Abstract
The ongoing deterioration of the earth's natural resources and the increase in the use of chemical fertilizers pose serious concerns about the future of agriculture. Biofertilizers are increasingly being used as a substitute for synthetic fertilizers because they are perceived as being more sustainable. The objective of this study was to evaluate the effect of plant growth-promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) on the growth, biochemical properties, and nutritional status of potato (Solanum tuberosum L.) minitubers. A pot experiment was conducted via inoculation of PGPR at five levels (Azotobacter chroococcum and Pseudomonas putida at rates of 100 and 200 mL, and control) separately and in interaction with AMF at three levels (Funneliformis mosseae, Rhizophagus intraradices, and control) on potato plants. The factorial experiment was designed based on a completely randomized design with 15 treatments and five replicates. Co-inoculation of PGPR and AMF significantly improved plant growth and yield of potato minitubers. Accordingly, the interaction of P. putida at 100 mL and R. intraradices led to increase minituber number (116%), minituber weight (181%), shoot dry weight (248%), root dry weight (120%), chlorophyll (Chl) content (57%), carotenoid content (10%), ascorbic acid (8%), proline (18%), total soluble solids (TSS, 49%), TSS to titration acidity (TA, 46%), phosphorus (72%), potassium (27%), zinc (24%), and Fe (17%) compared with the control. Heat map analysis indicated that minitubers weight, shoot dry weight, and TSS had the higher variation in potato tubers with AMF and PGPR inoculations, which can be identified as indicators for further investigations. The interaction of R. intraradices and P. putida is effective in improving minituber yield of potato plants, which can be beneficial for producers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Potato (Solanum tuberosum L.) is from the Solanaceae family and is considered the most important food product in the world after wheat, corn, and rice because the tubers of this plant have high nutritional value (Mushtaq et al. 2022). Producing large quantities of mini-tubes with high quality and low cost, in a relatively short time and in an environmentally friendly way, is considered a challenge in many countries (Wasilewska-Nascimento et al. 2020) because potato tubers can carry many pathogenic organisms that either directly attack the underground parts of the plant or first infect the leaves and then accumulate in the tubers (Mushtaq et al. 2022). Disease-free microtubers produced under tissue culture conditions have been shown to increase potato yield. In addition, these tubers can be used in potato breeding programs, such as the use of methods for creating mutations or for gene transfer by agrobacterium. Microtubers can be produced and stored in the laboratory in large numbers throughout the year and they can be transported directly to the market without being transferred to a new environment. Additionally, microtubers can be grown directly in the field without an acclimatization (Wasilewska-Nascimento et al. 2020).
The lack of nutrients, particularly N and P, is one of the challenges that potato plants encounter when trying to develop. Beneficial soil microorganisms can improve the rhizosphere and aid in plant nutrition (Fasusi et al. 2021). Biofertilizers contain a selection of beneficial live microorganisms that, when applied to the surface of a plant, seed, or soil, can colonize the rhizosphere or interior of the host plant and promote plant growth by increasing nutrient availability (Riaz et al. 2021). Plant growth-promoting rhizobacteria (PGPR) live in close relationships with plants and can stimulate the absorption of nutrients and increase the level of plant hormones in tissues. Some of these bacteria can significantly increase the solubility of P, stable N, make antibiotics, produce siderophores, synthesize enzymes involved in ethylene production, increase the release and absorption of Mn, Zn, and, and improve the growth conditions (Fasusi et al. 2021). Azotobacter is important in the biological fixation of N and Pseudomonas are important in converting the insoluble forms of P into soluble forms available to the plant (Dar et al. 2021). The benefits of inoculating plants with PGPR have been shown to improve germination speed, root and shoot growth, control of pathogens, soil microbial activity, and the availability of nutrients for the plant. Soil bacteria are particularly important due to their catabolic diversity, high ability in root colonization, and their ability to produce a wide range of enzymes, siderophores, and metabolic substances (Begum et al. 2022; Riaz et al. 2021). In addition, PGPR can produce growth hormones such as indole acetic acid, cytokinins, and gibberellins (Riaz et al. 2021).
Using microorganisms, which are crucial in meeting the nutritional demands of plants, is an effective way to accomplish the aims of sustainable agriculture. Arbuscular mycorrhizal fungi (AMF) have the capacity to develop and penetrate compact soils and small soil pores through increasing access to immobile nutrients (Giovannini et al. 2020). The extensive network of external hyphae plays an important role in strengthening the absorption of water and nutrients from the soil, which is needed for the growth and increase of plant production. Additionally, AMF symbiosis improves phytoremediation efficiency, which can aid in the detoxification of soil contaminants and ultimately enhance plant performance (Sheteiwy et al. 2022). It has been reported that AMF can interfere with the stomatal conductance and alleviate the oxidative damage by promoting enzymatic and non-enzymatic antioxidant activities. Furthermore, AMF can regulate patterns of expression of aquaporin genes and osmolyte accumulations in plant tissues.
As a relatively simple and low-cost alternative strategy, the use of PGPR and AMF is a promising tool to improve plant growth. Due to the problems caused by the use of chemical fertilizers, the use of biological fertilizers has become increasingly important. Agriculture based on the use of biofertilizers with the goal of ceasing or drastically lowering the use of chemical inputs has received attention in recent decades (Daniel et al. 2022). Accordingly, various investigations have demonstrated the improvement of potato growth and yield by inoculation with AMF (Yooyongwech et al. 2016; Baradar et al. 2021; Mushtaq et al. 2022) and PGPR (Pathak et al. 2019; Ekin 2019; Batool et al. 2020). Several factors, such as soluble sugar content and titration acidity (TA), can be used to evaluate the quality of fruits and vegetables. The balance between these components greatly influences how the products taste and appear (Mikiciuk et al. 2019). However, the data about the simultaneous use of different types of AMF and PGPR on minitubers of potato is not available; therefore, this work aimed to discover the changes in growth, photosynthesis pigments, osmolytes, and minerals in minitubers of potato plants treated with an integrated application of AMF and PGPR. The findings can be beneficial to produce high quality minitubers for potato growers, which is a global issue.
2 Materials and Methods
2.1 Materials
The potato tuberlets of Agria cultivar were purchased from Zare Ghosta company, Iran. The PGPR were obtained from the Water and Soil Research Institute of Iran.
2.2 Growth Conditions and Experimental Treatments
This work was conducted as factorial based on a completely randomized design with five replicates. The tuberlets were cultivated in 3-L pots in a greenhouse with a photoperiod of 16/8 (light/ dark) and relative humidity of 65%-80% in the Mohagheg Ardabili University, Iran. The mean temperatures of day and night during the experiment were 27 ± 1 and 16 ± 9 °C, respectively. The soil was first sterilized with autoclaving at 180 °C for 2 h and 1 atmosphere steam pressure. Soil used for this study was loamy-clay with pH = 8.1, K = 21.2%, P = 0.83%, N = 0.12%, organic matter = 0.58%, and calcium carbonate = 15%.
The experiment was designed as factorial with PGPR (first agent) at five levels including Azotobacter chroococcum (67B) and Pseudomonas putida (P169) at 100 and 200 mL, and control. The second agent was AMF, which had three levels: F. mosseae (BEG 119), R. intraradices (DAOM 197,198), and control. Therefore, 15 treatments of the interactions of PGPR and AMF were obtained. All strains of AMF and PGPR were provided by the Soil and Water Research Institute, Karaj, Iran. The strains of A. chroococcum (67B) and p. putida had previously been isolated from wheat fields that had been intensively cultivated, and they had been recorded with the World Catalogue of Microorganisms under the designation CCSM (Culture Collection of Soil Microorganisms) at the Soil and Water Research institute in Karaj, Iran. A. chroococcum has multiple plant growth promotion activities like N 2-fixing activity, synthesis of siderophores, and indole-3-aceticacid stand (Viscardi et al. 2016) and P. putida has an ability to solubilize phosphates and produce auxin, which aid in the growth of plants (Safari et al. 2020). Both species of AMF had a close symbiosis with plants through providing water and fundamental nutrients for corresponding plants (Darakeh et al. 2022). For inoculation with AMF inoculum containing spores, hyphae, inoculated root segments of sorghum, and sand was applied at the rate of 50 g per plant around the roots (30 spores per g of soil) when plants were cultivated (El-Sawah et al. 2021). After that, PGPR suspension was also prepared with a concentration of 109 CFU/mL around the roots. The inoculum was made by re-culturing in nutrient broth (Merck) and shaking (120 rpm) for 36 h at 28 °C. The bacteria culture was then centrifuged at 5,000 xg for 10 min at 4 °C. Freshly generated pallet was suspended in sterile distilled water, and the bacterial suspension's concentration was adjusted to 109 colony-forming units (CFU) mL−1. Irrigation continued until the end of the experiment (90 days) with distilled water based on soil field capacity (Darakeh et al. 2022).
3 Weight of Plant Tissues
After harvesting the plants, shoot parts were cut from underground parts, and then the shoots and roots were separately dried in an oven at of 60 °C for 48 h to measure the weights by a digital scale (0.001 g) (Ibrahim and El-Sawah 2022).
4 Chlorophyll (Chl) Assay
Leaf Chl contents were measured according to the method of Arnon (1949). 0.5 g of fresh leaves were ground using liquid nitrogen. The ground leaves were then mixed with 20 mL acetone (80%) and centrifuged for 10 min at 6000 xg. The supernatants were measured at 645 and 663 nm by a spectrophotometer (Shimadzu UV-160) and used in the following equations:
Where A645 and A663 are the absorbance values read at 645 and 663 nm, respectively; V is the final volume of acetone consumed in mL; W is fresh tissue weight.
4.1 Proline Concentration
To determine the proline content, 0.1 g of fresh leaf tissue was mixed with 10 mL of sulfosalicylic acid (3% w / v) and centrifuged at 4000 xg for 20 min. Then, the mixture was supplied with 2 mL of ninhydrin acid and 2 mL of glacial acetic acid. Simultaneously, 2 mL of standard 0, 4, 8, 12, 16, 20 mg of proline were mixed with 2 mL of ninhydrinic acid and 2 mL of acetic acid. The samples were read at 520 nm using a spectrophotometer (Bates et al. 1973).
4.2 Ascorbic Acid (Vitamin C) Measurement
The titration method was used to measure ascorbic acid (Chua et al. 2000). Accordingly, 2 mL of filtered minituber extract was mixed with 2 mL of stabilizing solution of trichloroacetic acid and then titrated with indophenol reagent using a magnetic stirrer until the color changed pink. The stability of the pink color was based on the amount of indophenol consumed in the sample and the ascorbic acid standard (Merck, Germany), the amount of ascorbic acid was measured.
4.3 Preparation of Minituber Extraction
The tissue samples of minitubers were frozen in liquid nitrogen. Then, 5 g tissue was homogenized in 10 mL 50 mmol L–1 phosphate buffer at pH 7.8. The homogenate was centrifuged at 15,000 × g at 4 °C for 20 min and the supernatant (minituber extract) was obtained to determine further analyses.
4.4 Titration Acidity (TA) Determination
In an Erlenmeyer flask, TA was determined by combining 5 mL of minitubers extract with 20 mL of distilled water. The pH meter electrode was inserted into the solution, and titration with NaOH (0.1 N) was then performed at 20 °C. When the solution's pH reached 1.8, the titration process was considered complete. The amount of acid in the minituber extract was determined as 1 g of acid per 100 mL of minituber extract (%) based on the amount of NaOH used during the titration (Yaghoubi Farani et al. 2019):
Where A is the percentage of TA in minituber extract (g/100 mL); V1 is the volume of NaOH (mL); N is the normality of NaOH, and V0 is the amount of minituber extract (mL).
4.5 Total Soluble Solids (TSS)
To measure TSS, some drops of minituber extract were put in a refractometer. Before the measurement, the refractometer was calibrated with distilled water and the results were recorded as Brix (McCready et al. 1950).
4.6 Soil Minerals
For the available N status of the soil, the method outlined in Subbiah and Asija (1956) was used. The standard approach developed by Olsen (1954) was used to estimate the amount of accessible P in the soil. Diethylene triamine penta acetic acid (DTPA) was utilized following the method outlined in Lindsay and Norvell (1978) to estimate the amount of Zn in the soil.
4.7 Tuber Minerals
Mineral contents in potato minitubers were measured using the dry ash technique. Nitrogen was measured by the Kjeldahl method (Bremner 1996) and P was measured by Olsen's method at a wavelength of 720 nm (Shimadzu UV3100 spectrophotometer) (Olsen 1954). According to Mason (1963), the photometric method was used to estimate K. To determine the content of Fe and Zn, an atomic absorption device (AAS) was used (Karadjova et al. 2002).
4.8 Data Analysis
For data analysis, the Windows version of SAS (SAS, version 9.3, SAS Institute, Cary, NC) was used. LSD test was used to compare the mean values and the data were statistically assessed at a 5% probability level (P ≤ 0.05). Principal component analysis (PCA) and agglomerative hierarchical clustering (AHC) were performed by XLSTAT. In PCA, the data were mainly determined by two factors; the first factor was axis 1 (F1) and the second one was axis 2 (F2).
5 Results
5.1 Minituber Number and Weight
The number and weight of potato minitubers significantly increased with the PGPR and AMF. The minitubers number ranged from 2 tubers in non-application of the biofertilizers to 4.33 minitubers in the interaction of P. putida at 100 mL and R. intraradices. All plants treated with the PGPR and AMF represented the higher minitubers number compared with control plants (Table 1). In addition, tuber weight increased with the PGPR and AMF. The highest minitubers weight was obtained in plants treated with the interaction of P. putida at 100 mL and R. intraradices, with a 181% increase relative to the control. In non-AMF inoculated plants, P. putida at 100 and 200 mL increased minitubers weight by 83 and 100%, and A. chroococcum at 100 and 200 enhanced this parameter by 55 and 73%, respectively, compared with the control (Table 1). As a result, the simultaneous use of P. putida at 100 mL and R. intraradices led to notable enhancement of minituber yield.
5.2 Shoot and Root Weight
Shoot and root weight were significantly (P ≤ 0.05) improved by the PGPR and AMF. The interaction of F. mosseae and P. putida at 100 yielded a higher shoot dry weight (3.3 g) compared with other plants. Under non-PGPR application, R. intraradices and F. mosseae yielded the higher shoot dry weight by 81 and 85%, respectively. Moreover, the simultaneous use of AMF and P. putida resulted in the optimum shoot weight of potato plants. P. putida at 100 mL along with R. intraradices and F. mosseae led to 248 and 266% raises in shoot weight relative to control plants (Table 1). Like shoot weight, a notable enhancement in root weight was observed when plants were treated with simultaneous use of PGPR and AMF. Plants treated with P. putida at 100 mL along with R. intraradices and F. mosseae represented 120 and 101% increases in root weight, respectively, compared to the control. Compared to the non-PGPR inoculation treatment, R. intraradices and F. mosseae respectively elevated the root weight of potato plants by 70 and 52% (Table 1). Hence, shoot and root weight showed higher amounts when P. putida at 100 mL was used with AMF.
5.3 Photosynthesis Pigments
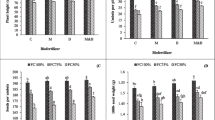
Chlorophyll (Chl) content increased with inoculation of PGPR and AMF, particularly when the plants were treated with a combination of PGPR and AMF. In plants inoculated with only AMF, total Chl increased by 32, 53, and 24%, respectively, when compared with control. For plants inoculated with F. mosseae, the 16 and 25% enhancements in total Chl for A. chroococcum at 100 and 200 mL and 36 and 22% raise in this trait for P. putida at 100 and 200 mL, respectively (Fig. 1a). Carotenoid showed the higher amounts when plants were treated with the PGPR and AMF, ranging from 0.30 mg g−1 in the control to 0.35 mg g−1 in the interaction of A. chroococcum at 200 mL and F. mosseae (Fig. 1b). Hence, co-application of PGPR and AMF can improve Chl amount more effectively than using either one alone.
Total chlorophyll (a) and carotenoid (b) contents of potato plants inoculated with plant growth-promoting rhizobacteria (PGPR) and Arbuscular mycorrhizal fungi (AMF). Values are means ± standard deviation (SD). Different letters show statistically significant differences among treatments at P ≤ 0.05. AC: Azotobacter chroococcum, PP: Pseudomonas putida
5.4 Ascorbic Acid and Proline
The biofertilizers used in the present study elevated ascorbic acid content. Accordingly, the interaction of P. putida at 200 mL and F. mosseae inoculation, and A. chroococcum and F. mosseae demonstrated 15 and 11% raises of ascorbic acid relative to the control (Fig. 2a). Proline showed the same trend of ascorbic acid upon the PGPR and AMF. The maximum proline accumulation (67. 1 µmol g−1) was observed in plants treated with P. putida at 100 mL and R. intraradices, with an 18% improvement as compared to the control (Fig. 2b). As a result, all PGPR and AMF had a positive role in improving ascorbic acid and proline accumulation.
Ascorbic acid (a) and poline (b) contents of potato plants inoculated with plant growth-promoting rhizobacteria (PGPR) and Arbuscular mycorrhizal fungi (AMF). Values are means ± standard deviation (SD). Different letters show statistically significant differences among treatments at P ≤ 0.05. AC: Azotobacter chroococcum, PP: Pseudomonas putida
5.5 Total Soluble Solid (TSS), Titration Acidity (TA), and TSS/TA
The PGPR and AMF led to increased TSS especially when plants were inoculated with both PGPR and AMF. In plants inoculated with R. intraradices, 31, 34, 46, and 50% increases in TSS relative to the control were observed with A. chroococcum at 100 and 200 mL and P. putida at 100 and 200 mL, respectively. In addition, for plants treated with P. putida at 200 mL, R. intraradices and F. mosseae elevated the TSS by 30 and 18%, respectively, compared to without AMF inoculation (Fig. 3a). In addition, TA of potato minituber ranged from 0.37% in control to 0.42% in R. intraradices without PGPR inoculation (Fig. 3b). Accordingly, TSS/TA showed an increasing trend with the use of PGPR and AMF. In plants inoculated with R. intraradices, the use of A. chroococcum at 100 and 200 mL and P. putida at 100 and 200 mL increased by 41, 42, 64, and 56% compared to non-PGPR treatment, respectively. Among the AMF, R. intraradices possessed a notable role in boosting TSS/TA (Fig. 3c). Therefore, the inoculation with R. intraradices and PGPR significantly improved TSS/TA, a key indicator of potato quality.
Total soluble solids (a), titration acidity (b), and TSS/TA (c) of potato minitubers upon plant growth-promoting rhizobacteria (PGPR) and Arbuscular mycorrhizal fungi (AMF). Values are means ± standard deviation (SD). Different letters show statistically significant differences among treatments at P ≤ 0.05. AC: Azotobacter chroococcum, PP: Pseudomonas putida
5.6 Minerals
The PGPR and AMF led to notable changes in the minitubers minerals of potato plants. A. chroococcum was associated with increasing N content of potato minitubers. In addition, A. chroococcum at 200 mL along with F. mosseae resulted in a 17% improvement in the N content in the potato minitubers compared with the control (Table 2). All biofertilizers, particularly P. putida, raised P content in potato minitubers, with the greatest increase occurring with the treatment that combined P. putida at 100 mL and R. intraradices. In addition, K significantly increased with concurrent use of all biofertilizers. The treatment that combined P. putida at 100 mL and F. mosseae increased K content by 40% (Table 2). Zinc, a main micronutrient for plants, was substantially enhanced by bacterial and fungal inoculations. The treatment combining P. putida at 100 mL and R. intraradices led to optimum improvement (24%) in Zn concentration in potato minitubers relative to the control (Table 2). Moreover, Fe content showed higher amounts when plants were treated with bacterial and fungal inoculation. It represented a 17% modification with a difference from 1.18 mg g−1 in the control plants to 1.39 mg g−1 in plants inoculated with P. putida at 100 mL and R. intraradices (Table 2). Both AMF and PGPR are useful for improving minitubers minerals, but they have different effects.
5.7 Multivariate Analysis
According to PCA, F1 mainly justified the changes (75.15%), while F2 only expanded 12.6% of the variations under PGPR. All studied traits except titration acidity and minituber nitrogen were justified by F1. Furthermore, F1 determined all treatments except A. chroococcum at 200 mL (Fig. 4a). The similar pattern was obtained for AMF with 87.08% for F1 and 12.92% for F2. All studied treatments and traits except N were justified by F1 (Fig. 4b).
Principal component analysis for traits and treatments of potato plants upon plant growth-promoting rhizobacteria (a) and Arbuscular mycorrhizal fungi (b). TN: tuber number, TW: tuber weight; SDW: shoot dry weight, RDW: root dry weight, Chl: Chlorophyll; AA: Ascorbic acid; TSS: total soluble solids; TA: titration acidity; Az: Azotobacter chroococcum; PP: Pseudomonas putida
According to AHC analyses, four different clusters were determined for PGPR including cluster 1: control, cluster 2: A. chroococcum at 100 and 200 mL, cluster 3: P. putida at 100 mL, and cluster 4: P. putida at 200 mL (Fig. 5a). Moreover, three cluster were specified for the three treatments of AMF (Fig. 5b).
5.8 Heat Map
Heat map results indicated the changes of experimental traits upon the treatments, varying from blue (the minimum value) to red (the maximum value). Accordingly, minituber P, minituber weight, and shoot dry weight posseted the maximum variability under PGPR (Fig. 6a). In addition, root and shoot dry weight, minituber weight, TSS, and total Chl showed the higher modifications upon the AMF inoculation (Fig. 6b).
Heat map for traits and treatments of potato plants upon plant growth-promoting rhizobacteria (a) and Arbuscular mycorrhizal fungi (b). TN: tuber number, TW: tuber weight; SDW: shoot dry weight, RDW: root dry weight, Chl: Chlorophyll; AA: Ascorbic acid; TSS: total soluble solids; TA: titration acidity; Az: Azotobacter chroococcum; PP: Pseudomonas putida
6 Discussion
The present study indicated that combining AMF and PGPR is more effective than their separate application in improving plant yield. As a result, the results suggest there is a synergistic relationship between PGPR and AMF, which results in an increase in the activity of microorganisms in the soil. The greater microbial activity likely increases the absorption of mineral elements, especially P and N, which improves photosynthetic processes and ultimately results in greater tuber number and weight (Pathak et al. 2019). It appears that inoculating potato tubers with PGPR and AMF, in addition to stimulating the production of growth regulators, improved the root system, increased the plant's access to water and nutrients, and finally increased tuber weight (Yasmeen et al. 2019). It is also possible that these microorganisms can stimulate the production of siderophore and solubilize P (Giovannini et al. 2020). Moreover, PGPR and AMF directly affect the physical and chemical properties of the soil, thereby increasing the weight and number of potato tubers. Past research has also shown an increase in tuber number and weight in potatoes when inoculated by AMF alone (Yooyongwech et al. 2016; Ghobadi et al. 2020), and PGPR (Ekin 2019; Batool et al. 2020; Mushtaq et al. 2022), and with PGPR (Pathak et al. 2019).
The substantial increase in plant weight in the presence of biofertilizers may be due to the stimulation of physicochemical and biological properties of the soil, as well as significant impacts on the water holding capacity, which enhances plant growth and development (Tahiri et al. 2022). The interaction between PGPR with AMF enhances the growth of the plant's vegetative and reproductive organs, which leads to an increase in biomass yield by delivering the proper amounts of essential elements including N, P, and K (Pathak et al. 2019). Additionally, as nitrogen plays a crucial function in the development of chlorophyll and is the most effective element in the construction of proteins, a symbiotic relationship between plants and biostimulants boosts N availability and improves plant weight (Mishra et al. 2016). PGPRs are able to synthesize phytohormones that stimulate cell growth and cell division. Abscisic acid (ABA), Indole-3-acetic acid (IAA), gibberellins, and cytokinins (CKs), which are crucial for plant growth, are only a few examples of growth regulators that are promoted by the biostimulators (Meena et al. 2020). Abscisic acid (ABA) controls plant responses to diverse environmental challenges and plays a crucial role in several phases of the plant life cycle, including seed development and dormancy. IAA is a key shoot signal that controls all facets of plant vascular development. It plays a crucial part in controlling plant growth and manages cell elongation, the formation of vascular tissue, and apical dominance. Furthermore, cytokines (CKs) are essential for a variety of plant growth and development processes, such as cell division, chloroplast biogenesis, apical dominance, leaf senescence, vascular differentiation, nutrient mobilization, shoot differentiation, anthocyanin synthesis, and photomorphogenic development. Gibberellic acid (GA) is a class of tetracyclic diterpenes that has a significant impact on a number of biological processes, including seed germination, leaf growth, stem lengthening, and fruit development. As a result, by supporting phytohormones, PGPR and AMF significantly contribute to plant growth. Similarly, Baradar et al. (2021) demonstrated the interaction between P. fluorescens and AMF (F. mosseae and R. intraradices) had a positive role in the growth of potato plants.
The present study showed that the combined use of PGPR and AMF has a notable role in photosynthesis pigments. The quantity of photosynthetic pigments is one of the most important factors affecting photosynthetic performance (Meena et al. 2020). It has been discovered that AMF and PGPR play a beneficial function in expanding leaf surfaces, which in turn increases the amount of chlorophyll on the leaves. An improved stomatal function may improve the uptake of CO2 by leaves. The symbiotic relationship between AMF and PGPR and plants can increase the activity of the Rubisco enzyme, chloroplast activity, ATP availability, ribulose 1,5-bs phosphate production, and leaf N (Zahra et al. 2022). By providing essential elements for the plant and increasing the amount of water in the plant cells, biostimualtors may increase the chlorophyll in plant leaves (Begum et al. 2022). The parameters rated to plant growth like Chl and carotenoid were greatly improved by P. putida at 100 mL, but they were not further changed when the concentration increased to 200 mL. It demonstrated that the amount of PGPR has a significant impact on the ability for photosynthesis. Perhaps more than 200 mL of P. putida imbalances ion uptake and causes a reduction in Chl and carotenoid concentration. The beneficial role of AMF and PGPR in improving photosynthesis content has been reported on tobacco (Begum et al. 2022) and Russian knapweed (Rasouli-Sadaghiani et al. 2019).
TSS and TA content act as a proxy for fruit flavor and the TSS/TA ratio is primarily used to assess the flavor and physiological ripeness of fruits and tubers (Mikiciuk et al. 2019). The reaction of plants to biofertilizers was increased TSS and TSS/TA, which was previously demonstrated in strawberry (Mikiciuk et al. 2019). Although TSS and TA have primarily been studied in relation to fruits (Mikiciuk et al. 2019; Ait Rahou et al. 2022), the current experiment suggests that combining PGPR and AFM may improve the quality of potato tubers. Although the role of AMF and PGPR in TSS and TA of potato tubers has only rarely been addressed, Maruf et al. (2019) indicated the increase of TSS during storage time and a decrease by vermicompost. Because of this, TSS may react differently to organic and biofertilizers.
The interaction of AMF and PGPR increases the nutrients including N, P, Zn, and Fe in potato minituber. These elements play an essential part in the development of higher plants as well as several biochemical processes that take place within plant cells. AMF's large network of exterior hyphae excels at giving plants the necessary nutrients. Plant nutrition, which is commonly thought of as a plant mechanism to increase growth, may be improved by the AMF and PGPR symbiosis (Weisany et al. 2016). Mycorrhizae are beneficial for P nutrition and the influx of P in AMF-colonized roots can be 3–5-fold higher compared to that in non-mycorrhizal roots (Wipf et al. 2019). AM contributes to plant growth via the assimilation of immobile soil nutrients such as P and Zn. It has been reported that AMF hyphae can transfer immobile P resources to the roots, and thus play a more important role in P uptake by their host plants. AMF can create a network with plant roots to enhance inorganic P acquisition by providing organic acids and phosphatases (Weisany et al. 2016). The increased minerals in potato tubers because of the symbiosis of AMF and plants were demonstrated by Lone et al. (2020). Moreover, Darakeh et al. (2022) showed that Bio and organic fertilizers significantly improve the root and leaf nutrients of black cumin. In addition, PGPR play a significant role in growth and development of plant through providing the availability of nutrients in rhizosphere (Baradar et al. 2021). For instance, most PGPR can solubilize P, which increases the amount of P ions accessible in the soil for plant absorption (Giovannini et al. 2020). Similar to our findings, the improvement of mineral nutrients by PGPR have been reported in different plant species (Tinna et al. 2020; Bourles et al. 2020; Mushtaq et al. 2022). This enhancement could also be attributed to the rhizosphere's organic acid synthesis by bacteria and plants, which lowers soil pH and increases P, Ca, Fe, and Mn availability (Mushtaq et al. 2022). Among the biofertilizers, A. chroococcum had a significant role in enhancing N, which enhances mineralization of organic forms of N in soil and increases root systems in plants (Mushtaq et al. 2022).
Inoculation with AMF and PGPR enhanced the concentrations of ascorbic acid and proline. Ascorbic acid is a cofactor for many enzymes in plants. This vitamin is also involved in a variety of processes, including photosynthesis, cell wall growth, cell growth and division, resistance to environmental stresses, ethylene and gibberellin synthesis, anthocyanins, and hydroxyproline synthesis (Veljović-Jovanović et al. 2017). A biostimulator symbiosis can change the metabolic pathways of ascorbic acid (Yasmeen et al. 2019; Rasouli et al. 2022). Similarly, increased ascorbic acid was observed in lettuce plants exposed to AMF and seaweed extract (Rasouli et al. 2022). Proline functions not only as an osmotic protector but also as an antioxidant, stabilizing the interaction of membrane and protein structures, balancing cytoplasmic acidity, maintaining NADP/NADPH in interaction with metabolism, especially during stress, and providing the necessary regeneration factors to maintain mitochondrial oxidative phosphorylation in the direction of ATP production (Cacefo et al. 2023). The differences in proline levels between treatments can be attributed to the concentration and type of biostimulators, which produce varying amounts of proline. Darakeh et al. (2022) on black cumin reported increased proline under biostimulators, which is consistent with the current findings. Eshaghi Gorgi et al. (2022) also addressed the improvement of proline in Melissa officinalis L. after PGPR and AMF inoculations.
According to PCA, there was a discernible correlation between F1 and ascorbic acid, TSS, Zn, and tuner number. Negative correlations were found between the control and A. chroococcum at 200 mL and P. putida at 100 and 200 mL. In the first quadrant, N, K, P, ascorbic acid, TA, Chl, and Zn serve as the notable proxies for R. intraradices in the fourth quadrant, while others provide the description of F. mosseae in the fourth quadrant. It can be discovered that AMF symbioses were negatively correlated with the control. According to the heat map, tuber weight, shoot dry weight, and TSS were the higher modifications of potato tubers upon the AMF and PGPR inoculations; therefore, they can be determined as indicators for further investigations in potato tuber. Like our results, the most variable traits under different treatments of fertilizers have been reported by Nasirzadeh et al. (2022), Ghasemzadeh et al. (2022).
7 Conclusions
The present study showed that the co-application of arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria is more effective than their separate application on the growth and yield of potato minitubers. In addition, based on the type and concentration of the biostimulators, different results can be obtained on plant yield, and accordingly, the interaction of Rhizophagus intraradices and Pseudomonas putida at 100 mL was more successful. This treatment also increased the ratio of total soluble solids, which is critical in determining potato quality and ripening. It is possible to establish a scenario for potato farmers based on economic and qualitative factors using the nutritional value, total solids, and titration acid under beneficial biostimulators.
References
Ait Rahou Y, Douira A, Tahiri AI, Cherkaoui EM, Benkirane R, Meddich A (2022) Application of plant growth-promoting rhizobacteria combined with compost as a management strategy against Verticillium dahliae in tomato. Can J Plant Pathol 30:1–22. https://doi.org/10.1080/07060661.2022.2089235
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Baradar A, Saberi-Riseh R, Sedaghati E, Vatankhah M (2021) Interaction between Pseudomonas fluorescens, Arbuscular mycorrhizae and iron chelates and their potential for controlling Rhizoctonia solani on potato. J Plant Pathol 103:1221–1230. https://doi.org/10.1007/s42161-021-00894-2
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Batool T, Ali S, Seleiman MF, Naveed NH, Ali A, Ahmed K, Mubushar M (2020) Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci Rep 10:1–19. https://doi.org/10.1038/s41598-020-73489-z
Begum N, Wang L, Ahmad H, Akhtar K, Roy R, Khan MI, Zhao T (2022) Co-inoculation of arbuscular mycorrhizal fungi and the plant growth-promoting rhizobacteria improve growth and photosynthesis in tobacco under drought stress by up-regulating antioxidant and mineral nutrition metabolism. Microb Ecol 83:971–988. https://doi.org/10.1007/s00248-021-01815-7
Bourles A, Guentas L, Charvis C, Gensous S, Majorel C, Crossay T, Amir H (2020) Co-inoculation with a bacterium and arbuscular mycorrhizal fungi improves root colonization, plant mineral nutrition, and plant growth of a Cyperaceae plant in an ultramafic soil. Mycorrhiza 30:121–131. https://doi.org/10.1007/s00572-019-00929-8
Bremner JM (1996) Nitrogen‐total. Methods of soil analysis: Part 3 Chemical methods. 5:1085–1121. https://doi.org/10.2136/sssabookser5.3.c37
Cacefo V, Ribas AF, Vieira LGE (2023) Proline metabolism as a mechanism for the energy dissipation in VaP5CSF129A transgenic tobacco plants under water deficit. J Plant Physiol 283:153964. https://doi.org/10.1016/j.jplph.2023.153964
Chua KJ, Chou SK, Ho JC, Mujumdar AS, Hawlader MNA (2000) Cyclic air temperature drying of guava pieces: effects on moisture and ascorbic acid contents. Food Bioprod Process 78:72–78. https://doi.org/10.1205/096030800532761
Daniel AI, Fadaka AO, Gokul A, Bakare OO, Aina O, Fisher S, Klein A (2022) Biofertilizer: the future of food security and food safety. Microorganisms 10:1–16. https://doi.org/10.3390/microorganisms10061220
Dar SA, Bhat RA, Dervash MA, Dar ZA, Dar GH (2021) Azotobacter as biofertilizer for sustainable soil and plant health under saline environmental conditions. In: Hakeem KR, Dar GH, Mehmood MA, Bhat RA (eds) Microbiota and Biofertilizers. 1st edn. Springer, Cham, pp 231–254. https://doi.org/10.1007/978-3-030-48771-3_14
Darakeh SASS, Weisany W, Tahir NAR, Schenk PM (2022) Physiological and biochemical responses of black cumin to vermicompost and plant biostimulants: Arbuscular mycorrhizal and plant growth-promoting rhizobacteria. Ind Crop Prod 188:115557. https://doi.org/10.1016/j.indcrop.2022.115557
Ekin Z (2019) Integrated use of humic acid and plant growth promoting rhizobacteria to ensure higher potato productivity in sustainable agriculture. Sustainability 11:3417. https://doi.org/10.3390/su11123417
El-Sawah AM, El-Keblawy A, Ali DFI, Ibrahim HM, El-Sheikh MA, Sharma A, Sheteiwy MS (2021) Arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria enhance soil key enzymes, plant growth, seed yield, and qualitative attributes of guar. Agriculture 11:1–19. https://doi.org/10.3390/agriculture11030194
Eshaghi Gorgi O, Fallah H, Niknejad Y, Barari Tari D (2022) Effect of Plant growth promoting rhizobacteria (PGPR) and mycorrhizal fungi inoculations on essential oil in Melissa officinalis L. under drought stress. Biologia 77:11–20
Fasusi OA, Amoo AE, Babalola OO (2021) Characterization of plant growth-promoting rhizobacterial isolates associated with food plants in South Africa. Antonie Van Leeuwenhoek 114:1683–1708. https://doi.org/10.1007/s10482-021-01633-4
Ghasemzadeh N, Iranbakhsh A, Oraghi-Ardebili Z, Saadatmand S, Jahanbakhsh-Godehkahriz S (2022) Cold plasma can alleviate cadmium stress by optimizing growth and yield of wheat (Triticum aestivum L.) through changes in physio-biochemical properties and fatty acid profile. Environ Sci Pollut Res 29:35897–35907. https://doi.org/10.1007/s11356-022-18630-3
Ghobadi M, Dehnavi MM, Yadavi AR, Parvizi K, Zafari D (2020) Reduced P fertilization improves Fe and Zn uptake in potato when inoculated with AMF in P Fe and Zn deficient soil. Rhizosphere 15:100239. https://doi.org/10.1016/j.rhisph.2020.100239
Giovannini L, Palla M, Agnolucci M, Avio L, Sbrana C, Turrini A, Giovannetti M (2020) Arbuscular mycorrhizal fungi and associated microbiota as plant biostimulants: research strategies for the selection of the best performing inocula. Agronomy 10:1–14. https://doi.org/10.3390/agronomy10010106
Ibrahim HM, El-Sawah AM (2022) The mode of integration between azotobacter and rhizobium affect plant growth, yield, and physiological responses of pea (Pisum sativum L.). J Soil Sci Plant Nutr 22:1238–1251. https://doi.org/10.1007/s42729-021-00727-2
Karadjova I, Izgi B, Gucer S (2002) Fractionation and speciation of Cu, Zn and Fe in wine samples by atomic absorption spectrometry. Spectrochimica Acta - Part b: At Spectrosc 57:581–590. https://doi.org/10.1016/S0584-8547(01)00386-X
Lindsay WL, Norvell W (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428. https://doi.org/10.2136/sssaj1978.03615995004200030009x
Lone R, Alaklabi A, Malik JA, Koul KK (2020) Mycorrhizal influence on storage metabolites and mineral nutrition in seed propagated potato (Solanum tuberosum L.) plant. J Plant Nut 43:2164–2175. https://doi.org/10.1080/01904167.2020.1766075
Maruf M, Roy TS, Rajesh C, Jannatul F, Nowroz F, Rehana N (2019) Effect of vermicompost and tuber size on total soluble solids, sucrose and skin color of potato under ambient storage condition. Azari J Agric 6:58–66. https://doi.org/10.29252/azarinj.008
Mason JL (1963) Flame photometric determination of potassium in Unashed Plant Leaves. Anal Chem 35:874–875. https://doi.org/10.1021/ac60200a032
McCready RM, Guggolz J, Silviera V, Owens HS (1950) Determination of starch and amylose in vegetables. Anal Chem 22:1156–1158. https://doi.org/10.1021/ac60045a016
Meena M, Swapnil P, Divyanshu K, Kumar S, Zehra TYN, A, Upadhyay RS, (2020) PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J Basic Microbiol 60:828–861. https://doi.org/10.1002/jobm.202000370
Mikiciuk G, Sas-Paszt L, Mikiciuk M, Derkowska E, Trzciński P, Głuszek S, Rudnicka J (2019) Mycorrhizal frequency, physiological parameters, and yield of strawberry plants inoculated with endomycorrhizal fungi and rhizosphere bacteria. Mycorrhiza 29:489–501. https://doi.org/10.1007/s00572-019-00905-2
Mishra V, Gupta A, Kaur P, Singh S, Singh N, Gehlot P, Singh J (2016) Synergistic effects of Arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria in bioremediation of iron contaminated soils. Int J Phytoremediation 697–703. https://doi.org/10.1080/15226514.2015.1131231
Mushtaq Z, Asghar H, Zahir ZA, Maqsood M (2022) The interactive approach of rhizobacteria and l-tryptophan on growth, physiology, tuber characteristics, and iron concentration of potato (Solanum tuberosum L.). J Plant Growth Regul 41:1359–1366. https://doi.org/10.1007/s00344-021-10395-2
Nasirzadeh L, Kvarnheden A, Sorkhilaleloo B, Hervan EM, Fatehi F (2022) Foliar-applied selenium nanoparticles can alleviate soil-cadmium stress through physio-chemical and stomatal changes to optimize yield, antioxidant capacity, and fatty acid profile of wheat (Triticum aestivum L.). J Soil Sci Plant Nutr 1–12. https://doi.org/10.1007/s42729-022-00821-z
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate (No. 939). US Department of Agriculture
Pathak D, Lone R, Khan S, Koul KK (2019) Isolation, screening and molecular characterization of free-living bacteria of potato (Solanum tuberosum L.) and their interplay impact on growth and production of potato plant under mycorrhizal association. Sci Hort 252:388–397. https://doi.org/10.1016/j.scienta.2019.02.072
Rasouli F, Amini T, Asadi M, Hassanpouraghdam MB, Aazami MA, Ercisli S, Mlcek J (2022) Growth and Antioxidant Responses of Lettuce (Lactuca sativa L.) to Arbuscular Mycorrhiza Inoculation and Seaweed Extract Foliar Application. Agronomy 12:401. https://doi.org/10.3390/agronomy12020401
Rasouli-Sadaghiani MH, Barin M, Khodaverdiloo H, Siavash Moghaddam S, Damalas CA, Kazemalilou S (2019) Arbuscular mycorrhizal fungi and rhizobacteria promote growth of Russian knapweed (Acroptilon repens L.) in a Cd-contaminated soil. J Plant Growth Regulation 38:113–121. https://doi.org/10.1007/s00344-018-9815-x
Riaz U, Murtaza G, Anum W, Samreen T, Sarfraz M, Nazir MZ (2021) Plant Growth-Promoting Rhizobacteria (PGPR) as biofertilizers and biopesticides. In: Hakeem KR Dar GH, Mehmood MA, Bhat RA (eds) Microbiota and Biofertilizers. 1st edn. Springer, Cham, pp. 181–196. https://doi.org/10.1007/978-3-030-48771-3_11
Safari M, Kari Dolatabad H, Rivera NduU, NA, (2020) Protective effect of Pseudomonas spp. isolates and zinc on seed germination and β-amylase activity in wheat cultivars under cadmium stress. Acta Physiol Plant 42:1–10. https://doi.org/10.1007/s11738-020-03038-8
Sheteiwy MS, Ahmed M, Korany SM, Alsherif EA, Mowafy AM, Chen J, AbdElgawad H (2022) Arbuscular Mycorrhizal Fungus “Rhizophagus irregularis” impacts on physiological and biochemical responses of ryegrass and chickpea plants under beryllium stress. Environ Pollut 315:1–12. https://doi.org/10.1016/j.envpol.2022.120356
Subbiah BV, Asija GL (1956) A rapid method for the estimation of nitrogen in soil. Current Sci 26:259–260
Tahiri AI, Meddich A, Raklami A, Alahmad A, Bechtaoui N, Anli M, Oufdou K (2022) Assessing the potential role of compost, PGPR, and AMF in improving tomato plant growth, yield, fruit quality, and water stress tolerance. J Soil Sci Plant Nutr 22:743–764. https://doi.org/10.1007/s42729-021-00684-w
Tinna D, Garg N, Sharma S, Pandove G, Chawla N (2020) Utilization of plant growth promoting rhizobacteria as root dipping of seedlings for improving bulb yield and curtailing mineral fertilizer use in onion under field conditions. Sci Hort 270:1–8. https://doi.org/10.1016/j.scienta.2020.109432
Veljović-Jovanović S, Vidović M, Morina F (2017) Ascorbate as a key player in plant abiotic stress response and tolerance. In: Hossain M, Munné-Bosch S, Burritt D, Diaz-Vivancos P, Fujita M, Lorence A (eds) Ascorbic acid in plant growth, development and stress tolerance. Springer, Cham, pp. 49–109. https://doi.org/10.1007/978-3-319-74057-7_3
Viscardi S, Ventorino V, Duran P, Maggio A, De Pascale S, Mora ML, Pepe O (2016) Assessment of plant growth promoting activities and abiotic stress tolerance of Azotobacter chroococcum strains for a potential use in sustainable agriculture. J Soil Sci Plant Nutr 16:848–863. https://doi.org/10.4067/S0718-95162016005000060
Wasilewska-Nascimento B, Boguszewska-Mańkowska D, Zarzyńska K (2020) Challenges in the production of high-quality seed potatoes (Solanum tuberosum L.) in the tropics and subtropics. Agronomy 10:260–272. https://doi.org/10.3390/agronomy10020260
Weisany W, Zehtab-Salmasi S, Raei Y, Sohrabi Y, Ghassemi-Golezani K (2016) Can arbuscular mycorrhizal fungi improve competitive ability of dill+ common bean intercrops against weeds? Eur J Agron 75:60–71. https://doi.org/10.1016/j.eja.2016.01.006
Wipf D, Krajinski F, van Tuinen D, Recorbet G, Courty PE (2019) Trading on the arbuscular mycorrhiza market: from arbuscules to common mycorrhizal networks. New Phytol 223:1127–1142. https://doi.org/10.1111/nph.15775
Yaghoubi Farani A, Mohammadi Y, Ghahremani F (2019) Modeling farmers’ responsible environmental attitude and behaviour: A case from Iran. Environ Sci Pollut Res 26:28146–28161. https://doi.org/10.1007/s11356-019-06040-x
Yasmeen T, Tariq M, Iqbal S, Arif MS, Riaz M, Shahzad SM, Li T (2019) Ameliorative capability of plant growth promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) against salt stress in plant. In: Hasanuzzaman M, Hakeem K, Nahar K, Alharby H (eds) Plant Abiotic Stress Tolerance. Springer, Cham, pp 409–448. https://doi.org/10.1007/978-3-030-06118-0_17
Yooyongwech S, Samphumphuang T, Tisarum R, Theerawitaya C, Cha-Um S (2016) Arbuscular mycorrhizal fungi (AMF) improved water deficit tolerance in two different sweet potato genotypes involves osmotic adjustments via soluble sugar and free proline. Sci Hort 198:107–117. https://doi.org/10.1016/j.scienta.2015.11.002
Zahra N, Al Hinai MS, Hafeez MB, Rehman A, Wahid A, Siddique KH, Farooq M (2022) Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol Biochem 178:55–69. https://doi.org/10.1016/j.plaphy.2022.03.003
Author information
Authors and Affiliations
Contributions
The data were prepared by Roghieh Barzgari-Barogh, Davoud Hassnpanah, Behrouz Esmaeilpour, Sodabeh Jahanbakhsh Godehkahri, Sepideh Kalatejari; The initial draft was prepared by Roghieh Barzgari-Barogh and revised by the others.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barzegari Barogh, R., Hassanpanah, D., Esmaeilpour, B. et al. Co-Inoculation of Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Improve Growth, Biochemical Attributes, and Nutritional Status of Potato (Solanum tuberosum L.) Minitubers. J Soil Sci Plant Nutr 23, 3447–3460 (2023). https://doi.org/10.1007/s42729-023-01262-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01262-y