Abstract
Boron (B) is an essential micronutrient for vascular plants, and its availability in the soil represents a limiting factor for world agricultural production. 24-Epibrassinolide (EBR) is a biodegradable and eco-friendly plant steroid hormone, with multiple benefits connected to growth and development. We hypothesized that inadequate B supplementation could limit plant development, causing damage to leaf and root structures. Therefore, the objective of this research was to verify the possible contributions of EBR in root and leaf structures and biomass accumulation in soybean plants under inadequate B supplies (deficiency or toxicity). The experiment followed a completely randomized factorial design with two concentrations of 24-epibrassinolide (0 and 100 nM EBR, described as − EBR and + EBR, respectively) combined with three B supplies (0.6, 30, and 1500 µM B, described as low, control, and high supply of B). EBR alleviated the damages occasioned by the inadequate B supplies on root tissues, specifically maximizing the vascular cylinder, metaxylem, and epidermis, improving the nutritional status. This steroid also minimized the harmful effects of B stress on leaf anatomy, stimulating the epidermis on both leaf sides, palisade parenchyma, and spongy parenchyma; structures intrinsically related to protection and carbon dioxide availability to the photosynthetic process. Concomitantly, this steroid had a positive impact on biomass accumulation. These results are explained by beneficial actions on leaf structures and photosynthetic machinery. Therefore, our results demonstrate that the EBR application can improve soybean plants’ tolerance under inadequate B supplementation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soybean (Glycine max) is a leguminous species with high nutritional value, rich in proteins (Bamji and Corbitt 2017; Kim et al. 2006; Wang et al. 2008), being a commodity with broad importance in human and animal nutrition and industrial products (Goldsmith 2008; Nishinari et al. 2014). In the 2017/2018 harvest, world soybean production was around 336 million tons, with Brazil, the USA, and Argentina accounting for around 82% of world production. The root is the organ responsible for water and nutrient uptake, fixing the plant in the soil (Seago and Fernando 2013; Zeng et al. 2019), and is covered by the root epidermis. This tissue has direct contact with the soil solution, contributing to fluxes of ions (Du and Wei 2018; Javelle et al. 2011; Thomas et al. 2007). The cortex is adjacent to the epidermis, consisting of parenchymal cells with spaces between them, working as storage tissue (Enstone et al. 2003; Pérez Chaca et al. 2014). The endodermis corresponds to the last layer of the cortex, acting as a barrier aiming to modulate the water and nutrient supplies due to the presence of Casparian strips (Barberon et al. 2016; Lux et al. 2011; Purushothaman et al. 2013).
The leaf is the main organ responsible for photosynthesis, frequently presenting stomata in abaxial and adaxial faces (Maia et al. 2018), and is covered by the epidermis that protects this organ against abiotic stresses (Gao et al. 2019; Nemeskéri and Helyes 2019; Wyka et al. 2019). Stomata modulate essential processes, such as CO2 absorption, transpiration, and thermal regulation (Vezza et al. 2018). In soybean plants, the mesophyll is formed by the palisade and spongy parenchyma (Gonçalves et al. 2017; Lansing and Franceschi 2000) being found in chloroplasts and other organelles that act during the photosynthesis process (Ju et al. 2017).
Boron (B) is an essential micronutrient for vascular plants, and its availability in the soil represents a limiting factor for world agricultural production (Kobayashi et al. 2018). B deficiency can be triggered by inadequate soil conditions, such as high pH and low organic matter content, and environmental factors, such as drought, high temperature, and light (Atique-ur-Rehman et al. 2018). Another problem is that B is susceptible to leaching in soil because it is highly soluble and mobile, which can easily lead to plant deficiency after excessive rainfall (Goli et al. 2019). On the other hand, in arid and semi-arid regions, there can be problems related to the toxicity of this micronutrient (Nawaz et al. 2020), where B can be taken to the surface layers of the soil due to the combined actions linked to the evaporation process of the soil water and capillary process (Yau and Ryan 2008).
In plants, B has structural and metabolic functions, being intrinsically related to calcium in cell wall development, acting in protein synthesis, sugar transport, respiration, and carbohydrate metabolism (Reid 2014; Shireen et al. 2018). Additionally, the mobility and permeability of this nutrient can vary by species (Wimmer and Eichert 2013). The B deficiency reflects mainly on meristematic tissues (Hänsch and Mendel 2009), affecting the root growth and the development of seeds, flowers, and fruits (Herrera-Rodríguez et al. 2010; Oiwa et al. 2013), also limiting absorption and transport of water and nutrients, due to damages to the root structures, including the xylem vessels (Li et al. 2017). On the other hand, B excess is associated with oxidative stress, in which B toxicity causes the overproduction of reactive oxygen species (ROS), such as superoxide (O2−) and hydrogen peroxide (H2O2), causing an imbalance in metabolism, affecting the division and expansion of the plant cells (Farghaly et al. 2021; Sakamoto et al. 2011).
Brassinosteroids (BRs) are steroidal phytohormones that have multiple benefits on plant metabolism, including regulation of ion channels in the plasma membrane (Zhang et al. 2005), improvements in photosynthetic apparatus performance (Shahbaz et al. 2008), antioxidant metabolism (Zhang et al. 2008), and regulation of flowering and cell expansion (Clouse 2002). These steroids induce positive repercussions on root and leaf tissues (Maia et al. 2018; Oliveira et al. 2019; Ribeiro et al. 2019), being detected in plants exposed to nutritional stress (deficiency and/or excess) (Lima et al. 2018; Vriet et al. 2012; Zhang et al. 2008). Among the different types of BRs, 24-epibrassinolide (EBR) is the most used BR because it is considered the natural bioactive form and is biodegradable and highly efficient (Khripach 2000; Maia et al. 2018).
We hypothesize that inadequate B supplementation can limit plant development, causing damage to leaf and root structures (Huang et al. 2014; Li et al. 2017; Mei et al. 2016). Recent studies have demonstrated that EBR can play an important role in plant development, especially under stressful conditions, because this steroid triggers benefits in root metaxylem (Santos et al. 2020), in nutritional status (Lima et al. 2018), also favoring the maintenance of chloroplastic pigments (Rodrigues et al. 2020) and improving the growth (Ribeiro et al. 2020). Therefore, the objective of this research was to verify the possible contributions of EBR in root and leaf structures and biomass accumulation in soybean plants under inadequate B supplies.
2 Materials and Methods
2.1 Location and Growth Conditions
The experiment was performed at the Campus of Paragominas of the Universidade Federal Rural da Amazônia, Paragominas, Brazil (2°55′ S, 47°34′ W). The study was conducted in a greenhouse with temperature and humidity-controlled. The minimum, maximum, and median temperatures were 24, 33, and 25.1 °C, respectively. The relative humidity during the experimental period varied between 60 and 80%.
2.2 Plants, Containers, and Acclimation
Seeds of Glycine max (L.) Merr. var. M8644RR Monsoy™ were germinated and grown in 1.2-L pots filled with a mixed substrate of sand and vermiculite at a ratio of 3:1. The plants were cultivated under semi-hydroponic conditions containing 500 mL of distilled water for 4 days. A nutritional solution described by Pereira et al. (2019) was used for plant nutrition, with ionic strength beginning at 50% (5th day) and later modified to 100% after 2 days (7th day). After this period, the nutritional solution remained at total ionic strength.
2.3 Experimental Design
The experiment followed a completely randomized factorial design with two concentrations of 24-epibrassinolide (0 and 100 nM EBR, described as − EBR and + EBR, respectively) and three B supplies (0.6, 30, and 1500 µM B, described as low, control, and high supply of B). With five replicates for each of the six treatments, a total of 30 experimental units were used in the experiment, with one plant in each unit.
2.4 24-epibrassinolide Preparation and Application
Eight-day-old seedlings were sprayed with EBR or Milli-Q water (containing a proportion of ethanol equal to that used to prepare the EBR solution; described as − EBR) at 5-day intervals until day 28. Solution of EBR (Sigma-Aldrich, USA) (100 nM) was prepared by dissolving the solute in ethanol followed by dilution with Milli-Q water [ethanol: water (v/v) = 1:10000] (Ahammed et al. 2013a).
2.5 Plant Conduction and Boron Supplies
Plants received the following macro- and micronutrients contained in the nutrient solution in agreement with Pereira et al. (2019). For B treatments, H3BO3 was used at concentrations of 0.6 μM (low), 30 μM (control), and 1500 μM (high) applied over 12 days (days 16–28 after the start of the experiment). During the study, the nutrient solutions were changed at 07:00 h at 3-day intervals, with the pH adjusted to 5.5 using HCl or NaOH. On day 28 of the experiment, physiological and morphological parameters were measured for all plants, and leaf tissues were harvested for anatomical, biochemical, and nutritional analyses.
2.6 Chlorophyll Fluorescence, Gas Exchange, and Anatomical Variables
Chlorophyll fluorescence was measured as described by Maia et al. (2018). Gas exchange was evaluated following the calibration procedures described by Pereira et al. (2019). Samples were collected following the methodology of Oliveira et al. (2019) and O’Brien et al. (1964). Stomatal characterization was carried out according to Segatto et al. (2004).
2.7 Determination of Antioxidant Enzymes, Superoxide, and Soluble Proteins
Antioxidant enzymes (SOD, CAT, APX, and POX), superoxide, and soluble proteins were extracted from root tissues according to the method of Badawi et al. (2004). Total soluble proteins were quantified using the methodology described by Bradford (1976). The SOD assay was measured at 560 nm (Giannopolitis and Ries 1977), and the SOD activity was expressed in unit mg−1 protein. The CAT assay was detected at 240 nm (Havir and McHale 1987), with CAT activity expressed as μmol H2O2 mg−1 protein min−1. The APX assay was measured at 290 nm (Nakano and Asada 1981), and the APX activity was expressed in μmol AsA mg−1 protein min−1. The POX assay was detected at 470 nm (Cakmak and Marschner 1992), with the activity expressed in μmol tetraguaiacol mg−1 protein min−1. The determination of O2− was measured at 530 nm (Elstner and Heupel 1976).
2.8 Quantification of Hydrogen Peroxide, Malondialdehyde, and Electrolyte Leakage
Stress indicators (H2O2 and MDA) were extracted using the methodology described by Wu et al. (2006). H2O2 was measured with the procedures defined by Velikova et al. (2000). MDA was determined by the method of Cakmak and Horst (1991), using the extinction coefficient of 155 mM−1 cm−1. EL was measured according to Gong et al. (1998) and is calculated by the formula EL (%) = (EC1/EC2) × 100.
2.9 Determination of Photosynthetic Pigments, Nutrient Contents, and Biomass
The chlorophyll and carotenoid determinations were performed using a spectrophotometer (model UV-M51; Bel Photonics), according to the methodology of Lichtenthaler and Buschmann (2001). The determination of B, K, Ca, S, Cu, Mn, and Mo was carried out using an inductively coupled plasma mass spectrometer (model ICP-MS 7900; Agilent). The growth of roots, stems, and leaves was measured based on constant dry weights (g) after drying in a forced-air ventilation oven at 65 °C.
2.10 Data Analysis
The normality of residues was checked using Shapiro–Wilk test. Data were subjected to two-way ANOVA, and significant differences between the means were determined using the Scott–Knott test at a probability level of 5% (Steel et al. 2006). All statistical procedures used the Assistat 7.7 software.
3 Results
Steroid application increased B contents in treatments control and with low B supply
The low and high B supplies promoted changes in B contents in the root, stem, and leaf tissues of soybean plants (Table 1). Plants sprayed with EBR and submitted to low B presented increases in B contents of 56%, 2%, and 19% in root, stem, and leaf, respectively, compared with the same treatment without EBR. The control + EBR treatment had increases of 36%, 26%, and 4% in root, stem, and leaf, respectively. However, plants submitted to high B and sprayed with EBR presented reductions of 26%, 12%, and 6% in root, stem, and leaf, respectively.
EBR maximized the protection of roots and CO2 availability in leaves under B stress.
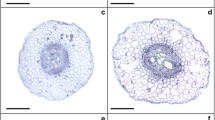
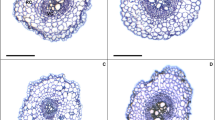
Low and high B supplies occasioned decreases in root anatomy (Table 2 and Fig. 1). Plants sprayed with EBR and exposed to low B treatment had increased RET (root epidermis thickness), RDT (root endodermis thickness), RCD (root cortex diameter), VCD (vascular cylinder diameter), and RMD (root metaxylem diameter) by 6%, 5%, 29%, 14%, and 4%, respectively when compared to the same treatment without EBR. While control + EBR treatment presented increases of 12% (RET), 15% (RDT), 21% (RCD), 6% (VCD), and 4% (RMD). To high B with EBR, the variables RET, RDT, RCD, VCD, and RMD had increases of 21%, 34%, 8%, 10%, and 12%, respectively. To leaf structures (Table 2 and Fig. 1), low and high B supplies promoted decreases, except for the PPT/SPT ratio. For ETAd, ETAb, PPT, and SPT plants sprayed with EBR under the low B treatment, we detected increases of 14%, 18%, 27%, and 32%, respectively, while the control treatment had increases of 3%, 18%, 6%, and 10%, respectively. High B presented increases of 20%, 8%, 14%, and 17% respectively. While for PPT/SPT, there were reductions of 4% (low B), 4% (control), and 4% (high B) in plants treated with EBR, if compared to equal treatment without EBR.
Root and leaf cross sections in soybean plants sprayed with 24-epibrassinolide (EBR) and exposed to different boron (B) supplies. B low/ − EBR (A), B low/ + EBR (B), B control/ − EBR (C), B control/ + EBR (D), B high/ − EBR (E), and B high/ + EBR (F). RE root epidermis, RC root cortex, RD root endodermis, VC vascular cylinder, RM root metaxylem, EAd adaxial epidermis, EAb abaxial epidermis, PP palisade parenchyma, SP spongy parenchyma. Black bars, 300 µm; grey bars, 150 µm
3.1 Nutritional Status was Improved after EBR Pretreatment
The low and high B supplies promoted reductions in nutrient contents in root, stem, and leaf tissues (Table 3). Plants sprayed with EBR and exposed to low B presented increases in K, Ca, S, Cu, Mn, and Mo contents of 21%, 1%, 16%, 33%, 17%, and 3% in the root; 6%, 2%, 21%, 25%, 1%, and 21% in the stem; and 9%, 14%, 10%, 13%, 7%, and 10% in leaf, respectively, when compared to the same treatment without EBR. The control treatment with EBR also had increases in K, Ca, S, Cu, Mn, and Mo of 21%, 14%, 24%, 9%, 7%, and 7% (root); 4%, 1%, 5%, 12%, 33%, and 9% (stem); and 8%, 6%, 11%, 11%, 14%, and 18% (leaf) in this order, when compared to the same treatment without EBR. Plants submitted to high B with EBR presented increments in K, S, Cu, Mn, and Mo of 8%, 16%, 5%, 25%, and 5%, but Ca suffered a reduction of 2% in the root. In the stem, there were verified increases in K, Ca, S, Cu, Mn, and Mo of 5%, 7%, 9%, 14%, 8%, and 11%. To leaf, we detected increases of 11%, 9%, 7%, 9%, 5%, and 14%, respectively, when compared to the same treatment without EBR.
3.2 B Stressed Plants Suffered Minor Oxidative Damage on Photosynthetic Machinery
The low and high supplementation with B reduced the photosynthetic pigments considering Chl a, Chl b, Total Chl, and Car variables (Table 4), causing increases in Chl a/Chl b and Chl/Car when compared to the control treatment. Plants sprayed with EBR and exposed to low B treatment had increased Chl b, Total Chl, and Car (by 8%, 2%, and 6%, respectively) compared to the same treatment without EBR; the control treatment showed increases of 26%, 33%, 28%, and 27%, respectively; and in the high B supply, there were increases of 5%, 24%, 9%, and 16% in Chl a, Chl b, Total Chl, and Car, respectively. In plants sprayed with EBR, the Chl a/Chl b, and Chl/Car ratios showed reductions compared to non-pulverized plants. Concerning chlorophyll fluorescence (Fig. 2), low and high B treatments promoted reductions, except F0. For F0, plants sprayed with EBR had reductions of 11%, 13%, and 2% for the low, control, and high B treatments, respectively, compared to the same treatment without EBR (Fig. 2A). In relation to Fm (Fig. 2B), Fv (Fig. 2C), and Fv/Fm (Fig. 2D) under the application of EBR, the low B supplement had increases of 1%, 4%, and 3%, respectively; in the control B + EBR treatment there were increases of 1%, 4%, and 4%, respectively; and the high B with EBR treatment presented increments of 4%, 6%, and 2%, respectively. On photosystem II (Table 4), low and high B supplies induced reductions in ΦPSII, qp, and ETR, while increases in NPQ, EXC, and ETR/PN presented increases compared to the control treatment. Plants sprayed with EBR and exposed to low treatment had increased ΦPSII, qp, and ETR by 21%, 6%, and 19%, respectively, whereas for NPQ, EXC, and ETR/PN decreased by 9%, 10%, and 32%, respectively. In the control treatment + EBR, ΦPSII, qp, and ETR presented increases of 12%, 10%, and 13%, respectively, whereas NPQ, EXC, and ETR/PN presented reductions of 5%, 5%, and 7%, respectively. The high B supply + EBR treatment showed increases of 13%, 14%, and 2% for the variables ΦPSII, ETR, and NPQ, respectively, but for qp, EXC, and ETR/PN, there were reductions of 7%, 6%, and 9%, respectively. Concerning gas exchange (Table 4), low and high B supplies caused reductions. Plants sprayed with EBR and exposed to low treatment resulted in increases for E, gs, PN, and PN/Ci of 77%, 133%, 79%, and 123%, respectively, whereas for Ci, there was a reduction of 20%. Control plants with EBR, PN, WUE, and PN/Ci presented increases of 21%, 55%, and 24%, respectively, while Ci, E, and gs had reductions of 3%, 21%, and 18%, respectively. The treatment with high B + EBR presented increases of 24%, 38%, and 25% in PN, WUE, and PN/Ci, respectively, and for Ci, E, and gs, there were reductions of 2%, 4%, and 8%, respectively.
Minimal fluorescence yield of the dark-adapted state F0 (A), maximal fluorescence yield of the dark-adapted state Fm (B), variable fluorescence Fv (C), and maximal quantum yield of PSII photochemistry Fv/Fm (D) in soybean plants sprayed with 24-epibrassinolide (EBR) and exposed to different boron (B) supplies. Columns with different uppercase letters between B supplies (low, control, and high B supply under equal EBR level) and lowercase letters between EBR level (with and without EBR under equal B supply) indicate significant differences from the Scott-Knott test (P < 0.05). Means ± SD, n = 5
3.3 Stomatal Performance was Upregulated after EBR Application
The low and high B supplies reduced stomatal characteristics, except for PDS and EDS (Table 5). Plants sprayed with EBR under the low treatment B on the adaxial face presented increases for SD, SF, and SI of 30%, 6%, and 42%, respectively, compared to the same treatment without EBR. Under the control treatment, the adaxial face had increased for SD and SI by 9% and 18%, respectively; in high B treatment, there were increases of 23%, 5%, and 28% in SD, SF, and SI, respectively. For PDS and EDS in the abaxial face, EBR + low B induced decreases of 13% and 12%, respectively. Under control treatment combined with EBR suffered decreases of 2% (PDS) and 6% (EDS) and under the high B with EBR had reductions of 13% in PDS and 12% in EDS.
3.4 EBR Spray Increased the Activities of Antioxidant Enzymes in Plants Exposed to B Stress
Low and high B supplies increased enzyme activities (Fig. 3). For SOD, plants sprayed with EBR had increases of 176%, 115%, and 73% in supplements low, control, and high B, respectively, relative to the same treatment without EBR (Fig. 3A). Concerning CAT, plants exposed to EBR presented increases of 9%, 17%, and 27% in the low, control, and high B treatments, respectively (Fig. 3B) To APX, plants pretreated with EBR presented elevations of 21%, 13%, and 42% in the low, control, and high B treatments, respectively (Fig. 3C). In POX, plants with EBR had increases of 21% (low B), 48% (control), and 48% (high B) (Fig. 3D). On stress indicators (Fig. 4), supplies with low and high B levels caused increases. For O2−, plants sprayed with EBR had reductions of 1%, 2%, and 26% in low, control, and high B, respectively, compared to the same treatment without EBR (Fig. 4A). Concerning H2O2, plants sprayed with EBR had decreases of 2%, 14%, and 10% in the low, control, and high B treatments, respectively (Fig. 4B). To MDA, plants pretreated with EBR presented reductions of 9%, 3%, and 33% in treatments using low, control, and high B, respectively (Fig. 4C). EL (plants with EBR) was decreased by 1%, 19%, and 5% under low, control, and high B supplies, respectively (Fig. 4D).
Activities of superoxide dismutase SOD (A), catalase CAT (B), ascorbate peroxidase APX (C), and peroxidase POX (D) in soybean plants sprayed with 24-epibrassinolide (EBR) and exposed to different boron (B) supplies. Columns with different uppercase letters between B supplies (low, control, and high B supply under equal EBR level) and lowercase letters between EBR level (with and without EBR under equal B supply) indicate significant differences from the Scott-Knott test (P < 0.05). Means ± SD, n = 5
Superoxide O2.− (A), hydrogen peroxide H2O2 (B), malondialdehyde MDA (C), and electrolyte leakage EL (D) in soybean plants sprayed with 24-epibrassinolide (EBR) and exposed to different boron (B) supplies. Columns with different uppercase letters between B supplies (low, control, and high B supply under equal EBR level) and lowercase letters between EBR level (with and without EBR under equal B supply) indicate significant differences from the Scott-Knott test (P < 0.05). Means ± SD, n = 5
3.5 EBR Mitigated the B Stress-Induced Effects on Biomass
The low and high B supplies promoted significant reductions in growth compared to the control treatment (Fig. 5), except for RDM. For LDM, plants sprayed with EBR and exposed to the control treatment had a 6% increase compared with equal treatment without EBR (Fig. 5A). Concerning RDM, the treatments under low, control, and high B + EBR had increases of 15%, 8%, and 61%, in this order (Fig. 5B). To SDM (Fig. 5C) and TDM (Fig. 5D), the low B supply + EBR promoted increases of 7% and 1%, respectively; whereas the control supply with EBR presented increases of 1% and 5%, respectively; and under high B + EBR, increases of 5% and 10%, respectively.
Leaf dry matter LDM (A), root dry matter RDM (B), stem dry matter SDM (C), and total dry matter TDM (D) in soybean plants sprayed with 24-epibrassinolide (EBR) and exposed to different boron (B) supplies. Columns with different uppercase letters between B supplies (low, control, and high B supply under equal EBR level) and lowercase letters between EBR level (with and without EBR under equal B supply) indicate significant differences from the Scott-Knott test (P < 0.05). Means ± SD, n = 5
4 Discussion
Our findings provide evidence supporting the ability of EBR to alleviate the negative effects triggered by B stress (deficiency or toxicity) in soybean plants. The EBR application reduced B contents in plants subjected to toxicity and increased B contents in control and low B treatments. The positive effects detected in the control and low B can be attributed to improvements in the transport mechanisms of this element in the plant, especially to inflow channels and transporters BOR1, BOR2, and NIP5. The BOR2 transporter promotes the cross-linking of the pectin polysaccharide rhamnogalacturonan II (RG-II) and root elongation, maximizing nutrient uptake, including B (Takada et al. 2014). The NIP5 influx channels are located in the epidermis, cortex, and endoderm cells, while NIP6 is required for transport between the xylem and phloem (Robert and Friml 2009; Takano et al. 2008; Tanaka et al. 2008).
On the other hand, under B toxicity, EBR probably stimulated the BOR4 transporter, which is located in the endoderm and pericycle, being responsible for the detoxification mechanism of B in the plant via secretion (Miwa et al. 2007; Takano et al. 2008). Concomitantly, EBR-induced benefits on antioxidant metabolism, contributing to homeostasis and reducing reactive oxygen species (ROS) often found in plants exposed to stress conditions, including B toxicity (Bajguz and Hayat 2009). B is a micronutrient with high plant mobility (Takano et al. 2008), and multiple B transport mechanisms have been described in the literature (Robert and Friml 2009). This element is a small molecule presenting high permeability relative to other nutrients, with uptake linked to several transporters and broad distribution in plant tissues (Reid 2014). Landi et al. (2012) reported that B toxicity causes oxidative stress due to B accumulation in leaf cell walls, causing imbalances of cytoplasmic metabolism. Devi et al. (2012), evaluating soybean responses under different concentrations of B (0.5, 1.0, 1.5, and 2.0 kg ha−1), observed increases in B contents in leaves and stems.
Soybean plants exposed to deficiency and toxicity of B suffered reductions related to root anatomy variables, but the use of EBR mitigated these effects, reflected in increases in RET, RDT, RCD, VCD, and RMD. The epidermis, endodermis, and cortex are specialized tissues linked to the protection and ionic transport of the apoplast in the direction of the symplast (Scheres et al. 2002). The endoderm still acts as an apoplastic barrier controlling water and nutrient uptake (Enstone et al. 2003; Lux et al. 2004). Increases in RET, RDT, and RCD indicate that EBR spray reduced the damages caused by the B stresses, promoting the increase of the cell expansion rate in these tissues and stimulating the mitotic cycle to maintain cell proliferation in the meristem (Hacham et al. 2011). The effects of EBR on RMD indicate that it promotes benefits on root protection, with gradual increase and consequent improvement in hydraulic conductivity (Hameed et al. 2009). With the thickening of the root, higher absorption of water and nutrients can occur due to positive repercussions on vascularization (Meyer et al. 2011). Ghanati et al. (2005) demonstrated that soybean seedlings exposed to B toxicity (5 mM) had inhibition of root growth due to hypodermis formation and suberin deposition in cortical cell walls corroborated by our research. A study conducted by Aquea et al. (2012) investigating the effects of B toxicity on Arabidopsis thaliana demonstrated that there were cellular changes in the root meristem, inhibiting root growth.
EBR had positive effects on leaf anatomy (ETAd, ETAb, PPT, and SPT). These results evidenced the ability of this steroid to mitigate the damage to leaf structures exposed to B toxicity. On the other hand, B deficiency interferes negatively with cell wall structures (Meriño-Gergichevich et al. 2017), causing imbalances in water relations and reductions in leaf elongation rate (Wimmer and Eichert 2013). EBR spray on plants with B deficiency, or toxicity promoted leaf integrity and anatomy (Shahbaz and Ashraf 2007). Sotiropoulos et al. (2002) studied Actinidia deliciosa and Actinidia arguta species submitted to five B treatments (20, 50, 100, 200, and 500 µM B) and observed a reduced thickness of the leaf cross-section due to the reduction of PPT and SPT.
In general, EBR treatment causes increases in macronutrient and micronutrient contents in low, adequate, and high B supplies because EBR is efficient in regulating the absorption of ions in the plant cell (Khripach 2000; Shahbaz and Ashraf 2007). B modulates the secretory activities in the membranes, exerting influence on proton extrusion and electric potential generation, which is essential for ATPase enzyme activities, causing membrane hyperpolarization and consequent stimulation in the absorption of K and Ca ions (Ahmad et al. 2009; Brown et al. 2002). In plants under B deficiency and toxicity, the membrane properties linked to nutrient uptake often modified, explaining the reductions in nutritional contents (K, Ca, S, Cu, Mn, and Mo). Davis et al. (2003), evaluating the responses of tomato plants under the soil and leaf application of B, observed increases in K and Ca contents in the shoot, similar to our results.
EBR spray in plants under B stress (low and high supplies) increased the photosynthetic pigments (Chl a, Chl b, Total Chl, and Car), suggesting that the EBR helped to pigment membrane damages, which was confirmed by the reductions of MDA and EL. In other words, ROS accumulation causes oxidative damage and lipid peroxidation, producing MDA with subsequent increments of EL, signaling deleterious effects on membrane integrity (Genisel et al. 2013; Li et al. 2018; Mito et al. 2019; Sun et al. 2015; Yao et al. 2017). Similar results were found by Zhang et al. (2014) and Dong et al. (2017), where EBR spraying increased the levels of Chl a, Chl b, and Total Chl in melon plants subjected to high temperature and in wheat plants under saline stress (120 mM NaCl).
EBR application in plants exposed to different B concentrations promoted better results concerning chlorophyll fluorescence. Low and high B supplies caused inhibition of the processes linked to the electron transport chain. However, our results revealed that the EBR treatment was able to increase Fm, Fv, and Fv/Fm values and reduce F0, demonstrating beneficial effects on photochemical reactions of PSII, with greater protection to the photosynthetic apparatus (Zhang et al. 2013). Additionally, lower F0 values associated with increased Fm in plants exposed to EBR application indicate a positive action of this steroid over the light-harvesting complex (Melo et al. 2017). Wang et al. (2009), evaluating maize plants exposed to 550 mg Mn kg−1 soil combined with 0.1 mg L−1 EBR found increases in Fv/Fm, compared with equal treatment without EBR. Lima and Lobato (2017), investigating cowpea plants sprayed with 100 nM EBR and submitted to water deficit, reported an increase in Fm (32%) and reduction in F0 (15%), respectively, similar to results found in this research.
EBR promoted increases in ΦPSII, qp, and ETR values and reductions in NPQ, EXC, and ETR/PN in plants exposed to high and low B supplies. Increases in ΦPSII values indicate that EBR application improved energy capture efficiency and increased the proportion of open reaction centers in the PSII (qp) (Yu et al. 2004). Higher qp values are associated with increased capture capacity of the PSII electron-acceptor molecule (plastoquinone) frequently associated with reducing power and ATP consumption and avoiding photoinhibition (Khamsuk et al. 2018). Additionally, reductions in NPQ values in low B + EBR-treated plants demonstrate the protective effect of this steroid on PSII to excess energy, alleviating damage to thylakoid membranes (Wu et al. 2014). Thussagunpanit et al. (2015) studied rice plants under high-temperature conditions, and the application of EBR (1 nM) also promoted increases in ΦPSII, qp, and ETR, in agreement with our results. Shu et al. (2016), evaluating tomato seedlings under low irradiance (180 µmol m−2 s−1) and low temperature (15 °C day/7 °C night), observed beneficial effects of exogenous EBR application (0.1 µmol L−1) on the parameters of ΦPSII and qp, indicating that EBR alleviated the photoinhibition caused by the simulated stress.
An increase in ETR and a decrease in EXC in response to EBR application indicated that this steroid improved the electron transport in PSII. Reductions in ETR/PN of EBR-treated plants indicate an increase in carboxylation efficiency and probably lower carbon losses by photorespiration (Ahammed et al. 2013a; Pereira et al. 2019). Increases in ETR and ΦPSII were found by Dobrikova et al. (2014), investigating the effects of three EBR concentrations (0.01, 0.1, and 1 mg L−1) on the membrane structures of thylakoids in pea plants, obtaining increases of 25% and 35% in ETR under 0.01 and 0.1 mg L−1 EBR, respectively, when compared to the control treatment.
EBR application mitigated the effects of low and high B treatments on gas exchange. Higher values of PN, E, gs, and PN/Ci under low B conditions and increases in PN, WUE, and PN/Ci in high B treatment are associated with positive actions of the EBR on photochemical and diffusional aspects found in this study. Additionally, there is a linear relationship between the ability to capture, utilize, and dissipate light and CO2 uptake (Wong et al. 2012). We detected increases in Fv/Fm, ΦPSII, and ETR values proving higher photosynthetic efficiency despite low and high B concentrations. Parallelly, increases in PN can be associated with lower Ci values, resulting in higher PN/Ci, suggesting a higher activity of Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and, consequently, increases in CO2 uptake in the Calvin-Benson cycle. Increases in gs values combined with higher SD, SI, and SF and lower PDS and EDS suggested higher photosynthetic capacity in EBR-treated plants due to more uniform CO2 diffusion to Rubisco sites (Tanaka et al. 2013). Our results are consistent with the findings by Hu et al. (2013), evaluating pepper plants, being found significant increases in PN, gs, and E and a decrease in Ci in plants under water deficit and treated with EBR (0.01 mg L−1), compared to plants without steroid.
Plants submitted to low and high B in association with the application of EBR presented a better performance concerning stomatal characteristics than plants not treated with EBR. Increases in stomatal density (SD), stomatal functionality (SF), and stomatal index (SI) on the adaxial face of leaves under the influence of EBR demonstrate that this steroid acts on stomatal development, activating proteins involved in the signaling pathway of the stomatal mechanism (Lin et al. 2013). Changes in stomatal, such as shape, size, and quantity, are efficient strategies of plants to cope with stressful conditions because the increase in the density and reduction in the size of the stomata contribute to a higher diffusion of CO2 to the carboxylation sites and increases in WUE (Devi and Reddy 2018; Franks and Beerling 2009). Our results show that EBR played an important role against the effects of low and high B, contributing to the increases in stomata quantity and efficiency (SD and SI) while reducing the size (smaller PDS and EDS) and positively reflecting on the PN values (Zhao et al. 2015). Increases in SD were observed in grape leaves subjected to water deficit and exogenous EBR applications (0.05, 0.10, and 0.20 mg L−1), promoting increases of 43%, 15%, and 53%, respectively, compared to plants without EBR (Wang et al. 2015). Oliveira et al. (2018) observed positive effects of 50 nM EBR against saline stress in young Eucalyptus urophylla plants, with increases of 23%, 7%, and 24% in SD, SF, and SI, respectively, and reductions of 8% and 10% in PDS and EDS, in this order, compared to stress treatment without EBR, being similar to our results.
Plants treated with EBR under low and high B supplies had increases in the activities of antioxidant enzymes (SOD, CAT, APX, and POX), indicating beneficial interference of the EBR on the antioxidant defense system. These results are related to the increases in Fv/Fm and ETR because the maximization of the photosynthetic potential reduces the availability of NADPH and ATP (Zhou et al. 2004), which are consumed in the Calvin cycle, facilitating the transport of electrons and reducing the possibility of ROS formation (Ogweno et al. 2008). Li et al. (2015) studying the antioxidant system in pepper seedlings submitted to two thermal regimes (28/18 °C and 15/5 °C) and spray with EBR (0.1 μM) showed significant increases of 29%, 31%, 25%, and 9% after the EBR application for SOD, POX, CAT, and APX, respectively. Surgun et al. (2016) analyzed the EBR effects on the B-tolerance mechanisms in Arabidopsis thaliana plants submitted to three B concentrations (0, 0.80, and 1.60 mM) combined with two EBR levels (0.01, and 1 mM) and described that the application of EBR maximized the activities linked to antioxidant enzymes (SOD, CAT, APX, and POX).
The stress promoted by the high B stress-induced increases in O2, H2O2, MDA, and EL, but these effects were reduced after the application of EBR. EBR spray increased the activities of antioxidant enzymes, as previously detected. The antioxidant enzymes act in the neutralization of the ROS (Dalyan et al. 2018; Zhang et al. 2008), with the SOD enzyme acting at the beginning of the defense process, catalyzing the conversion of O2− to H2O2, which is subsequently degraded by the CAT, APX, and POX enzymes (Zhou et al. 2018). Ahammed et al. (2013b), working with tomato plants exposed to EBR and three concentrations of polychlorinated biphenyls (0.4, 2.0, and 10.0 μg L−1), reported decreases in O2−, H2O2, and MDA levels, similar to the results found in this research. Ogweno et al. (2008) investigated the photosynthetic efficiency and oxidative stress in tomato plants under two temperature conditions, normal (25/18 °C) and high (40/30 °C), and three EBR concentrations (0.01, 0.1, 1.0 mg L−1), reporting reductions in H2O2 and MDA levels in plants treated with EBR.
Plants subjected to deficiency or toxicity of B when sprayed with EBR had increased related to growth (LDM, RDM, SDM, and TDM). These responses can be attributed to the beneficial roles of EBR on anatomical responses, chlorophyll fluorescence, and gas exchange, evidenced by the increases in mesophyll cells (palisade parenchyma and spongy parenchyma), ΦPSII, ETR, and PN, as presented in this study. The application of EBR also favored increases in photosynthetic pigments and gas exchange, positively influencing the biomass (Xie et al. 2011; Naz et al. (2015);. Hayat et al. (2011) studied tomato plants under the three concentrations of EBR (10−6, 10−8, 10−10 M) and the three concentrations of HBL (10−6, 10−8, 10−10 M) and reported that plants treated with EBR had more intense effects on length, fresh matter, dry matter, and foliar area when compared to plants treated with HBL. A study conducted by Liu et al. (2018) analyzing the B toxicity in Puccinellia tenuiflora seedlings submitted to three different B levels described reductions of 69% and 40% in root dry matter and shoot dry matter, respectively, in plants treated with high B.
5 Conclusions
EBR pretreatment promoted improvements in leaf and root structures, also inducing increases in biomass accumulation. EBR alleviated the damages occasioned by the inadequate B supplies on root tissues, more specifically maximizing the vascular cylinder, metaxylem, and epidermis, improving the nutritional status. This steroid also minimized the harmful effects of B stress on leaf anatomy, stimulating the epidermis on both leaf sides, palisade parenchyma, and spongy parenchyma; both structures are intrinsically related to protection and CO2 availability to the photosynthetic process. Concomitantly, this steroid had a positive impact on biomass accumulation. These results are explained by beneficial actions on leaf structures and photosynthetic machinery. Therefore, our results demonstrate that the EBR application can improve soybean plants’ tolerance under inadequate B supplementation.
References
Ahammed GJ, Choudhary SP, Chen S, Xia X, Shi K, Zhou Y, Yu J (2013a) Role of brassinosteroids in alleviation of phenanthrene–cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J Exp Bot 64:199–213. https://doi.org/10.1093/jxb/ers323
Ahammed GJ, Ruan YP, Zhou J, Xia XJ, Shi K, Zhou YH, Yu JQ (2013b) Brassinosteroid alleviates polychlorinated biphenyls-induced oxidative stress by enhancing antioxidant enzymes activity in tomato. Chemosphere 90:2645–2653. https://doi.org/10.1016/j.chemosphere.2012.11.041
Ahmad W, Niaz A, Kanwal S, Khalid Rasheed M (2009) Role of boron in plant growth: a review. J Agric Res 47:329–338
Aquea F, Federici F, Moscoso C, Vega A, Jullian P, Haseloff J, Arce-Johnson P (2012) A molecular framework for the inhibition of Arabidopsis root growth in response to boron toxicity. Plant, Cell Environ 35:719–734. https://doi.org/10.1111/j.1365-3040.2011.02446.x
Atique-ur-Rehman FM, Rashid A, Nadeem F, Stuerz S, Asch F, Bell RW, Siddique KHM (2018) Boron nutrition of rice in different production systems. A review. Agron Sustain Dev 38:25. https://doi.org/10.1007/s13593-018-0504-8
Badawi GH, Yamauchi Y, Shimada E, Sasaki R, Kawano N, Tanaka K, Tanaka K (2004) Enhanced tolerance to salt stress and water deficit by overexpressing superoxide dismutase in tobacco (Nicotiana tabacum) chloroplasts. Plant Sci 166:919–928. https://doi.org/10.1016/j.plantsci.2003.12.007
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8. https://doi.org/10.1016/j.plaphy.2008.10.002
Bamji SF, Corbitt C (2017) Glyceollins: soybean phytoalexins that exhibit a wide range of health-promoting effects. J Funct Foods 34:98–105. https://doi.org/10.1016/j.jff.2017.04.020
Barberon M, Vermeer JEM, De Bellis D, Wang P, Naseer S, Andersen TG, Humbel BM, Nawrath C, Takano J, Salt DE, Geldner N (2016) Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164:447–459. https://doi.org/10.1016/j.cell.2015.12.021
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brown PH, Bellaloui N, Wimmer MA, Bassil ES, Ruiz J, Hu H, Pfeffer H, Dannel F, Römheld V (2002) Boron in plant biology. Plant Biol 4:205–223. https://doi.org/10.1055/s-2002-25740
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468. https://doi.org/10.1111/j.1399-3054.1991.tb00121.x
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227. https://doi.org/10.1104/pp.98.4.1222
Clouse SD (2002) Brassinosteroids. Arabidopsis Book 2–23. https://doi.org/10.1199/tab.0009
Dalyan E, Yüzbaşıoğlu E, Akpınar I (2018) Effect of 24-epibrassinolide on antioxidative defence system against lead-induced oxidative stress in the roots of Brassica juncea L. Seedlings. Russ J Plant Physiol 65:570–578. https://doi.org/10.1134/S1021443718040118
Davis JM, Sanders DC, Nelson PV, Lengnick L, Sperry WJ (2003) Boron improves growth, yield, quality, and nutrient content of tomato. J Am Soc Hortic Sci 128:441–446
Devi KN, Singh LNK, Sumarjit Singh M, Basanta Singh S, Khamba Singh K (2012) Influence of sulphur and boron fertilization on yield, quality, nutrient uptake and economics of soybean (Glycine max) under upland conditions. J Agric Sci 4. https://doi.org/10.5539/jas.v4n4p1
Devi MJ, Reddy VR (2018) Transpiration response of cotton to vapor pressure deficit and its relationship with stomatal traits. Front Plant Sci 871:1–12. https://doi.org/10.3389/fpls.2018.01572
Dobrikova AG, Vladkova RS, Rashkov GD, Todinova SJ, Krumova SB, Apostolova EL (2014) Effects of exogenous 24-epibrassinolide on the photosynthetic membranes under non-stress conditions. Plant Physiol Biochem 80:75–82. https://doi.org/10.1016/j.plaphy.2014.03.022
Dong YJ, Wang WW, Hu GQ, Chen WF, Zhuge YP, Wang ZL, He MR (2017) Role of exogenous 24-epibrassinolide in enhancing the salt tolerance of wheat seedlings. J Soil Sci Plant Nutr 17:554–569. https://doi.org/10.4067/S0718-95162017000300001
Du X, Wei X (2018) Definition of fine roots on the basis of the root anatomy, diameter, and branch orders of one-year old Fraxinus mandshurica seedlings. J for Res 29:1321–1327. https://doi.org/10.1007/s11676-017-0561-x
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620. https://doi.org/10.1016/0003-2697(76)90488-7
Enstone DE, Peterson CA, Ma F (2003) Root endodermis and exodermis: structure, function, and responses to the environment. J Plant Growth Regul 21:335–351. https://doi.org/10.1007/s00344-003-0002-2
Farghaly FA, Salam HK, Hamada AM, Radi AA (2021) The role of benzoic acid, gallic acid and salicylic acid in protecting tomato callus cells from excessive boron stress. Sci Hortic (amsterdam) 278:109867. https://doi.org/10.1016/j.scienta.2020.109867
Franks PJ, Beerling DJ (2009) Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc Natl Acad Sci 106:10343–10347. https://doi.org/10.1073/pnas.0904209106
Gao L, Tian Y, Chen MC, Wei L, Gao TG, Yin HJ, Zhang JL, Kumar T, Liu LB, Wang SM (2019) Cloning and functional characterization of epidermis-specific promoter MtML1 from Medicago truncatula. J Biotechnol 300:32–39. https://doi.org/10.1016/j.jbiotec.2019.05.003
Genisel M, Turk H, Erdal S (2013) Exogenous progesterone application protects chickpea seedlings against chilling-induced oxidative stress. Acta Physiol Plant 35:241–251. https://doi.org/10.1007/s11738-012-1070-3
Ghanati F, Morita A, Yokota H (2005) Deposition of suberin in roots of soybean induced by excess boron. Plant Sci 168:397–405. https://doi.org/10.1016/j.plantsci.2004.09.004
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. occurrence in higher plants. Plant Physiol 59:309–314
Goldsmith PD (2008) Economics of soybean production, marketing, and utilization. Soybeans Chem Prod Process Util 117–150https://doi.org/10.1016/B978-1-893997-64-6.50008-1
Goli E, Hiemstra T, Rahnemaie R (2019) Interaction of boron with humic acid and natural organic matter: experiments and modeling. Chem Geol 515:1–8. https://doi.org/10.1016/j.chemgeo.2019.03.021
Gonçalves C, Junior A, Pereira M, Gasparino E, Matins D (2017) Morphological modifications in soybean in response to soil water management. Plant Growth Regul 83:105–117. https://doi.org/10.1007/s10725-017-0287-y
Gong M, Li Y-J, Chen S-Z (1998) Abscisic acid-induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant systems. J Plant Physiol 153:488–496. https://doi.org/10.1016/S0176-1617(98)80179-X
Hacham Y, Holland N, Butterfield C, Ubeda-Tomas S, Bennett MJ, Chory J, Savaldi-Goldstein S (2011) Brassinosteroid perception in the epidermis controls root meristem size. Development 138:839–848. https://doi.org/10.1242/dev.061804
Hameed M, Ashraf M, Naz N (2009) Anatomical adaptations to salinity in cogon grass [Imperata cylindrica (L.) Raeuschel] from the Salt Range, Pakistan. Plant Soil 322:229–238. https://doi.org/10.1007/s11104-009-9911-6
Hänsch R, Mendel RR (2009) Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr Opin Plant Biol 12:259–266. https://doi.org/10.1016/j.pbi.2009.05.006
Havir EA, McHale NA (1987) Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol 84:450–455. https://doi.org/10.1104/pp.84.2.450
Hayat S, Yadav S, Wani AS, Irfan M, Ahmad A (2011) Comparative effect of 28-homobrassinolide and 24-epibrassinolide on the growth, carbonic anhydrase activity and photosynthetic efficiency of Lycopersicon esculentum. Photosynthetica 49:397–404. https://doi.org/10.1007/s11099-011-0051-x
Herrera-Rodríguez M, González-Fontes A, Rexach J, Camacho-Cristóbal J, Maldonado J, Navarro-Gochicoa M (2010) Role of boron in vascular plants and response mechanisms to boron stresses. Plant Stress 4:115–122
Hu WH, Yan XH, Xiao YA, Zeng JJ, Qi HJ, Ogweno JO (2013) 24-Epibrassinosteroid alleviate drought-induced inhibition of photosynthesis in Capsicum annuum. Sci Hortic (amsterdam) 150:232–237. https://doi.org/10.1016/j.scienta.2012.11.012
Huang JH, Cai ZJ, Wen SX, Guo P, Ye X, Lin GZ, Chen LS (2014) Effects of boron toxicity on root and leaf anatomy in two citrus species differing in boron tolerance. Trees - Struct Funct 28:1653–1666. https://doi.org/10.1007/s00468-014-1075-1
Javelle M, Vernoud V, Rogowsky PM, Ingram GC (2011) Epidermis: the formation and functions of a fundamental plant tissue. New Phytol 189:17–39. https://doi.org/10.1111/j.1469-8137.2010.03514.x
Ju S, Wang L, Zhang C, Yin T, Shao S (2017) Alleviatory effects of silicon on the foliar micromorphology and anatomy of rice (Oryza sativa L.) seedlings under simulated acid rain. PLoS One 12:1–18. https://doi.org/10.1371/journal.pone.0187021
Khamsuk O, Sonjaroon W, Suwanwong S, Jutamanee K, Suksamrarn A (2018) Effects of 24-epibrassinolide and the synthetic brassinosteroid mimic on chili pepper under drought. Acta Physiol Plant 40:106. https://doi.org/10.1007/s11738-018-2682-z
Khripach V (2000) Twenty years of Brassinosteroids: steroidal plant hormones warrant better crops for the XXI century. Ann Bot 86:441–447. https://doi.org/10.1006/anbo.2000.1227
Kim SL, Berhow MA, Kim JT, Chi HY, Lee SJ, Chung IM (2006) Evaluation of soyasaponin, isoflavone, protein, lipid, and free sugar accumulation in developing soybean seeds. J Agric Food Chem 54:10003–10010. https://doi.org/10.1021/jf062275p
Kobayashi M, Miyamoto M, Matoh T, Kitajima S, Hanano S, Sumerta IN, Narise T, Suzuki H, Sakurai N, Shibata D (2018) Mechanism underlying rapid responses to boron deprivation in Arabidopsis roots. Soil Sci Plant Nutr 64:106–115. https://doi.org/10.1080/00380768.2017.1416670
Landi M, Degl’Innocenti E, Pardossi A, Guidi L (2012) Antioxidant and photosynthetic responses in plants under boron toxicity: a review. Am J Agric Biol Sci 7:255–270. https://doi.org/10.3844/ajabssp.2012.255.270
Lansing AJ, Franceschi VR (2000) The paraveinal mesophyll: a specialized path for intermediary transfer of assimilates in legume leaves. Aust J Plant Physiol 27:757–767
Li J, Yang P, Gan Y, Yu J, Xie J (2015) Brassinosteroid alleviates chilling-induced oxidative stress in pepper by enhancing antioxidation systems and maintenance of photosystem II. Acta Physiol Plant 37:1–11. https://doi.org/10.1007/s11738-015-1966-9
Li M, Zhao Z, Zhang Z, Zhang W, Zhou J, Xu F, Liu X (2017) Effect of boron deficiency on anatomical structure and chemical composition of petioles and photosynthesis of leaves in cotton (Gossypium hirsutum L.). Sci Rep 7:1–10. https://doi.org/10.1038/s41598-017-04655-z
Li T, Yun Z, Wu Q, Zhang Z, Liu S, Shi X, Duan X, Jiang Y (2018) Proteomic profiling of 24-epibrassinolide-induced chilling tolerance in harvested banana fruit. J Proteomics 187:1–12. https://doi.org/10.1016/j.jprot.2018.05.011
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Current Protocols in Food Analytical Chemistry. John Wiley & Sons Inc, Hoboken, NJ, USA, pp 431–438
Lima JV, Lobato AKS (2017) Brassinosteroids improve photosystem II efficiency, gas exchange, antioxidant enzymes and growth of cowpea plants exposed to water deficit. Physiol Mol Biol Plants 23:59–72. https://doi.org/10.1007/s12298-016-0410-y
Lima MDR, Barros Junior UO, Batista BL, Lobato AKS (2018) Brassinosteroids mitigate iron deficiency improving nutritional status and photochemical efficiency in Eucalyptus urophylla plants. Trees 32:1681–1694. https://doi.org/10.1007/s00468-018-1743-7
Lin LL, Wu CC, Huang HC, Chen HJ, Hsieh HL, Juan HF (2013) Identification of microRNA 395a in 24-epibrassinolide-regulated root growth of Arabidopsis thaliana using microRNA arrays. Int J Mol Sci 14:14270–14286. https://doi.org/10.3390/ijms140714270
Liu C, Dai Z, Xia J, Chang C, Sun H (2018) Combined effect of salt and drought on boron toxicity in Puccinellia tenuiflora. Ecotoxicol Environ Saf 157:395–402. https://doi.org/10.1016/j.ecoenv.2018.03.061
Lux A, Luxová M, Abe J, Morita S (2004) Root cortex: structural and functional variability and responses to environmental stress. Root Res 13:117–131
Lux A, Martinka M, Vaculik M, White P (2011) Root responses to cadmium in the rhizosphere: a review. J Exp Bot 62:1–17. https://doi.org/10.1093/jxb/erq281
Maia CF, Silva BRS, Lobato AKS (2018) Brassinosteroids positively modulate growth: physiological, biochemical and anatomical evidence using two tomato genotypes contrasting to dwarfism. J Plant Growth Regul 37:1–14. https://doi.org/10.1007/s00344-018-9802-2
Mei L, Li Q, Wang H, Sheng O, Peng S (2016) Boron deficiency affects root vessel anatomy and mineral nutrient allocation of Poncirus trifoliata (L.) Raf. Acta Physiol Plant 38:1–8. https://doi.org/10.1007/s11738-016-2099-5
de Melo HF, de Souza ER, Cunha JC (2017) Fluorescence of chlorophyll a and photosynthetic pigments in Atriplex nummularia under abiotic stresses. Rev Bras Eng Agrícola e Ambient 21:232–237. https://doi.org/10.1590/1807-1929/agriambi.v21n4p232-237
Meriño-Gergichevich C, Reyes-Díaz M, Guerrero J, Ondrasek G (2017) Physiological and nutritional responses in two highbush blueberry cultivars exposed to deficiency and excess of boron. J Soil Sci Plant Nutr 17:307–318. https://doi.org/10.4067/S0718-95162017005000024
Meyer CJ, Peterson CA, Steudle E (2011) Permeability of Iris germanica’s multiseriate exodermis to water, NaCl, and ethanol. J Exp Bot 62:1911–1926. https://doi.org/10.1093/jxb/erq380
Mito MS, Silva AA, Kagami FL, Almeida JD, Mantovanelli GC, Barbosa MC, Kern-Cardoso KA, Ishii-Iwamoto EL (2019) Responses of the weed Bidens pilosa L. to exogenous application of the steroidal saponin protodioscin and plant growth regulators 24-epibrassinolide, indol-3-acetic acid and abscisic acid. Plant Biol 21:326–335. https://doi.org/10.1111/plb.12927
Miwa K, Takano J, Omori H, Seki M, Shinozaki K, Fujiwara T (2007) Plants tolerant of high boron levels. Science (80) 318:1417. https://doi.org/10.1126/science.1146634
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nawaz M, Ishaq S, Ishaq H, Khan N, Iqbal N, Ali S, Rizwan M, Alsahli AA, Alyemeni MN (2020) Salicylic acid improves boron toxicity tolerance by modulating the physio-biochemical characteristics of maize (Zea mays L.) at an early growth stage. Agronomy 10:2013. https://doi.org/10.3390/agronomy10122013
Naz FS, Yusuf M, Khan TA, Fariduddin Q, Ahmad A (2015) Low level of selenium increases the efficacy of 24-epibrassinolide through altered physiological and biochemical traits of Brassica juncea plants. Food Chem 185:441–448. https://doi.org/10.1016/j.foodchem.2015.04.016
Nemeskéri E, Helyes L (2019) Physiological responses of selected vegetable crop species to water stress. Agron J 9:1–19
Nishinari K, Fang Y, Guo S, Phillips GO (2014) Soy proteins: a review on composition, aggregation and emulsification. Food Hydrocoll 39:301–318. https://doi.org/10.1016/j.foodhyd.2014.01.013
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373. https://doi.org/10.1007/BF01248568
Ogweno JO, Song XS, Shi K, Hu WH, Mao WH, Zhou YH, Yu JQ, Nogués S (2008) Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J Plant Growth Regul 27:49–57. https://doi.org/10.1007/s00344-007-9030-7
Oiwa Y, Kitayama K, Kobayashi M, Matoh T (2013) Arabidopsis thaliana. Soil Sci Plant Nutr 59(4):621–627
Oliveira JL, Silva JN, Martins MA, Pereira EG, Oliveira MCTB (2018) Gasification of waste from coffee and eucalyptus production as an alternative source of bioenergy in Brazil. Sustain Energy Technol Assessments 27:159–166. https://doi.org/10.1016/j.seta.2018.04.005
Oliveira VP, Lima MDR, Silva BRS, Batista BL, Lobato AKS (2019) Brassinosteroids confer tolerance to salt stress in Eucalyptus urophylla plants enhancing homeostasis, antioxidant metabolism and leaf anatomy. J Plant Growth Regul 38:557–573. https://doi.org/10.1007/s00344-018-9870-3
Pereira YC, Rodrigues WS, Lima EJA, Santos LR, Silva MHL, Lobato AKS (2019) Brassinosteroids increase electron transport and photosynthesis in soybean plants under water deficit. Photosynthetica 57:1–11
Pérez Chaca MV, Vigliocco A, Reinoso H, Molina A, Abdala G, Zirulnik F, Pedranzani H (2014) Effects of cadmium stress on growth, anatomy and hormone contents in Glycine max (L.) Merr. Acta Physiol Plant 36:2815–2826. https://doi.org/10.1007/s11738-014-1656-z
Purushothaman R, Zaman-Allah M, Mallikarjuna N, Pannirselvam R, Krishnamurthy L, Gowda CLL (2013) Root anatomical traits and their possible contribution to drought tolerance in grain legumes. Plant Prod Sci 16:1–8. https://doi.org/10.1626/pps.16.1
Reid R (2014) Understanding the boron transport network in plants. Plant Soil 385:1–13. https://doi.org/10.1007/s11104-014-2149-y
Ribeiro AT, de Oliveira VP, de Oliveira Barros Junior U, da Silva BRS, Batista BL, da Silva Lobato AK (2020) 24-Epibrassinolide mitigates nickel toxicity in young Eucalyptus urophylla S.T. Blake plants: nutritional, physiological, biochemical, anatomical and morphological responses. Ann For Sci 77:5. https://doi.org/10.1007/s13595-019-0909-9
Ribeiro DGS, Silva BRS, Lobato AKS (2019) Brassinosteroids induce tolerance to water deficit in soybean seedlings: contributions linked to root anatomy and antioxidant enzymes. Acta Physiol Plant 41:1–11. https://doi.org/10.1007/s11738-019-2873-2
Robert HS, Friml J (2009) Auxin and other signals on the move in plants. Nat Chem Biol 5:325–332. https://doi.org/10.1038/nchembio.170
da Rodrigues WS, Pereira YC, de Souza ALM, Batista BL, da Lobato AKS (2020) Alleviation of oxidative stress induced by 24-epibrassinolide in soybean plants exposed to different manganese supplies: upregulation of antioxidant enzymes and maintenance of photosynthetic pigments. J Plant Growth Regul. https://doi.org/10.1007/s00344-020-10091-7
Sakamoto T, Inui YT, Uraguchi S, Yoshizumi T, Matsunaga S, Mastui M, Umeda M, Fukui K, Fujiwara T (2011) Condensin II alleviates DNA damage and is essential for tolerance of boron overload stress in Arabidopsis. Plant Cell 23:3533–3546. https://doi.org/10.1105/tpc.111.086314
Santos LR, da Silva BRS, Pedron T, Batista BL, da Lobato AKS (2020) 24-Epibrassinolide improves root anatomy and antioxidant enzymes in soybean plants subjected to zinc stress. J Soil Sci Plant Nutr 20:105–124. https://doi.org/10.1007/s42729-019-00105-z
Scheres B, Benfey P, Dolan L (2002) Root development primer. Arabidopsis Book 1:e0101. https://doi.org/10.1199/tab.0101
Seago Jr. JL, Fernando DD (2013) Anatomical aspects of angiosperm root evolution. Ann Bot 112(2):223–238. https://doi.org/10.1093/aob/mcs266
Segatto FB, Bisognin DA, Benedetti M, Costa LC, Rampelotto MV, Nicoloso FT (2004) A technique for the anatomical study of potato leaf epidermis. Ciência Rural 34:1597–1601. https://doi.org/10.1590/S0103-84782004000500042
Shahbaz M, Ashraf M (2007) Influence of exogenous application of brassinosteroid on growth and mineral nutrients of wheat (Triticum Aestivum L.) under saline conditions. Pakistan J Bot 39:513–522
Shahbaz M, Ashraf M, Athar H-U-R (2008) Does exogenous application of 24-epibrassinolide ameliorate salt induced growth inhibition in wheat (Triticum aestivum L.)? Plant Growth Regul 55:51–64. https://doi.org/10.1007/s10725-008-9262-y
Shireen F, Nawaz M, Chen C, Zhang Q, Zheng Z, Sohail H, Sun J, Cao H, Huang Y, Bie Z (2018) Boron: functions and approaches to enhance its availability in plants for sustainable agriculture. Int J Mol Sci 19:1856. https://doi.org/10.3390/ijms19071856
Shu S, Tang Y, Yuan Y, Sun J, Zhong M, Guo S (2016) The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined low temperature and weak light stress. Plant Physiol Biochem 107:344–353. https://doi.org/10.1016/j.plaphy.2016.06.021
Sotiropoulos TE, Therios IN, Dimassi KN, Bosabalidis A, Kofidis G (2002) Nutritional status, growth, CO2 assimilation, and leaf anatomical responses in two kiwifruit species under boron toxicity. J Plant Nutr 25:1249–1261. https://doi.org/10.1081/PLN-120004386
Steel RG, Torrie JH, Dickey DA (2006) Principles and procedures of statistics: a biometrical approach, 3rd edn. Academic Internet Publishers, Moorpark
Sun S, An M, Han L, Yin S (2015) Foliar application of 24-epibrassinolide improved salt stress tolerance of perennial ryegrass. HortScience 50:1518–1523
Surgun Y, Çöl B, Bürün B (2016) 24-epibrassinolide ameliorates the effects of boron toxicity on Arabidopsis thaliana (L.) Heynh by activating an antioxidant system and decreasing boron accumulation. Acta Physiol Plant 38:71. https://doi.org/10.1007/s11738-016-2088-8
Takada S, Miwa K, Omori H, Fujiwara T, Naito S, Takano J (2014) Improved tolerance to boron deficiency by enhanced expression of the boron transporter BOR2. Soil Sci Plant Nutr 60:341–348. https://doi.org/10.1080/00380768.2014.881705
Takano J, Miwa K, Fujiwara T (2008) Boron transport mechanisms: collaboration of channels and transporters. Trends Plant Sci 13:451–457. https://doi.org/10.1016/j.tplants.2008.05.007
Tanaka M, Wallace IS, Takano J, Roberts DM, Fujiwara T (2008) NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. Plant Cell 20:2860–2875. https://doi.org/10.1105/tpc.108.058628
Tanaka Y, Sugano SS, Shimada T, Hara-Nishimura I (2013) Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol 198:757–764. https://doi.org/10.1111/nph.12186
Thomas R, Fang X, Ranathunge K, Anderson TR, Peterson CA, Bernards MA (2007) Soybean root suberin: anatomical distribution, chemical composition, and relationship to partial resistance to Phytophthora sojae. Plant Physiol 144:299–311. https://doi.org/10.1104/pp.106.091090
Thussagunpanit J, Jutamanee K, Sonjaroon W, Kaveeta L, Chai-Arree W, Pankean P, Suksamrarn A (2015) Effects of brassinosteroid and brassinosteroid mimic on photosynthetic efficiency and rice yield under heat stress. Photosynthetica 53:312–320. https://doi.org/10.1007/s11099-015-0106-5
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Vezza ME, Llanes A, Travaglia C, Agostini E, Talano MA (2018) Arsenic stress effects on root water absorption in soybean plants: physiological and morphological aspects. Plant Physiol Biochem 123:8–17. https://doi.org/10.1016/j.plaphy.2017.11.020
Vriet C, Russinova E, Reuzeau C (2012) Boosting crop yields with plant steroids. Plant Cell 24:842–857. https://doi.org/10.1105/tpc.111.094912
Wang HH, Feng T, Peng XX, Yan ML, Zhou PL, Tang XK (2009) Ameliorative effects of brassinosteroid on excess manganese-induced oxidative stress in Zea mays L. leaves. Agric Sci China 8:1063–1074. https://doi.org/10.1016/S1671-2927(08)60314-4
Wang W, Bringe NA, Berhow MA, De Mejia EG (2008) Β-conglycinins among sources of bioactives in hydrolysates of different soybean varieties that inhibit leukemia cells in vitro. J Agric Food Chem 56:4012–4020. https://doi.org/10.1021/jf8002009
Wang Z, Zheng P, Meng J, Xi Z (2015) Effect of exogenous 24-epibrassinolide on chlorophyll fluorescence, leaf surface morphology and cellular ultrastructure of grape seedlings (Vitis vinifera L.) under water stress. Acta Physiol Plant 37:1–12. https://doi.org/10.1007/s11738-014-1729-z
Wimmer MA, Eichert T (2013) Review: mechanisms for boron deficiency-mediated changes in plant water relations. Plant Sci 203–204:25–32. https://doi.org/10.1016/j.plantsci.2012.12.012
Wong SL, Chen CW, Huang HW, Weng JH (2012) Using combined measurements of gas exchange and chlorophyll fluorescence to investigate the photosynthetic light responses of plant species adapted to different light regimes. Photosynthetica 50:206–214. https://doi.org/10.1007/s11099-012-0027-5
Wu Q-S, Xia R-X, Zou Y-N (2006) Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J Plant Physiol 163:1101–1110. https://doi.org/10.1016/j.jplph.2005.09.001
Wu X, Yao X, Chen J, Zhu Z, Zhang H, Zha D (2014) Brassinosteroids protect photosynthesis and antioxidant system of eggplant seedlings from high-temperature stress. Acta Physiol Plant 36:251–261. https://doi.org/10.1007/s11738-013-1406-7
Wyka TP, Bagniewska-Zadworna A, Kuczyńska A, Mikołajczak K, Ogrodowicz P, Żytkowiak M, Surma M, Adamski T (2019) Drought-induced anatomical modifications of barley (Hordeum vulgare L.) leaves: an allometric perspective. Environ Exp Bot 166: https://doi.org/10.1016/j.envexpbot.2019.103798
Xie L, Yang C, Wang X (2011) Brassinosteroids can regulate cellulose biosynthesis by controlling the expression of CESA genes in Arabidopsis. J Exp Bot 62:4495–4506. https://doi.org/10.1093/jxb/err164
Yao Y, Zhao N, Xian T, Tu S, Pan L, Tu K (2017) Effect of 2,4-epibrassinolide treatment on the postharvest quality and physiological metabolism of fresh daylily flower buds during storage. Sci Hortic (amsterdam) 226:110–116. https://doi.org/10.1016/j.scienta.2017.08.039
Yau SK, Ryan J (2008) Boron toxicity tolerance in crops: a viable alternative to soil amelioration. Crop Sci 48:854. https://doi.org/10.2135/cropsci2007.10.0539
Yu JQ, Huang L-F, Hu WH, Zhou YH, Mao WH, Ye SF, Nogués S (2004) A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J Exp Bot 55:1135–1143. https://doi.org/10.1093/jxb/erh124
Zeng HY, Deng X, Zeng QR (2019) Basal and foliar treatment using an organic fertilizer amendment lowers cadmium availability in soil and cadmium uptake by rice on field micro-plot experiment planted in contaminated acidic paddy soil. Soil Sediment Contam Int J 28(1):1–14. https://doi.org/10.1080/15320383.2018.1525336
Zhang M, Zhai Z, Tian X, Duan L, Li Z (2008) Brassinolide alleviated the adverse effect of water deficits on photosynthesis and the antioxidant of soybean (Glycine max L.). Plant Growth Regul 56:257–264. https://doi.org/10.1007/s10725-008-9305-4
Zhang YP, He J, Yang SJ, Chen YY (2014) Exogenous 24-epibrassinolide ameliorates high temperature-induced inhibition of growth and photosynthesis in Cucumis melo. Biol Plant 58:311–318. https://doi.org/10.1007/s10535-014-0395-8
Zhang YP, Zhu XH, Ding HD, Yang SJ, Chen YY (2013) Foliar application of 24-epibrassinolide alleviates high-temperature-induced inhibition of photosynthesis in seedlings of two melon cultivars. Photosynthetica 51:341–349. https://doi.org/10.1007/s11099-013-0031-4
Zhang Z, Ramirez J, Reboutier D, Brault M, Trouverie J, Pennarun AM, Amiar Z, Biligui B, Galagovsky L, Rona JP (2005) Brassinosteroids regulate plasma membrane anion channels in addition to proton pumps during expansion of Arabidopsis thaliana cells. Plant Cell Physiol 46:1494–1504. https://doi.org/10.1093/pcp/pci162
Zhao W, Sun Y, Kjelgren R, Liu X (2015) Response of stomatal density and bound gas exchange in leaves of maize to soil water deficit. Acta Physiol Plant 37https://doi.org/10.1007/s11738-014-1704-8
Zhou YH, Yu JQ, Huang LF, Nogués S (2004) The relationship between CO2 assimilation, photosynthetic electron transport and water-water cycle in chill-exposed cucumber leaves under low light and subsequent recovery. Plant, Cell Environ 27:1503–1514. https://doi.org/10.1111/j.1365-3040.2004.01255.x
Zhou YL, Huo SF, Wang LT, Meng JF, Zhang ZW, Xi ZM (2018) Exogenous 24-Epibrassinolide alleviates oxidative damage from copper stress in grape (Vitis vinifera L.) cuttings. Plant Physiol Biochem 130:555–565
Acknowledgements
This research had financial support from Fundação Amazônia de Amparo a Estudos e Pesquisas (FAPESPA/Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), and Universidade Federal Rural da Amazônia (UFRA/Brazil) to AKSL. YCP had a scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil). ANSN and BTSA were supported with scholarships from Programa de Educação Tutorial (PET/Brazil).
Author information
Authors and Affiliations
Contributions
AKSL was the advisor for this project, planned all phases of the research, and critically revised the manuscript. YCP, ANSN, BTSA, BRSS, and MAMB conducted the experiments, performed physiological, biochemical, anatomical, and morphological determinations, and wrote and edited the manuscript. BLB carried out the nutritional determinations and critically revised the manuscript. AB critically revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pereira, Y.C., Nascimento, A.N., Aguiar, B.T.d. et al. Anatomical Modifications Modulated by Pretreatment with 24-Epibrassinolide Alleviate Boron Stress in Soybean Plants: Valuable Repercussions on Nutrient Contents, Photosynthesis, and Biomass. J Soil Sci Plant Nutr 22, 4533–4550 (2022). https://doi.org/10.1007/s42729-022-01053-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-01053-x