A, Jackson BG, Pritchard WJ, De Long JR, Oakley S, Mason KE, Ostle NJ, Baggs EM, Johnson D, Fierer N, Bardgett RD (2018) Fungal diversity regulates plant-soil feedbacks in temperate grassland. Sci Adv 4: eaau4578. https://doi.org/10.1126/sciadv.aau4578
Shao Y, Bao W, Chen D, Eisenhauer N, Zhang W, Pang X, Xu G, Fu S (2015) Using structural equation modeling to test established theory and develop novel hypotheses for the structuring forces in soil food webs. Pedobiologia 58:137–145. https://doi.org/10.1016/j.pedobi.2015.06.001
Shaw EA, Boot CM, Moore JC, Wall DH, Baron JS (2019) Long-term nitrogen addition shifts the soil nematode community to bacterivore-dominated and reduces its ecological maturity in a subalpine forest. Soil Biol Biochem 130:177–184. https://doi.org/10.1016/j.soilbio.2018.12.007
Shipley B (2009) Confirmatory path analysis in a generalized multilevel context. Ecology 90:363–368. https://doi.org/10.1890/08-1034.1
Taylor LS, Phillips G, Bernard EC, DeBruyn JM (2020) Soil nematode functional diversity, successional patterns, and indicator taxa associated with vertebrate decomposition hotspots. PLoS ONE 15:e0241777. https://doi.org/10.1371/journal.pone.0241777
Timonen S, Christensen S, Ekelund F (2004) Distribution of protozoa in scots pine mycorrhizospheres. Soil Biol Biochem 36:1087–1093. https://doi.org/10.1016/j.soilbio.2004.02.019
Ullah MR, Carrillo Y, Dijkstra FA (2023) Relative contributions of fungi and bacteria to litter decomposition under low and high soil moisture in an Australian grassland. Appl Soil Ecol 182. https://doi.org/10.1016/j.apsoil.2022.104737
van den Hoogen J, Geisen S, Routh D et al (2019) Soil nematode abundance and functional group composition at a global scale. Nature 572:194–198. https://doi.org/10.1038/s41586-019-1418-6
van den Hoogen J, Geisen S, Wall DH et al (2020) A global database of soil nematode abundance and functional group composition. Sci Data 7:103. https://doi.org/10.1038/s41597-020-0437-3
van der Putten WH, Bardgett RD, Bever JD, Bezemer TM, Casper BB, Fukami T, Kardol P, Klironomos JN, Kulmatiski A, Schweitzer JA, Suding KN, Van de Voorde TFJ, Wardle DA, Hutchings M (2013) Plant-soil feedbacks: the past, the present and future challenges. J Ecol 101:265–276. https://doi.org/10.1111/1365-2745.12054
Wang J, Wang H, Lin Q, Wu Y, He X, Chen X, Yan W, Zhao J (2022) Legume biological nitrogen fixation improves but chemical nitrogen fertilizer suppresses soil nematode communities in a Camellia oleifera plantation. Land Degrad Dev 34:1403–1414. https://doi.org/10.1002/ldr.4542
Wang L, Wu Q, Jiang G (2020) The effect of desertification on frozen soil on the Qinghai-Tibet plateau. Sci Total Environ 711:134640. https://doi.org/10.1016/j.scitotenv.2019.134640
Wang X, Nielsen UN, Yang X, Zhang L, Zhou X, Du G, Li G, Chen S, Xiao S (2018) Grazing induces direct and indirect shrub effects on soil nematode communities. Soil Biol Biochem 121:193–201. https://doi.org/10.1016/j.soilbio.2018.03.007
Wang X, Xiao S, Yang X, Liu Z, Zhou X, Du G, Zhang L, Guo A, Chen S, Nielsen UN (2019) Dominant plant species influence nematode richness by moderating understory diversity and microbial assemblages. Soil Biol Biochem 137:107566. https://doi.org/10.1016/j.soilbio.2019.107566
Wardle DA (2002) Communities and ecosystems: linking the aboveground and belowground components (MPB-34). Princeton University Press
Wickham H, Chang W, Wickham MH (2016) Package ‘ggplot2’. Create elegant data visualisations using the grammar of graphics Version 2:1–189.
Wilschut RA, Geisen S (2021) Nematodes as drivers of plant performance in natural systems. Trends Plant Sci 26:237–247. https://doi.org/10.1016/j.tplants.2020.10.006
Xue H, Luo DQ, Duo B, Xue Q, Qu XL, Guo WW (2022) Community structure and diversity of soil nematodes around Lake Paiku in Tibet. China Ecomont (Journal on Protected Mountain Areas Research) 14:37–47. https://doi.org/10.1553/eco.mont-14-2s37
Yang X, Wang X, Xiao S, Liu Z, Zhou X, Du G, Liu K, Wang Y, Chen S, Nielsen UN (2021) Dominant plants affect litter decomposition mainly through modifications of the soil microbial community. Soil Biol Biochem 161:108399. https://doi.org/10.1016/j.soilbio.2021.108399
Yeates G (1999) Effects of plants on nematode community structure. Annu Rev Phytopathol 37:127
Yeates GW, Bongers T, De Goede RGM, Freckman DW, Georgieva SS (1993) Feeding habits in soil nematode families and genera-an outline for soil ecologists. J Nematol 25:315–331
Yin H, Su Y, Liu S, Li X, Li X, Fan C, Guan P, Xie Z, Wang S, Scheu S, Krashevska V (2022) Consistent response of nematode communities to management of coniferous plantations. For Ecosyst 9. https://doi.org/10.1016/j.fecs.2022.100045
Zhang C, Wang J, Ren Z, Hu Z, Tian S, Fan W, Chen X, Griffiths BS, Hu F, Liu M (2020) Root traits mediate functional guilds of soil nematodes in an ex-arable field. Soil Biol Biochem 151. https://doi.org/10.1016/j.soilbio.2020.108038
Zhang J, Hu Z, Zhang C, Tao Y, Chen X, Griffiths BS, Liu M (2022) Roots with larger specific root length and C: N ratio sustain more complex rhizosphere nematode community. Plant Soil 477:693–706. https://doi.org/10.1007/s11104-022-05465-7
Zhang N, Zhong B, Zhao C, Wang E, Wang Y, Chen D, Shi F (2020b) Change of soil physicochemical properties, bacterial community and aggregation during desertification of grasslands in the Tibetan Plateau. Eur J Soil Sci 72:274–288. https://doi.org/10.1111/ejss.12939
Zhang P, Li B, Wu J, Hu S, Seabloom E (2018) Invasive plants differentially affect soil biota through litter and rhizosphere pathways: a meta-analysis. Ecol Lett 22:200–210. https://doi.org/10.1111/ele.13181
Zubek S, Majewska ML, Błaszkowski J, Stefanowicz AM, Nobis M, Kapusta P (2016) Invasive plants affect arbuscular mycorrhizal fungi abundance and species richness as well as the performance of native plants grown in invaded soils. Biol Fertil Soils 52:879–893. https://doi.org/10.1007/s00374-016-1127-3
Zuo Y, Wang K, Dou Z, Wang N, Liu T, Lu Q (2020) A review of soil nematodes as biological indicators for the assessment of soil health. Front Agric Sci Eng 7:275. https://doi.org/10.15302/j-fase-2020327
Funding
This work was supported by the National Natural Science Foundation of China (projects 41830321, 32071532, and 31870412), the “111 Project” (BP0719040), the Natural Science Foundation of Gansu Province (22JR5RG564), and the Second Tibetan Plateau Scientific Expedition and Research Program (2019QZKK0302). We are grateful to X.Z. and Z.R. for his assistance in field work, and we would like to thank I.A. for his linguistic assistance during the preparation of this manuscript. We thank the Gansu Gannan Grassland Ecosystem National Field Scientific Observation and Research Station of Lanzhou University (Maqu Sub-station). Meanwhile, we thank the Core Facility of School of Life Sciences, Lanzhou University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by H.S., X.H., H.C., S.X., Z.L., J.C., J.W., A.Z., X.L., Y.W., Z.Y., K.L., L.A., and S.C. The first draft of the manuscript was written by H.S., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, H., Hou, X., Cui, H. et al. Long-Term Plant Community Removal Alters Soil Nematode Communities Mainly Through the Trophic Cascading Effects of Fungal Channel. J Soil Sci Plant Nutr 23, 6696–6706 (2023). https://doi.org/10.1007/s42729-023-01523-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01523-w