Abstract
Priorities in controlled-release fertilizer research include reducing the amount of epoxy resin needed in the coating, adjusting the solubility of urea and inhibitors, reducing the environmental impact of nitrogen fertilization, and ensuring that fertilizer is released at the same rate as crop requirements. In this study, sulfur-coated urea was combined with inhibitors to create composite-coated stabilized urea (CCSU) for improved fertilization efficiency, specifically four different methods of the coating were used: CCSU1 (HQ and DCD are sprayed on the surface of sulfur-coated urea, followed by an epoxy resin coating.), CCSU2 (HQ and DCD combined with epoxy resin are sprayed directly onto the sulfur-coated urea surface.), CCSU3 (spraying HQ on the sulfur-coated urea surface first, then spraying a mixture of DCD and epoxy resin), and CCSU4 (DCD is sprayed onto the sulfur-coated urea surface, followed by a mixture of HQ and epoxy resin.). The microstructure and controlled-release performance of CCSUs were assessed by means of scanning electron microscopy, hydrostatic release, and ammonia volatilization tests. The pot experiment was used to study the fertilization efficiency of CCSUs. The SEM results showed that the CCSUs have smooth, complete surfaces and a uniform thickness of film with tiny nitrogen release channels. The result of hydrostatic release tests showed that CCSUs delayed the time to 80% nitrogen release by 9–23 days in comparison with the epoxy resin coated urea (the treatment R). CCSU3 showed the best inhibition of ammonia volatilization, with a 16.24% reduction in ammonia volatilization at 28 days compared to R. Pot experiment studies with maize showed that CCSUs can continuously maintain N supply and improve corn yield and nitrogen use efficiency (NUE). In particular, the CCSU3 exhibited better agronomic effectiveness, with 23.81% higher yield and 23.79% higher NUE than R. It is recommended to mix DCD with epoxy resin and spray HQ alone onto the surface of sulfur-coated urea to form the CCSU, which could ensure the controlled release of nutrients while reducing the amount of epoxy resin and improve the release and conversion of nitrogen as well as the synchronization effect with the nitrogen demand of corn, thereby further enhancing the NUE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nitrogen fertilizer is the most extensively produced and applied type of fertilizer in the world, with urea being the most popular (Dubey et al. 2019). Because urea releases nutrients rapidly and has a short-lived effect, it cannot meet the nitrogen needs of crops at various stages of fertility (Chen et al. 2017). Urea is often overused in agricultural production. Leaching, runoff, and volatilization can have detrimental effects on soil, groundwater, and the atmosphere, as well as on the economic benefits to producers, when nitrogen utilization efficiency (NUE) is reduced (Zhao et al. 2009; Tian et al. 2019; An et al. 2020a).
It has been established that polymer-coated controlled-release urea is an effective remedy for low nitrogen consumption and nitrogen pollution (Lu et al. 2015). Controlled-release nitrogen fertilizer increased nitrogen consumption by 16.7–48.5% and 33.7–56.4% and yield by 9.1–21.0% and 3.89–15.7% in corn and wheat, respectively, as compared to treatments employing only conventional urea (Zheng et al. 2016; Zho et al. 2017; Zhang et al. 2021; Zhang et al. 2022). In addition, the researchers discovered that the use of controlled-release fertilizers (CRFs) resulted in greater crop yields for the same amount of nitrogen or economic inputs (Shen et al. 2022). In contrast to conventional urea, which is used multiple times during production, controlled-release urea is used only once and can reduce labor expenses. Environmentally having a conversation, CRFs increase nitrogen usage while decreasing NO3-N leaching (Zheng et al. 2017; L. Zhang et al. 2021).
Due to the varying way of diffusion of nitrogen over the membrane, the coating material becomes a limiting factor for the further development of CRFs. Currently, the inorganic materials used to make coatings, such as diatomaceous earth, biochar, phosphate powder, and sulfur, are readily available and inexpensive, with sulfur being more brittle, having poor water resistance, and exhibiting a poor controlled release effect after film production (Kassem et al. 2022). Besides, organic polymer materials, such as chitosan, natural rubber, and resin, are used to make fertilizer film. Epoxy resin is chemically stable, easy to process, and has good film-forming properties; however, it has high production costs, is difficult to degrade, and may be harmful to the environment (Li et al. 2016). Even though the variety of materials for fertilizer coating is expanding and the performance of controlled release is continually improving, the widespread commercialization of many organic synthetic films, which are difficult to produce and expensive, is still a way off.
The coating’s ability to control urea release is limited, and it cannot control the conversion of urea after leaching. To address this problem, urease/nitrification inhibitors have been added to nitrogen fertilizers. Both hydroquinone (HQ) and N-(n-Butyl) thiophosphoric triamide (NBPT) (urease inhibitors) significantly slowed urea hydrolysis and decreased the number of urea-degrading groups in the soil biotope, according to Li et al. (2019). The addition of dicyandiamide (DCD) nitrification inhibitors also yielded positive results, and Wang et al. (2021) demonstrated that the combination of urea and DCD lowered greenhouse gas emissions significantly. Chen et al. (2021) demonstrated that DCD and 3,4-dimethylpyrazolate phosphate (DMPP) significantly increased both NUE and maize yield. He et al. (2018) found that adding a blend of biochar, DCD, and HQ to urea successfully reduced NH3 emissions and increased rice output. However, there is also the possibility that the inhibitor will be rapidly fixed and degraded in the soil after application. To reduce the amount of difficult-to-degrade film materials and to improve the match between fertilizer release and crop demand, methods must be developed to better manage and control the release and conversion of nitrogen.
There have been numerous attempts to combine inorganic and organic envelopes, but relatively few studies have examined the combination of organic and inorganic materials with inhibitor coatings. Consequently, the objectives of this study were to (1) investigate the possibility of replacing resin materials with inorganic coating material sulfur and determine if there will be a significant change in the nitrogen release mechanism and controlled-release period; and (2) determine if and how different inhibitor coating methods affect the controlled-release performance, urea conversion, and nitrogen utilization of the sulfur-resin composite envelope. Based on these objectives, we hypothesized that the formation of a composite film shell of the inhibitor coated with sulfur and resin would have no negative effect on the controlled-release period of the fertilizer and would optimize the nitrogen release curve, thereby increasing crop yield and NUE.
2 Materials and Methods
2.1 Materials
Sulfur-coated urea was produced by the National Engineering Research Center for Efficient Utilization of Soil and Fertilizer Resources, Shandong Agricultural University, with particle diameter of 2–5 mm, nitrogen content ≥ 35% and sulfur content ≥ 15%. Hydroquinone (HQ) and dicyandiamide (DCD) were purchased from Shanghai Macklin Biochemical Co., Ltd. E-44 epoxy resin was purchased from Nantong Xingchen Synthetic Material Co. in China. Triethylenetetramine was purchased from Tianjin Kemiou Chemical Reagent Co., Ltd.
2.2 Preparation of the CCSUs (Composite-Coated Stabilized Urea)
Sulfur-coated urea was placed in the wrapping machine and heated to approximately 75 °C before being rotated and polished for 5 min (all treatments were done so). (1) To make the coating solution, epoxy resin was heated in 75 °C water, and triethylenetetramine was added in a mass ratio of 5:1. The coating solution was divided into 5 equal parts, stirred thoroughly before use, and sprayed onto the surface of the rotating sulfur-coated urea, which was then cooled and dried to make sulfur plus resin-coated urea (SR, contains 34.31% N). (2) To create sulfur + (HQ-DCD) + epoxy resin-coated urea (composite-coated stabilized urea, CCSU1), firstly, HQ and DCD were combined and heated using a magnetic stirrer, then dissolved in 75% ethanol and sprayed onto the rotating sulfur-coated urea several times. After the urea had dried completely, the epoxy resin coating solution was applied using the same way as the SR method. (3) To make sulfur + (HQ-DCD-epoxy resin)-coated urea (CCSU2), firstly, HQ and DCD were added to the epoxy resin that had been softened at 75 °C in a medium water bath, then thoroughly stirred, and this was the coating solution. Finally, the sulfur-coated urea was coated according to the SR. (4) To make sulfur + HQ + (DCD-epoxy resin)-coated urea (CCSU3), firstly, HQ was dissolved in 75% alcohol and sprayed onto the surface of sulfur-coated urea, then DCD was added to the epoxy resin that had been softened in a medium water bath at 75 °C and thoroughly stirred, and finally, the sulfur-coated urea was coated using the same way as the SR method. (5) To make sulfur + DCD + (HQ-epoxy resin) coated urea (CCSU4), we first switched the use of HQ and DCD, and then coated it using the CCSU3 method. Epoxy resin made up 2% of the mass of the sulfur-coated urea in all of the aforementioned CCSUs, HQ and DCD made up 0.4% and 2% of the pure N amount, respectively, and the final products all contained 34.03% N, 0.14% HQ, and 0.68% DCD. Additionally, the same process used to create SR was used to produce epoxy resin-coated urea (code R, 3% coating amount, 44.67% nitrogen content).
2.3 Microstructure Analyses of the CCSUs and Their Coatings
The surface and cross-sectional morphologies of the treatment of S, SR, and CCSUs were observed with a scanning electron microscope (SEM; JSM-6610LV, Japan) (Tian et al. 2019).
2.4 Characterization of N Release and Loss from the CCSUs
Nitrogen release tests were conducted to evaluate the controlled-release performance of SR and CCSUs. The nitrogen release characteristics of CCSUs were evaluated using the national standard method GB/T 23348-2009 (GAQS, IQPRC, SA 2009). In a nutshell, 200 ml of water at 25 °C were added to a glass vial containing 10 g of the CCSUs. Samples were taken on days 1, 3, 7, 14, 28, 42, and 56 as well as every 28 days after 56 days. The N content of the solution was calculated until the cumulative N release reached 80%.
The soil used in this study, including ammonia volatilization experiments and the pot experiment described later, was collected from the experimental station of the College of Resources and Environment, Shandong Agricultural University, China (36.16°N, 117.15°E). The basic physical and chemical properties of the top soil (0–20 cm) were characterized, which indicated a soil type of brown earth, and soil texture of clay loam, soil organic carbon of 11.25 g kg−1, available N of 38.14 mg kg−1, available P of 19.85 mg kg−1, available K of 104.16 g kg−1, pH of 6.99, and EC of 137.47 μS cm−1.
To assess the loss of NH3 volatilization, the urea (U), sulfur-coated urea (S), epoxy-coated urea (R), sulfur plus epoxy-coated urea (SR), and CCSUs were mixed with 300 g of soil at a dosage of 0.3 g N kg−1 dry soil in plastic boxes. Meanwhile, the CK without nitrogen fertilization was set. Additionally, the effect of S was eliminated by adding sulfur powder of equal mass to the sulfur film of the S to the CK, U, and R. After adjusting the soil moisture with distilled water to 60% of the field water holding capacity (WHC), a Petri dish containing 10 mL boric acid (3%) indicator solution was placed on the soil as a trap for capturing the volatilized NH3. The boxes were sealed and incubated in the dark at 25 °C. During the 60-day incubation period, the boric acid traps were replaced at regular intervals and titrated with a sulfuric acid standard solution (0.005 mol L−1) for NH3 quantification.
2.5 Pot Experiment
From June to September 2020, the pot experiment was conducted at the Shandong Agricultural University Resources and Environment Experimental Station. The pots were arranged in a completely random pattern (CRD). Nine treatments of different N fertilizations were set up in a randomized complete block design: CK (no N fertilizer), U (conventional urea, base fertilizer stage: jointing stage = 1:1), S (sulfur-coated urea), R (epoxy resin-coated urea), SR (sulfur plus resin-coated urea), and CCSU1-CCSU4 (different ways of using inhibitors). Each treatment was replicated 12 times with a total of 108 pots. Plastic pots (lower diameter 23.0 cm, upper diameter 35.0 cm, height 43.5 cm) were used in the experiment, with 20 kg of soil placed in each pot. N fertilizers were applied at a rate of 0.15 g N kg−1 soil. Calcium superphosphate (0.1 g P2O5 kg−1 dry soil) was used as a phosphorus fertilizer, and potassium chloride (0.1 g K2O5 kg−1 dry soil) was used as a potassium fertilizer. Prior to pot filling, 15 kg of topsoil (0–20 cm) was mixed with phosphorus and potassium fertilizers, CU, NCU, DCU, and NDCU, and urea was applied in splits as a basal fertilizer (50%) and top dressed (50%, at the jointing stage) and the effect of sulfur was eliminated by adding sulfur powder of equal mass to the sulfur film of the S treatment to the CK and R. On June 11, 2020, five maize seeds (Zea mays Ziyu 2) were sown in each pot and thinned to one seedling at the 5-leaf stage.
2.6 Collection and Determination of Potted Test Samples
Three pots were randomly selected and soil samples were taken with a soil auger from the top 0.2 m at the seedling, jointing, silking, and maturation stages. Following the removal of the maize roots, the fresh soil was thoroughly mixed and extracted with 1 mol L−1 KCL for NH4+-N and NO3-N determination using an AA3 autoanalyzer3 (SEAL Analytical, Germany). The moisture content of fresh soil was determined for calculation, and then, the remaining soil was air-dried. The sodium phenate-sodium hypochlorite colorimetric method (Santos et al. 2020) was used to measure urease activity in air-dried soil (1 mm). On September 22, 2022, three maize samples were harvested randomly of every treatment, and the ears per plant, grain number per ear, and 100-grain weight were measured. After sampling, all above ground samples were oven-dried at 105 °C for 30 min before being weighed at 75 °C to determine the amount of dry matter accumulation (DMA). After grinding, the dried samples were sieved (0.5-mm mesh) and digested in H2SO4/H2O2, followed by the Kjeldahl method of the overall N content (Kjeldahl 1883). Furthermore, the following formulas were used to calculate grain yield, N accumulation above ground (NA), N use efficiency (NUE), N fertilizer agronomic efficiency (NFAE), N partial factor productivity (NPFP), and N fertilizer apparent use efficiency (NFUE).
3 Results
3.1 Microstructure of the CCSUs
Figure 1 showed that the surface and cross-sectional (× 500) morphology of S, SR, and CCSUs subjected by electron microscopy scan. The surface of S (Fig. 1A) was rougher with many bumps, and the film layer (Fig. 1G) was thicker, but there were obvious boundaries between it and the urea core, and the wrapping was not tight; the surface of SR (Fig. 1B) was flatter, but there were many micropores of various sizes, and there were large gaps between the sulfur film and the resin film in the film layer (Fig. 1H), and the two had the poor binding ability. On the surface (Fig. 1C–F), although both used epoxy resin in the outer layer, compared to the SR, the CCSUs had fewer bumps and looked smoother and flatter, except for the CCSU1, which had more residue on the surface. This demonstrated that the addition of inhibitor by spraying had no negative impact on the resin film. On the contrary, spraying the inhibitor may fill the pits on the surface of the sulfur-coated urea, resulting in a smoother and more uniform resin film.
The cross-sectional view of CCSU1 (Fig. 1I) showed that no obvious demarcation between the inhibitor and the sulfur coating, and the two were fused into a thicker film layer, but the bonding with the resin was not ideal and there were obvious gaps; In the cross-sectional view of the CCSU2 (Fig. 1J), the compatibility of the inhibitor with the resin was excellent. The inhibitor mixed with resin forms a smooth, complete and uniform thickness film layer, and while the demarcation between it and the sulfur film remained visible, the gap between the two decreased.
The cross-sectional view of CCSU3 (Fig. 1K) showed that DCD bonded well with the epoxy resin and could form a complete and uniform coating, but there were a few pores in the resin film probably because air bubbles were not excluded when mixing DCD and resin. In addition, unlike CCSU2, HQ did not fuse with the sulfur film but instead coated its surface. This created an obvious separation between the resin layer, the inhibitor layer, and the sulfur layer, with the inhibitor layer being closer to the sulfur layer. However, due to the small amount of HQ that was filled in the uneven position of the sulfur layer, the HQ layer exhibited an irregular thickness and a little gap between it and the sulfur layer. It was worth noticing that on the sulfur layer, there were many irregular bumps, which might be due to the fertilizer being preheated for a long time and then sprayed with a hot HQ solution, resulting in a tiny amount of the sulfur layer melting. The cross-sectional view of CCSU4 (Fig. 1L) showed that, similar to DCD, HQ had good compatibility with the resin, but there were a few voids caused by air bubbles. Unlike the performance of CCSU3, the inhibitor layer formed by DCD was thicker and tightly bound to the resin, and the boundary between the two was not obvious, but there were more obvious cracks between the DCD layer and the sulfur layer. The DCD layer had not filled the pits on the surface of the sulfur layer and did not make it smoother and flatter as expected. This indicated that although the inhibitor has the effect of increasing the surface smoothness and reducing the interlayer gap, it will be affected by the type of inhibitor and the spraying method.
3.2 Characterization of N Release from the CCSUs
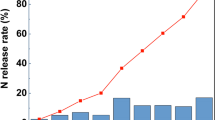
As shown in Fig. 2A, the differences in nitrogen release rates among treatments at 24 h were small, and the initial leaching rates were all well below 15%; N release from the S began to accelerate on day 3, and the differences between treatments began to increase, reaching about 30%, 22%, and 19% for the S, R, and SR treatments at day 14, respectively, while the CCSUs were all at a lower level of about 15%; N release from the S began to accelerate on day 3, and the differences between treatments began to increase, the cumulative nitrogen release rate from the S and R treatments exceeded 80% on day 84, while the values of the release rate among the CCSUs were very close, reaching 80% around day 112, with the CCSU3 having the lowest release rate of about 82%. Collectively the S treatment had the worst N release control ability, followed by the R, and the sulfur plus epoxy resin coating (SR) had slightly better release control performance than the epoxy resin coating alone. There was little difference in release control ability between CCSUs, but it could be seen that adding inhibitors to the sulfur and epoxy resin films not only did not reduce the release control performance but actually improved it.
According to Fig. 2B, the release rate of HQ was significantly higher than that of nitrogen (p<0.05), with the order being CCSU 4 > CCSU 1 > CCSU 2 > CCSU 3. On day 84, the HQ release rate for all treatments reached 80%, with the lowest rate of 80.79% for the CCSU3 and the highest rate of 92.43% for the CCSU4.
As illustrated in Fig. 2C compared to the release rate of HQ, DCD released more quickly, reaching 80% for all treatments on day 28, with the lowest release rate for the CCSU3 being 75.38% and the highest release rate for the CCSU1 being 88.45%. On day 42, the release rate of DCD exceeded 90% for all treatments, and all DCD was released for CCSU4.
In conclusion, despite the DCD release period of each treatment was less than half that of urea, the envelope could still achieve slow release of the inhibitor and extend the validity of the inhibitor, enabling the double controlled-release urea combined with the inhibitor to achieve the effect of controlling urea leaching as well as inhibiting urea conversion, reducing nitrogen loss and improving nitrogen uptake.
3.3 Characterization of N Loss from the CCSUs
As shown in Fig. 3A, the rate of ammonia volatilization increased first and then decreased after the urea was coated. The ammonia volatilization rate of the U increased rapidly at the start of incubation. It peaked on day 3, whereas the remaining treatments’ ammonia volatilization rate was relatively low in the first 5 days. The peak of ammonia volatilization rate was delayed by 11–14 days and decreased by 52.95–60.11% compared to that of U.
Peak ammonia volatilization rates of various coated controlled-release urea were CCSU 3 < CCSU 2 <CCSU1 < CCSU 4 < SR < R < S. The peak ammonia volatilization rate of the R appeared on day 14 of the incubation, whereas the CCSUs’ peak was delayed by three days to day 17, and the peak was reduced. The peak of ammonia volatilization from the SR was reduced by 3.78% and 7.22% than the R and the S, respectively, indicating that the double-layer coating of sulfur and epoxy resin had better release control performance than the single coating; additionally, the CCSUs reduced 0.06–8.64% than that of the SR, indicating that the coating method of adding inhibitors to the middle of sulfur and epoxy resin could reduce nitrogen loss more effectively.
The nitrogen release pattern in ammonia volatilization accumulation (Fig. 3B) was consistent with the trend in ammonia volatilization rate, with the CCSUs showing a plateau in ammonia volatilization accumulation around day 28 and a 42.46% to 55.05% decrease in ammonia volatilization accumulation compared to the U. Briefly, CCSU3 outperformed all other treatments.
3.4 Soil NH4 +-N, NO3 −-N, and Apparent Nitrification Rate of NH4 +-N
As shown in Fig. 4A, the trend of soil ammonium nitrogen content in the U treatment showed an increase followed by a decrease, and exceeded 6 mg kg−1 at both seedling and jointing stages, which was significantly higher than other treatments during the same period (p < 0.05). In contrast, the CCSUs significantly reduced the release of ammonium nitrogen from urea in the early stages of maize growth (p < 0.05), with the pattern exhibiting an increase followed by a relatively moderate growth rate, peaking at the silking stage and then rapidly decreasing. Among others, the CCSU3 inhibited ammonium nitrogen release more effectively in the early stages, with N release peaking at the spatulation stage, ensuring crop nitrogen requirements and maintaining soil ammonium nitrogen content above 3.8 mg kg−1 at maize maturity stage.
Changes of soil NH4+-N (A) and NO3−-N (B) content treated with the sulfur/resin-coated urea fertilizers. CK (no N fertilizer), U (conventional urea, base fertilizer stage: jointing stage = 1:1), S (sulfur-coated urea), R (epoxy resin-coated urea), SR (sulfur plus resin-coated urea), and CCSU1-CCSU4 (different ways of using inhibitors)
The soil nitrate-N content variation was similar to ammonium N. In Fig. 4B, the U showed a rapid release of nitrogen in the early stage, reaching a maximum of 24.99 mg kg−1 by the jointing stage. The SR exerted strong control over N release in the early stages, but the difference between it and the S and R treatments gradually faded in the later stages. With the addition of inhibitors, the CCSUs significantly reduced soil nitrate-N content in the early stage (p < 0.05), and soil nitrate-N content was elevated by 51.21 to 65.90% after the silking stage compared to the U. Although there was minimal variation between the CCSU, CCSU3 had the best-regulated release, with soil nitrate-N concentration of 29.46 mg kg−1 and 8.47 mg kg−1 at the silking and maturity stage, respectively.
As shown in Table 1, the apparent nitrification rate of the U remained high during the pre-growth period of maize, resulting in higher soil nitrate-N content. In contrast, the wrapper fertilizer treatments all showed a lower apparent nitrification rate. Noteworthy, at seedling and jointing stages, the apparent nitrification rates of CCSUs were lower than those of the S, R, and SR treatments. During the maize silking stage, the apparent nitrification rates of all wrapped fertilizers were increased to high levels, resulting in a rapid increase in soil nitrate-N content, but the differences across treatments were not significant (p > 0.05).
The CCSUs showed a marked decrease in the apparent nitrification rate at maturity, indicating that the membrane was still playing a controlling role. The CCSU3 showed the lowest apparent nitrification rate in all periods, decreasing by 4.94%, 11.56%, 0.64%, and 11.31%, respectively, compared to the SR. At the maturity stage, the CCSUs showed a significant decrease in apparent nitrification rate (p < 0.05), indicating that the film was still exerting control, in contrast to the U and R treatments, which exhibited almost no change. The CCSU3 displayed the lowest apparent nitrification rate throughout all periods, declining by 4.94%, 11.56%, 0.64%, and 11.31%, respectively, compared to the SR.
3.5 Urease Activity
As seen in Fig. 5, due to the depletion of urease inhibitors and the control of N release by the wrapper during the pre-growth stage, a large amount of nitrogen accumulated in the wrapper fertilizer was released at the silking stage, and with it, soil urease activity increased rapidly, 54.17 to 63.73% in the CCSUs compared to U and 2.60 to 8.97% in the SR treatment. At the maturity stage, the urease activity of the R was much reduced but still greater than the conventional urea treatment and higher than the seedling and nodulation stage, with the CCSU3 elevating 22.54% above SR. The huge amount of urea stored in the early stage was released at the silking stage and could be provided continually until maturity stage, consistent with changes in the nitrate and ammonium nitrogen content of the soil (Fig. 4A).
Soil urease activity changes treated with the sulfur/resin-coated urea fertilizers. CK (no N fertilizer), U (conventional urea, base fertilizer stage: jointing stage = 1:1), S (sulfur-coated urea), R (epoxy resin-coated urea), SR (sulfur plus resin-coated urea), and CCSU1-CCSU4 (different ways of using inhibitors)
3.6 Grain Yield
As shown in Table 2, the number of spikes in the S, R, and SR treatments was lower than that in the U treatment, but the number of spikes, 100-grain weight, and yield were considerably higher, with yields ranging from 16.25 to 29.35% higher than in the U. Furthermore, sulfur plus epoxy outperformed the single wrapper. The CCSU treatments did not significantly improve spike number, spike grain number, or 100-grain weight compared to the SR, but yield increased significantly, with the CCSU3 improving 55.79% and 20.44% compared to the U and SR treatments (p < 0.05), respectively, then CCSU2, CCSU1, and CCSU4 in that order. In terms of corn yield increase, the phenomena above showed that applying HQ to the surface of the sulfur membrane first and then mixing DCD with epoxy resin combined as the outer layer had the optimum performance.
3.7 NA, NUE, NFAE, NFPP, and NFUE
Table 3 displays the indicators for nitrogen absorption and utilization indexes of maize. The accumulation of nitrogen in the upper portion of the maize ground was greatly increased by nitrogen fertilizer application, with the SR showing modest improvement over the S or R treatments but 11.41% improvement over U. With the addition of inhibitors, the CCSUs shown a larger improvement, with NA advancing 15.44 to 29.53% over U and 3.61 to 16.27% over SR. Sulfur and epoxy resin coating did not greatly improve nitrogen use efficiency (NUE), but the addition of inhibitors made a bigger difference, with the CCSU3 outperforming the U and SR treatments by 55.79% and 20.42%, respectively. Compared to U, the coated N fertilizer’s NFAE was much higher. The SR treatment exhibited varying degrees of improvement to the S treatment, but the difference with the R treatment did not reach a significant level (p < 0.05); the CCSU3 treatment had the best effect, followed by CCSU2, CCSU1, and CCSU4. Urea coating increased yields for the same amount of nitrogen. However, as with NFAE, the augmentation of NPFP in the form of sulfur with resin coating was not appreciable when compared to epoxy coating, but the inclusion of inhibitors produced better results, with the largest advantage of the CCSU3 treatment with a 20.45% improvement over SR. The gain in NFUE varied greatly between fertilizer treatments, with the SR surpassing the S and R treatments and demonstrating a 19.52% increase over U. The CCSU treatments outperformed the U and SR treatments, with increases of 24.18 to 47.21% and 3.90 to 23.17%, respectively, with the highest rise at CCSU3. The form of the inhibitor used as an envelope filler resulted in a double control of N release and conversion, greatly improved nitrogen uptake and utilization as well as nitrogen accumulation in maize, and ultimately led to higher nitrogen fertilizer utilization, with the CCSU3 achieving the best fertilizer efficiency.
4 Discussion
It is possible to separate crop nutrient need into distinct curves, for instance, maize has an S-shaped need for nitrogen and requires nutrients urgently throughout the middle and late growth stages (Qi et al. 2021). Conventional agricultural production typically meets the nitrogen needs of crops at various stages of fertility through additional nitrogen fertilizer. Notably, stabilized coated nitrogen fertilizers with inhibitors can significantly improve the N release profile and better match crop requirements with a single base treatment, hence increasing crop yield and nitrogen utilization. According to An et al. (2020b), the N release from coated fertilizers involves three steps: water penetration into the wrapper, dissolution of the core, and N release via the membrane shell. The regulated release of coated fertilizers with extra inhibitors is affected by the coating material, soil moisture, pH, and temperature (Li et al. 2022). The type of coating influences the frequency of nitrogen release directly, whereas the manner of inhibitor addition controls the conversion efficiency after urea release. Numerous scientists have investigated the effects of combining urease and nitrification inhibitors. Sanz-Cobena et al. (2011) discovered that adding N-(n-Butyl) thiophosphoric triamide (NBPT) and dicyandiamide (DCD) to urea reduced N loss by 33% and increased maize yield by 18%. According to Martins et al. (2017), applying nitrapyrin (NP) and NBPT with urea to maize enhanced the uptake of total N by 47%, reduced soil NH3 and N2O volatilization, and increased yield by 23%. However, there have been fewer attempts to dissolve the inhibitor in membrane solutions and produce a composite envelope.
In this study, a composite envelope was constructed from three layers: a sulfur layer, an inhibitor coating layer, and an epoxy resin layer. The SEM results revealed that both the SR and CCSU surfaces were smoother, with just a slight distinction between the two, but the treatments exhibited statistically significant variances in the profiles (p < 0.05). The coating of CCSU1 exhibited more obvious cracks between the outer epoxy resin and the inhibitor coating, which might be attributed to the relatively short coating procedure, and that made it impossible for the two to integrate, resulting in a less-than-tight appearance. There were no evident fractures between the inner and outer layers on the CCSU2- and CCSU3-treated envelopes, showing that there was good compatibility between DCD and hydroquinone (HQ) and epoxy resin, and that the coating could be applied after proper mixing. Specifically, the CCSU3 membrane layer had a small number of more evenly distributed micropores than the CCSU2 membrane layer, which theoretically might be potentially unfavorable for controlled N release (Zhang, 2022), but did not behave as predicted in this experiment. Tan (2022) found a few micropores in the polylactic acid-coated urea film and concluded that these micropores permit water molecules to enter the inside of the fertilizer more slowly. More excessive porosity in the membrane layer would result in faster N release, according to Dong (2022). However, when the membrane layer has a dense structure or few pores, the nitrogen release does not fit the crop’s nutritional need pattern as nitrogen is difficult to be released in a timely manner.
In this study, it was shown that the existence of the inner sulfur membrane did not diminish the ability to regulate release and that the presence of these channels provided a pathway for the inhibitor in the inner layer and the nitrogen efflux from the core. The simultaneous release of the inhibitor and urea improved the action of the inhibitor. The cross-sectional view of CCSU4 revealed that DCD and sulfur were incompatible, as they were difficult to fit together tightly and exhibited significant delamination. In summary, it is plausible to expect that CCSU3’s nitrogen-controlled release mechanism is comparable to that of conventional resin-coated urea. The fertilizer is applied to the soil, and as a result of external soil forces and moisture, the pores in the membrane layer enlarge. As the osmotic pressure inside and outside the membrane rises, the pores transform into conduits for nutrient release along the concentration gradient and pressure gradient, working in conjunction with the osmotic gradient. This suggests that a fertilizer with controlled release could potentially increase crop output by delivering nutrients more efficiently.
The results of the hydrostatic release test were slightly different than anticipated, with the CCSU3 exhibiting exceptional release control. This composite film, composed of resin and DCD, not only conserved the integrity of the resin film, but also marginally enhanced its release control capabilities, so effectively extending the release period of HQ and DCD. According to Li et al. (2020), who utilized DCD and HQ to coat the epoxy resin-coated urea, the controlled-release period of urea was significantly longer than that of the inhibitor, and the ranking was urea > HQ > DCD, which was consistent with the results of the current experiment. In this test, however, the duration of the inhibitor-controlled release was considerably extended. In contrast, even though the middle layer of DCD was coated, the cumulative release rate of CCSU4 was not reduced, most likely for three reasons. First, DCD has poor film-forming skills and has difficulty adhering strongly to sulfur and resin films when employed individually, which reduces the film’s release control capability. Second, Liu et al. (2022) found that DCD was more soluble in water and soluble on its own, which is similar with the CCSU4 findings. Third, while the amount of DCD is relatively large in comparison to the amounts of HQ and epoxy resin, the mid-layer film shell is thicker, increasing the specific surface area of the fertilizer while thinning the outer layer, thereby decreasing the film’s water resistance and pressure resistance.
Nitrate and ammonium nitrogen, which plants may absorb and utilize directly, is a substantial source of nitrogen loss in agricultural output (Duff et al. 2022). When crop nutrient demand is minimal, urea given to the soil will be rapidly hydrolyzed, resulting in an oversupply of ammonium nitrogen. On the one hand, this ammonium nitrogen is easily converted to ammonia and lost, and on the other, it supplies sufficient substrate for nitrification to increase soil nitrification, resulting in nitrogen loss via leaching, N2O, and N2 (Tian et al. 2015). As urease and ammonia-oxidizing bacteria play a crucial role in this process, numerous studies have been conducted to regulate nitrogen conversion by adding urease inhibitors and nitrification inhibitors, which slow urea hydrolysis and conversion and enhance nitrogen utilization. Li et al. (2019) mixed HQ and NBPT with urea and found that their effects were comparable, limiting urea hydrolysis and ammonia volatilization in a similar fashion. Souza et al. (2019) co-applied DCD and 3,4-dimethylpyrazolate phosphate (DMPP) with urea and discovered that N2O and NH3 escaped largely from urea and that these nitrification inhibitors had a key role in limiting N losses and suppressing nitrification activities. In contrast, An et al. (2020a) and Pérez-Castillo et al. (2021) reported that inhibitors are ineffective in tropical and semi-arid regions and that dosages should be controlled carefully. Evidently, applying the inhibitor to the surface of the fertilizer is preferable than mixing or increasing the dosage. Recio et al. (2020) attempted this by coating the surface of urea with NBPT and 2-(3,4-dimethyl-lhH-pyrazol-l-yl) succinic acid (DMPSA), which boosted grain yield by 16% and decreased N2O emissions by more than 35%.
In this study, the ammonia volatilization trends of the SR and CCSUs were comparable to those of epoxy resin-coated urea. Consistent with previous research, blending urea with epoxy resin to create a coating solution encapsulated on the fertilizer surface significantly reduced the rate of ammonia volatilization and ammonia volatilization compared to conventional urea. Regarding the insignificance of the difference between CCSUs, the author posits two possible causes. First, the amount of inhibitor added was small, and there was no discernible difference in the rate of release. Second, because there were no crops in the soil culture experiment, the nitrogen loss reduced by the inhibitor could not be absorbed in time, and despite the fact that the N transformation was controlled, the accumulated ammonium nitrogen was eventually released in the form of ammonia, making it difficult to reflect the real situation.
Elrys et al. (2021) discovered that the inhibitory effect of DCD on nitrification leads to soil ammonium N accumulation and may eventually increase NH3 volatilization, similar to the results of this experiment. The results of the maize pot studies revealed apparent increases in yield and NFUE, which may possibly indicate that maize absorbed more nitrogen under the influence of inhibitors, hence increasing yield.
During maize development, the urease activity of CCSUs increased significantly in the middle and late stages compared to the R treatment, but not in the early stages. This may be due to the addition of urea to CCSUs at a ratio of 7:3 during basal application, which hydrolyzed quickly in both treatments during the early growth stages. Meanwhile, the inhibitors in the CCSUs treatment were released more slowly in the seeding stage and had a limited effect.
Observing the content of ammonium nitrogen in the soil revealed that the common urea blended at the beginning of the fertilizer application was the primary source of ammonium nitrogen. However, the addition of inhibitors to the treatment demonstrated some suppression effects. When maize was in its middle and late growth stages, when it required nutrients the most, the release of inhibitors from CCSUs was essentially complete, and the controlled-release effect of the membranes was rapidly diminishing. Consequently, a substantial amount of nutrients were released from the urea kernel, leading to an increase in soil urease activity as well as nitrate and ammonium nitrogen content. According to Keshavarz et al. (2018), inhibitors drastically altered the nutrient release profile and increased their usefulness for agricultural purposes.
During the maturation stage, the soil ammonium nitrogen concentration of CCSUs was significantly lower than that of SR, most likely as a result of the soil and other factors gradually releasing the inhibitors incorporated into the membrane shell. The apparent nitrification rate is a crucial determinant of soil nitrification severity (Wang et al. 2021). Cui et al. (2021) found that the apparent nitrification rate was closely related to the amount of nitrate-ammonium nitrogen in the soil and the soil type. The apparent nitrification rate of the soil exhibited comparable behavior to changes in soil nitrate-nitrogen content, and the CCSUs treatments considerably decreased soil nitrate-nitrogen content in the early stage, but the effect continued to diminish after the jointing stage. Small concentrations of inhibitors were unable to prevent the release of a substantial amount of nitrogen during the silking stage, resulting in a sudden increase in nitrate-nitrogen levels (Tian et al. 2015; Qi et al. 2021; Chen et al. 2021).
Yield and indicators characterizing nitrogen utilization efficiency are crucial indicators of fertilizer effectiveness. Previous research has demonstrated that controlled-release urea is more capable than conventional urea of addressing the long-term nitrogen needs of crops, hence increasing NUE and yield (Yang et al. 2011; Zheng et al. 2016; Tian et al. 2019). Ma et al. (2019) boosted wheat yield by 19.9% and NUE by 44.5% by combining urea with DCD and HQ as compared to urea alone. By analyzing the studies on inhibitors, it is possible to draw the following conclusions: (1) there is a problem with excessive inhibitor use; (2) inhibitor loss is possible; (3) inhibitors must be calculated and blended during administration, which increases the load. This study showed that the application of CCSUs resulted in a significant increase in yield relative to both U and SR, with CCSU3 having the greatest impact, which is consistent with previous research and expectations. It has been determined that the inhibitor and epoxy resin combination is viable and effective. The composite covering achieves dual management of urea leaching and conversion, allowing for a better-coordinated release of urea and inhibitors in response to soil and water factors. In summary, CCSUs enhance nitrogen utilization rate, reduce nitrogen loss and greenhouse gas emissions, align the nitrogen release curve with the crop demand curve, and boost crop output. It should be noted that coating with DCD alone may not be as effective as coating with DCD and epoxy resin.
5 Conclusions
This study showed that by applying a sulfur + resin coating, a controlled-release effect of 5% resin dosage could be accomplished with a resin containing just 2% mass of the urea core, while the urea still retained its control release diffusion capability. In addition to preserving the integrity of the film shell and the controlled-release performance of the fertilizer, the inclusion of the inhibitor in the form of a coating delayed the release of nitrogen. By modulating N release and decreasing the apparent nitrification rate of the soil, composite-coated stabilized urea (CCSU) better-coordinated nitrogen release and maize N demand, resulting in improved corn yields. Moreover, this is a novel and novel method for making the inhibitor more stable without causing it to be rapidly degraded and cured. Therefore, the substitution of epoxy resins with sulfur and combined inhibition to form a composite envelope is promising, as it could increase the compatibility between nitrogen release and crop needs, reduce production costs, and make the technology more environmentally friendly.
References
An OJ, Stoeckli J, Hao XY, Beres B, Li YJ (2020a) Nitrous oxide emissions following split fertilizer application on winter wheat grown on Mollisols of Southern Alberta, Canada. Geoderma 21:e00272. https://doi.org/10.1016/j.geodrs.2020.e00272
An X, Wu Z, Qin H, Liu X, Yu B (2020b) Integrated co-pyrolysis and coating for the synthesis of a new coated biochar-based fertilizer with enhanced slow-release performance. J Clean Prod 283:124642. https://doi.org/10.1016/j.jclepro.2020.124642
Chen JN, Cao FB, Xiong HR, Huang M, Zou YB, Xiong YF (2017) Effects of single basal application of coated compound fertilizer on yield and nitrogen use efficiency in double-cropped. Rice Crop J 5:265–270. https://doi.org/10.1016/j.cj.2017.01.002
Chen ZG, Li Y, Xu YH, Shu KL, Xia LL, Zhang N, Michael JC, Ding WX (2021) Spring thaw pulses decrease annual N2O emissions reductions by nitrification inhibitors from a seasonally frozen cropland. Geoderma 403:115310. https://doi.org/10.1016/j.geoderma.2021.115310
Cui L, Li DP, Wu ZJ, Xue Y, Xiao FR, Zhang K, Li YH, Zheng Y (2021) Effects of different nitrification inhibitors on nitrification,maize yield,and nitrogen use efficiency in red soil. Chinese J Eco 32:3953–3960. https://doi.org/10.13287/j.1001-9332.202111.022
Dong HP (2022) Preparation and properties of lignin/biochar/PLA composite coated sustained-release fertilizer. Central South University of Forestry and Technology, China, Hunan
Dubey A, Damodhara Rao M (2019) Zeolite coated urea fertilizer using different binders: fabrication, material properties and nitrogen release studies. Environ Technol Innovation 16:100452. https://doi.org/10.1016/j.eti.2019.100452
Duff AM, Patrick F, Israel I, Fiona B (2022) Assessing the long-term impact of urease and nitrification inhibitor use on microbial community composition, diversity and function in grassland. Soil Biol Biochem. 170:108709. https://doi.org/10.1016/j.soilbio.2022.108709
Elrys AS, Mohamed FAE, Enas Mohamed Wagdy A, Safaa MAIA, Khaled AE, El-Sayed MD (2021) Mitigate nitrate contamination in potato tubers and increase nitrogen recovery by combining dicyandiamide, moringa oil and zeolite with nitrogen fertilizer. Ecotox Environ Safe 209:111839. https://doi.org/10.1016/j.ecoenv.2020.111839
General Administration of Quality Supervision (2009) Inspection and quarantine of the People’s Republic of China, standardization administration (GAQS, IQPRC, SA), slow release fertilizer: GB/T 23348-2009. Standards Press of China, Beijing (in chinese)
He TH, Liu DY, Yuan JJ, Ni K, Mohammad Z, Luo JF, Stuart L, Ding WX (2018) A two years study on the combined effects of biochar and inhibitors on ammonia volatilization in an intensively managed rice field. Agric Ecosyst Environ 264:44–53. https://doi.org/10.1016/j.agee.2018.05.010
Kassem I, El-Houssaine A, Fatima-Zahra El B, Zineb K, Hassan H, Houssine S, Mounir El A (2022) Biodegradable all-cellulose composite hydrogel as eco-friendly and efficient coating material for slow-release MAP fertilizer. Prog Org Coat 162:106575. https://doi.org/10.1016/j.porgcoat.2021.106575
Keshavarz Afshar R, Lin RY, Mohammed YA, Chen CC (2018) Agronomic effects of urease and nitrification inhibitors on ammonia volatilization and nitrogen utilization in a dryland farming system: field and laboratory investigation. J Clean Prod 172:4130–4139. https://doi.org/10.1016/j.jclepro.2017.01.105
Kjeldahl J (1883) Neue methode zur bestimmung des stickstoffs in organischen körpern. Z Anal Chem 22:366–382. https://doi.org/10.1007/bf01338151
Li J, Wang YJ, Chen YB, Gu DR, Lin R, Yang XD (2022) Research advances on the accumulation and degradation of microcapsules derived from polymer-coated controlled-release fertilizers and their effects on soil quality. J Plant Nutr Fertil 28:1113–1121. https://doi.org/10.11674/zwyf.2021591
Li WY, Xiao Q, Hu CS, Liu BB and Sun RB (2019) A comparison of the efficiency of different urease inhibitors and their effects on soil prokaryotic community in a short-term incubation experiment. Geoderma 354: 113877. https://doi.org/10.1016/j.geoderma.2019.07.035
Li XD, Li Q, Xu X, Su Y, Yue QY, Gao BY (2016) Characterization, swelling and slow-release properties of a new controlled release fertilizer based on wheat straw cellulose hydrogel. J Taiwan Inst Chem Eng 60:564–572. https://doi.org/10.1016/j.jtice.2015.10.027
Li Y, He MR, Dai XL, Dong YJ (2020) Preparation of dual controlled release urea with nitrogen inhibitor and resine coating and the effects on soil nitrogen supply capacity and wheat yield. J Plant Nutr Fertil 26:1612–1624. https://doi.org/10.11674/zwyf.20030
Liu FB, Lang M, Ma X, Cheng TH, Zhang F, Guo GZ, Chen XP, Wang XZ (2022) Study on the mechanism of nitrification inhibitor type and dosage in different soils. Soil Fertil Sci in Chin 8:66–75. https://doi.org/10.11838/sfsc.1673-6257.21345
Lu P, Zhang Y, Jia C, Wang C, Li X, Zhang M (2015) Polyurethane from liquefied wheat straw as coating material for controlled release fertilizers. BioResour 10:7877–7888. https://doi.org/10.15376/biores.10.4.7877-7888
Ma Q, Wu ZJ, Yu WT, Zhou CR, Ning CC, Yuan HY, Xia ZQ (2019) Does the incorporation of dicyandiamide and hydroquinone with straw enhance the nitrogen supplying capacity in soil? Appl Soil Ecol 136:158–162. https://doi.org/10.1016/j.apsoil.2018.12.007
Martins MR, Sant’Anna SAC, Zaman M, Santos RC, Monteiro RC, Alves BJR, Jantalia CP, Boddey RM, Urquiaga S (2017) Strategies for the use of urease and nitrification inhibitors with urea: impact on N2O and NH3 emissions, fertilizer-15N recovery and maize yield in a tropical soil. Agric Ecosyst Environ 247:54–62. https://doi.org/10.1016/j.agee.2017.06.021
Pérez-Castillo AG, Chinchilla-Soto C, Elizondo-Salazar JA, Barboza R, Kim D-G, Müller C, Sanz-Cobena A, Borzouei A, Dawar K, Zaman M (2021) Nitrification inhibitor nitrapyrin does not affect yield-scaled nitrous oxide emissions in a tropical grassland. Pedosphere 31:265–278. https://doi.org/10.1016/S1002-0160(20)60070-4
Qi ZL, Dong YJ, He MR, Wang MY, Li Y, Dai XL (2021) Coated, stabilized enhanced-efficiency nitrogen fertilizers: preparation and effects on maize growth and nitrogen utilization. Front Plant Sci 12. https://doi.org/10.3389/fpls.2021.792262
Recio J, Montoya M, Álvarez JM, Vallejo A (2020) Inhibitor-coated enhanced-efficiency N fertilizers for mitigating NOx and N2O emissions in a high-temperature irrigated agroecosystem. Agric For Meteorol 292-293:108110. https://doi.org/10.1016/j.agrformet.2020.108110
Santos CF, Silva Aragão OO d, Silva DRG, Jesus E d C, Chagas WFT, Correia PS, Souza Moreira FM d (2020) Environmentally friendly urea produced from the association of n-(n-butyl) thiophosphoric triamide with biodegradable polymer coating obtained from a soybean processing byproduct. J Clean Prod 276. https://doi.org/10.1016/j.jclepro.2020.123014
Sanz-Cobena A, Misselbrook T, Camp V, Vallejo A (2011) Effect of water addition and the urease inhibitor NBPT on the abatement of ammonia emission from surface applied urea. Atmos Environ. 45:1517–1524. https://doi.org/10.1016/j.atmosenv.2010.12.051
Shen YZ, Wang B, Zhu SX, Xie W, Wang SQ, Zhao X (2022) Single application of a new polymer-coated urea improves yield while mitigates environmental issues associated with winter wheat grown in rice paddy soil. Field Crops Res 285:108592. https://doi.org/10.1016/j.fcr.2022.108592
Souza EFC, Rosen CJ, Venterea RT (2019) Contrasting effects of inhibitors and biostimulants on agronomic performance and reactive nitrogen losses during irrigated potato production. Field Crops Res 240:143–153. https://doi.org/10.1016/j.fcr.2019.05.001
Tan HY (2022) Preparation and performance study of slow-release fertilizer based on biodegradable polymer. Jilin University, China Jilin
Tian HY, Liu ZG, Zhang M, Guo YL, Zheng L, Li YC (2019) Biobased polyurethane, epoxy resin, and polyolefin wax composite coating for controlled-release fertilizer. ACS Appl Mater Interfaces 11:5380–5392. https://doi.org/10.1021/acsami.8b16030
Tian Z, Wang JJ, Liu S, Zhang ZQ, Dodla, Myers G (2015) Application effects of coated urea and urease and nitrification inhibitors on ammonia and greenhouse gas emissions from a subtropical cotton field of the Mississippi Delta Region. Sci Total Environ 533:329–338. https://doi.org/10.1016/j.scitotenv.2015.06.147
Wang MY, Qi ZL, Dai XL, He MR, Dong YJ (2021) Effects of different slow/controlled release urea on wheat growth and nitrogen utilization. J Plant Nutr Fertil 27:643–653. https://doi.org/10.11674/zwyf.20443
Yang YC, Zhang M, Zheng L, Cheng DD, Liu M, Geng YQ (2011) Controlled release urea improved nitrogen use efficiency, yield, and quality of wheat. Agron J. 103:479–485. https://doi.org/10.2134/agronj2010.0343
Zhang GX, Liu SJ, Dong YJ, Liao YC, Han J (2022) A nitrogen fertilizer strategy for simultaneously increasing wheat grain yield and protein content: Mixed application of controlled-release urea and normal urea. Field Crops Res 277:108405. https://doi.org/10.1016/j.fcr.2021.108405
Zhang LN (2022) Effects of multi-modification on coating structure and nitrogen release characteristics of bio-based polyurethane-coated urea. Shandong Agricultural Unversity, China Taian
Zhang L, Liang ZY, Hu YC, Schmidhalter, Zhang WS, Ruan SY, Chen XP (2021) Integrated assessment of agronomic, environmental and ecosystem economic benefits of blending use of controlled-release and common urea in wheat production. J Clean Prod 287:125572. https://doi.org/10.1016/j.jclepro.2020.125572
Zhao X, Xie YX, Xiong ZQ, Yan XY, Xing GX, Zhu ZL (2009) Nitrogen fate and environmental consequence in paddy soil under rice-wheat rotation in the Taihu Lake Region, China. Plant Soil 319:225–234. https://doi.org/10.1007/s11104-008-9865-0
Zheng W, Min Z, Liu Z, Zhou H, Chen B (2016) Combining controlled-release urea and normal urea to improve the nitrogen use efficiency and yield under wheat-maize double cropping system. Field Crops Res 197:52–62. https://doi.org/10.1016/j.fcr.2016.08.004
Zheng WK, Liu ZG, Zhang M, Shi YF, Zhu Q, Sun YB, Zhou HY, Li CL, Yang YC, Geng JB (2017) Improving crop yields, nitrogen use efficiencies, and profits by using mixtures of coated controlled-released and uncoated urea in a wheat-maize system. Field Crops Res 205:106–115. https://doi.org/10.1016/j.fcr.2017.02.009
Zho Z, Shen Y, Du C, Zhou J, Qin Y, Wu Y (2017) Economic and soil environmental benefits of using controlled-release bulk blending urea in the North China Plain. Land Degrad Dev 28:2370–2379. https://doi.org/10.1002/ldr.2767
Funding
This research was funded by the National Major Agricultural Science and Technology Program of China (No. NK2022180803) and the Agricultural fiber application project of Zibo Huayuan Science and Technology Innovation Development Co., Ltd. of China (No. 381192).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, G., Wang, M. & Dong, Y. Enhanced-Efficiency Nitrogen Fertilizers Coated with Sulfur, Inhibitor, and Epoxy Resin: Preparation and Effects on Maize Growth and Nitrogen Utilization. J Soil Sci Plant Nutr 23, 4091–4103 (2023). https://doi.org/10.1007/s42729-023-01326-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01326-z