Abstract
Soil extracellular enzyme activities (EEAs) and enzymatic stoichiometry (ES) play essential roles in plant invasion processes. Nevertheless, how soil EEAs and ES respond to Amaranthus palmeri invasion and what their driving mechanisms remain poorly understood. We compared 22 pairs of A. palmeri–invaded plots and native vegetation plots in North China. Soil physicochemical properties and the potential activities of C-acquiring enzyme (β-1,4-glucosidase, BG), N-acquiring enzyme (β-N-acetyl-glucosaminidase, NAG), and P-acquiring enzyme (alkaline phosphatase, AKP) were determined. Compared with native vegetation soil, invasion by A. palmeri significantly altered the soil total C, ammonium nitrogen (N-NH4) content, and C:N ratio. Pearson’s correlation and random forest analysis showed that soil EEAs and ES were mostly correlated with soil pH, total N, total C, and available phosphorus (P-PO4). Redundant analysis demonstrated that soil total C was the major factor significantly explaining the variations in soil EEAs and ES of A. palmeri. Our findings highlight the effects of invasive species (A. palmeri) on soil chemical and biological properties, and enhance the understanding of soil microbial nutrient limitation; they also serve to reveal the mechanism of nutrient cycling and underground ecosystem processes mediated by A. palmeri invasion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biological invasion has been recognized as an important threat to biodiversity, ecology, and the economy (Richardson and Ricciardi 2013; Fang et al. 2021). Invasive exotic plants may become vectors of diseases and even promote species extinction (van der Putten et al. 2007; Vilà et al. 2010). It is expected that in the coming decades, various anthropogenic activities such as global trade and agriculture, will further intensify plant invasion (Stefanowicz et al. 2019). Plant invasion often alters and benefits from microbially mediated root-soil processes (Hawkes et al. 2005; Yin et al. 2020). Root-soil-microbe interaction systems constitute the rhizosphere microenvironment, and soil extracellular enzymes, as the key intermediate medium, are sensitive to changes in the soil environment (Nardi et al. 2000; Hawes et al. 2002). Soil extracellular enzyme activities can reflect the magnitude and direction of the soil biogeochemical cycle, which is a vital biological indicator for evaluating soil fertility, soil quality, and plant invasion (Xiao et al. 2020 Xie et al. 2021; Araujo et al. 2022). Microbes secrete different enzymes, but only a few can explain the majority of measurable activities because they target the most abundant substrates in the environment (Moorhead et al. 2016). For example, β-1,4-glucosidase, β-1,4-N-acetyl-glucosaminidase, and acid (alkaline) phosphatase, which are linked to carbon, nitrogen, and phosphorus metabolism, have been well researched in recent years (Allison et al. 2010, 2018; Jian et al. 2021). These three enzymes are considered representative indicators of overall C, N, and P acquisition for the following two reasons: (1) the activities of other related enzymes are usually lower than those of the three enzymes and have a strong correlation with them; (2) these three enzymes are not easily denatured and have a long half-life (Moorhead et al. 2016; Jian et al. 2021).
Soil extracellular enzymes involved in C, N, and P cycling have been thought to reflect soil nutrient availability and stability in the process of plant invasion. For instance, invasion by Falcataria moluccana considerably improved the activity of acid phosphatase, consistent with phosphorus limitation in the invaded area (Allison et al. 2006). The soil EEAs of acid phosphatase, β-glucosidase, urease, and N-acetyl-glucosaminidase increased significantly with the chronosequence of Acacia dealbata invasion (Souza-Alonso 2015). Pennisetum setaceum altered the protease activity related to N cycling and the structure and composition of soil bacterial communities, thereby changing the function of invaded ecosystems (Rodríguez-Caballero et al. 2017). Chenopodium ambrosioides enhanced soil EEAs and soil microbial biomass at the fruit stage, creating favorable conditions for its reproduction (Wang et al. 2017). Invasion by Solidago canadensis (Canada goldenrod) influenced the extracellular enzymatic activities related to C, N, and P cycling, thereby changing the biogeochemical cycle (Hu et al. 2021).
Amaranthus palmeri, native to the Sonoran Desert in North America, has become a serious invasive plant in China. This species is commonly found in wastelands, roadsides, riverbanks, and other habitats. To date, it has been widely spread to various habitats and even economic crop areas, such as farmlands and orchards, seriously damaging the native ecosystem and causing vast economic losses (Ward et al. 2013; Zhang et al. 2022). However, few studies have focused on the impact of A. palmeri on the soil ecosystem, and previous studies on the EEAs of invasive plant soils were limited in scale. Here, we investigated soil extracellular enzyme activities and their dominant drivers in 22 major invaded areas of A. palmeri. The spatial distribution of extracellular enzymes in rhizosphere soil was analyzed, the impacts of A. palmeri invasion on soil extracellular enzyme activities were discussed, and the main driving factors of soil extracellular enzyme activities were explored. Our results provide important knowledge for further clarifying the change in soil nutrient availability and nutrient limitation during A. palmeri invasion as well as a theoretical basis for comprehensively evaluating the effect of plant invasion on ecosystems.
2 Materials and Methods
2.1 Description of the Study Area
We established 22 research sites (38.74°–40.04°N, 116.36°–117.85°E) in areas without obvious human interference in Beijing, Tianjin, and Hebei, China (Fig. 1). The geographic information, environmental properties, habitat types, and accompanying plants of the invasive and native plots in the sampling sites are listed in Table S1 (Supplemental material). A paired plot design was used to explore the differences between invasive and native species by pairing the elevation, slope, and landscape to minimize the differences in environmental properties (McLeod et al. 2016). The study plots included (1) A. palmeri invasion patches, where the invasion period of A. palmeri exceeded 5 years and its cover exceeded 80%. (2) Native vegetation patches (control) with no occurrence of A. palmeri, only native species. The native patches were dominated by Setaria viridis, Chenopodium album, Chloris virgata, and Digitaria sanguinalis. The native patches were approximately 2–5 m away from the invasive patches to ensure that factors other than vegetation were relatively consistent. A total of 44 patches were established, which included 22 replicates (sites) × 2 patches (invasive vs. native), and the patch areas ranged from 5 × 5 to 25 × 25 m.
2.2 Sample Collection
At the end of August 2021, three 1 m2 plots were set up in each patch. In each plot, three soil samples were collected by pulling plants and shaking roots. Specifically, 8–10 A. palmeri plants were harvested in each invasive plot and all dominant plants were harvested in each native plot, and shaken vigorously until ~ 80% of the soil was removed. The remaining soil that was tightly adhered to the roots was brushed off and collected as the rhizosphere soil (Fan et al. 2017). The soil samples were sieved through 2-mm mesh sieves to remove stones, visible roots, and plant material. Soil samples from the three plots were mixed into one sample, and 44 soil samples were ultimately obtained. The collected soil samples were placed in an ice box and transported to the laboratory. All samples were divided into two subsamples. One subsample was stored in a 4 °C refrigerator for the determination of enzyme activity and soil ammonium/nitrate nitrogen content. The other subsample was air-dried for the determination of physical and chemical properties.
2.3 Soil Physicochemical Properties and Extracellular Enzyme Assays
The soil pH was measured by a pH meter at a water/soil ratio of 2.5꞉1, and the soil total C and total N content were measured by a Vario MACRO cube elemental analyzer (Elementar, Germany). The contents of nitrate nitrogen (N-NO3) and ammonia nitrogen (N-NH4) were extracted with 200 mL of 2 mol/L potassium chloride (KCl) solution and determined by a colorimetric method. The soil’s total P content was analyzed by the H2SO4-HNO3 fusion method. The soil available phosphorus (P-PO4) was determined by molybdenum antimony colorimetry with a spectrophotometer.
The activities of β-1,4-glucosidase (BG, C-acquiring enzyme), β-N-acetyl-glucosaminidase (NAG, N-acquiring enzyme), and alkaline phosphatase (AKP, P-acquiring enzyme) were determined by test kits (Grace Biotechnology Co., Ltd, Suzhou). Briefly, for NAG, 0.0500 g of fresh soil was incubated at 37 °C for 1 h with 150 µL of fluorometric substrate (4-nitrophenyl-N-acetyl-β-D-glucosaminide) dissolved in buffer (pH 6.2), 350 µL of terminator solution was added, and the mixture was then centrifuged at 12,000 rpm for 10 min. The absorbance was measured at 405 nm using a spectrophotometer. BG was estimated similarly to NAG, except that p-nitrophenyl-β-D-glucopyranoside was used as the substrate. For AKP, the buffer solution (pH 10) and substrate (nitrophenol phosphate) differed from the method applied to determine the activity of NAG. The soil enzyme activity was expressed as mmol h−1 g−1 fresh soil.
2.4 Statistical Analyses
2.4.1 Calculation Formula of Soil Microbial Element Limitation
The enzymatic C:N, C:P, and N:P ratios were calculated as follows: ln(BG):ln(NAG), ln(BG):ln(AP), and ln(NAG):ln(AP), respectively. Vector length (VL) and vector angle (VA) were used to characterize the element limitation of soil microorganisms. The calculation formula was as follows (Moorhead et al. 2016):
X = BG/(BG + AP)
Y = BG/(BG + NAG)
Vector length = SQRT(X2 + Y2)
Vector angle = DEGREES ((ATAN2(X; Y))
The vector length represented the energy limitation relative to nutrient limitation, and the vector angle represented the phosphorus limitation relative to nitrogen limitation. A vector angle greater than 45° represented phosphorus limitation, and a vector angle less than 45° represented nitrogen limitation.
2.4.2 Data Analysis
The differences in physicochemical properties and extracellular enzymes in rhizosphere soil between invasive and native plants were analyzed with independent sample t tests in SPSS 23.0 (IBM, USA). We performed Pearson’s correlation analysis between soil physicochemical properties and soil extracellular enzyme activities (EEAs) and enzymatic stoichiometry (ES) using OriginLab 2021 (OriginLab, USA). The inverse distance weighted (IDW) method was applied to examine the spatial distribution of soil EEAs and ES using ArcGIS 10.2 (Environmental Systems Research Institute, USA). Redundancy analysis (RDA) using Canoco 4.5 (Microcomputer Power, USA) was to explore the constraint relationship between selected factors and extracellular enzyme activities. The interpretation variable soil’s total N was eliminated due to the variance inflation factor (VIF) exceeding 10, and the main variables affecting the variation in EEAs were obtained by the forward selection method and Monte Carlo replacement test (P < 0.05). Random forest (RF) analysis was used to determine the predominant drivers of the three soil extracellular enzyme activities. To estimate the importance of soil variables, we used percentage increases in the MSE (mean squared error) of variables: higher MSE% values imply more important variables (Jiao et al. 2018). The RF analysis was performed using the lm and calc.relimp functions in the “relaimpo” package using R software version 4.0.5.
3 Results
3.1 Soil Physicochemical Properties in Invasive Plots and Native Plots
The soil in the study area was alkaline, with an average value of 8.3 in the invasive plots and 8.33 in the native plots. The soil physicochemical property values and stoichiometric ratio were higher in the native plots than in the invasive plots for all but one property (soil nitrate content). Significant differences were observed between A. palmeri–invaded plots and native vegetation plots for soil total C (P = 0.002), soil N-NH4 content (P = 0.041), and soil C:N ratio (P = 0.026). The differences in the remaining indicators were marginally significant or nonsignificant (Table 1).
3.2 Differences in Soil EEAs and Their Acquisition Ratios Between A. palmeri and Native Vegetation
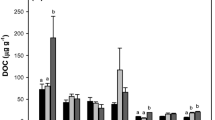
The average values of C-, N-, and P-acquiring enzyme activities were 168 mmol h−1 g−1 soil, 292 mmol h−1 g−1 soil, and 364 mmol h−1 g−1 soil in the rhizosphere of A. palmeri, respectively. The average values of C-, N-, and P-acquiring enzyme activities were 156 mmol h−1 g−1 soil, 267 mmol h−1 g−1 soil, and 375 mmol h−1 g−1 soil in the rhizosphere of native vegetation, respectively. The C- and N-acquiring enzyme activities in the rhizosphere soil of A. palmeri were slightly higher than those in the rhizosphere soil of native plants, whereas the P-acquiring enzyme activity exhibited the opposite patterns. These three EEAs did not show significant differences between A. palmeri and native vegetation soil (Fig. 2).
Inverse distance weight (IDW) analysis further indicated that N-acquiring enzyme activity in invasive and native plots showed similar spatial distribution patterns across the 22 sampling sites, with a higher activity in the eastern region. The C-acquiring enzyme activity in the invasive plots presented spatial distribution patterns similar to those of the N-acquiring enzyme activity. However, spatial variation in P- and C-acquiring enzyme activities was not observed across the native patches (Fig. 3).
The average values of soil enzyme C:N, C:P, and N:P ratio in the rhizosphere soil of A. palmeri were 1.85, 1.08, and 0.63, respectively. The average values of soil enzyme C:N, C:P, and N:P ratios in the rhizosphere soil of native vegetation were 2.05, 0.89, and 0.50, respectively (Fig. 2). Additionally, the C:N acquisition ratio showed similar spatial distribution patterns in the rhizosphere soil of A. palmeri and native vegetation (Fig. S2).
3.3 Pearson’s Correlation and Random Forest Analysis of the Soil Chemical and Biological Properties of Invasive and Native Plants
Pearson’s correlation analysis showed that N-acquiring enzyme activity in the rhizosphere soil of A. palmeri was significantly positively correlated with soil total N, total C, ammonia nitrogen content (N-NH4), and available phosphorus (P-PO4), and significantly negatively correlated with soil pH (P < 0.05). Soil N-acquiring enzyme activity responded to soil variables similarly in native plots. Significant correlations between C-acquiring enzyme activity and soil pH and P-PO4 and between P-acquiring enzyme activity and soil total C and P-PO4 were observed across the invaded plots. Moreover, the stoichiometric C:N imbalance was positively associated with soil pH and negatively associated with total N, total C, and N-NH4 content in invaded patches (P < 0.05). The three kinds of soil extracellular enzymes and their stoichiometric ratios were mainly related to soil pH, total N, total C, N-NH4 content, and P-PO4 (Fig. 4). Random forest analysis was used to test the relative importance of the variables affecting the extracellular enzymes (Fig. 5). Soil N-NH4 content and total C were the main variables driving the N-acquiring enzyme activity in invaded plots, and soil pH and total N were predominant for explaining N-acquiring enzyme (NAG) activity in native plots. C-acquiring enzyme (BG) activity in the invaded area was mainly affected by soil P-PO4 and total C, while soil N-NO3, pH, and P-PO4 mainly drove BG activity in the native area. The prevailing driver of P-acquiring enzyme (AKP) activity in invaded plots was soil total C, and AKP activity in native plots was mainly affected by soil pH. The selected factors can explain more than half of the variation in N-acquiring enzyme activity (A. palmeri, 62%; native vegetation, 53%), but most of the variation in P-acquiring enzyme activity has not been explained.
The importance of selected factors in explaining the variations of extracellular enzymatic activities of A. palmeri plots and native plots. Random forest was used to determine the variable importance. R2 (pseudo) represents the total variance that could be explained by selected factors. Negative R2 value indicates that the variance explained by the observed factors is less than that explained by their random values. **P < 0.01; *P < 0.05. Abbreviation: MAP, mean annual precipitation; MAT, mean annual temperature
3.4 Driving Variations in Soil Extracellular Enzymes and Enzymatic Stoichiometry of Invasive and Native Plants
Redundancy analysis (RDA) can reflect the constraint ability of selected variables on soil extracellular enzyme activities (Fig. 6). The first two axes (RDA1 and RDA2) explained 50.6% of the variation between extracellular enzyme activity and selected factors in the rhizosphere soil of A. palmeri, and 20.8% of the variation was significantly explained by soil total C (F = 5.25, P = 0.006). The first two axes (RDA1 and RDA2) explained 45.2% of the variation in extracellular enzyme activities in the rhizosphere soil of native plants, and 35.4% of the variation was significantly explained by soil pH (F = 6.06, P = 0.002) and P-PO4 (F = 3.66, P = 0.016).
4 Discussion
4.1 Response of Soil Physicochemical Properties to A. palmeri Invasion
The interaction between invasive plants and the underground ecosystem has been proposed as one of the potential mechanisms driving their establishment (Lee et al. 2012; Mincheva et al. 2014; Zhang et al. 2017). In this study, A. palmeri generally worsened the nutrient status of the invaded area (Table 1), but the change in nutrients also showed regional specificity (Fig. S1). Our results found that the soil total C content was significantly higher in native plots than in invasive plots (P = 0.002). This might be because most of the native sampling areas contained Poaceae species, such as Setaria viridis, which provided sufficient carbon sources for microorganisms and reduced the degree of microbial carbon restriction to native vegetation (Zhang et al. 2018). The soil C:N ratio is often inversely proportional to the decomposition rate of organic matter. The soil C:N ratio of native vegetation was significantly higher than that of invasive plants, indicating its lower decomposition rate of organic matter and consequent weakening or restriction of the metabolic activity of soil microorganisms (Allison and Vitousek 2005; Song et al. 2018). Invasive plants can affect the content of soil N-NH4 and N-NO3 through root activity (Kourtev et al. 2003; Rippel et al. 2020). The invasion by A. palmeri increased the N-NO3 content, but significantly reduced the N-NH4 content in rhizosphere soil (P < 0.05), indicating that A. palmeri may prefer to absorb and utilize N-NH4. Previous studies have indicated that the soil C:N:P ratio is mainly affected by the P content. Although the contents of C and N in soil change greatly, P deficiency is often the main reason for the increase in the soil C:P and N:P ratios (Tian et al. 2010). In the present study, the soil N:P ratio was higher than the mean value of land N:P in China (5.2), indicating the lack of phosphorus in the recipient ecosystems.
4.2 Response of Soil Extracellular Enzyme Activities to A. palmeri Invasion
The persistence of invasive plants can affect soil nutrient availability via the release of allelopathic chemicals to influence soil extracellular enzyme activities, making the soil environment not conducive to the growth of surrounding vegetation, thereby suppressing and excluding plants with higher nutrition requirements (Wang et al. 2017). The soil P- and C-acquiring enzyme activities in the current research were much higher than the N-acquiring enzyme activity, which suggested that microbes of A. palmeri allocate more resources for the production of enzymes related to P and C cycling. This result may be due to the higher carbon and nitrogen content in the rhizosphere of native plants reducing the secretion of microbial carbon and nitrogen hydrolases (Zhu et al. 2014). Compared with native vegetation, the increased C-acquiring enzyme activity (BG) consumed soil organic carbon and reduced the carbon source available for microorganisms. Moreover, more degradation products may be absorbed by plants to build biomass, underlying an intensified carbon limitation of A. palmeri (Lemanski et al. 2019; Zhong et al. 2020). This result was in accordance with the larger vector length of A. palmeri (Fig. 2). Resource allocation theory suggests that microorganisms increase the synthesis of EEAs corresponding to limited elements to meet their own nutrient requirements (Allison and Vitousek 2005; Waring et al. 2014). The enzymatic C:P and N:P ratios increased, and the enzymatic C:N ratio decreased significantly in the rhizosphere soil of A. palmeri, which further indicated that the carbon limitation in the invasive plots was relatively aggravated, while the P limitation was relatively alleviated. This was also confirmed by the vector angle and vector length analysis.
The soil N- and C-acquiring enzyme activities of the invasive plots and the N-acquiring enzyme activity of the native plots exhibited similar spatial distribution patterns, with a higher enzyme activity in the western parts of the sampling sites (Fig. 3), which was also reflected in the random forest analysis results. Only the N- and C-acquiring enzyme activities could be largely explained by the soil variables (Fig. 5). One possible reason for the lower explanation of the P-acquiring enzyme activity could be that the enzymes respond to the same factors in a site-specific manner, which may lead to neutral effects (Wang et al. 2022).
4.3 Drivers of Soil Extracellular Enzymes and Enzymatic Stoichiometry
Plant communities alter the soil nutrient content by stimulating nutrient metabolism, impacting soil enzyme activity (Pandey et al. 2014; Li et al. 2019). The N-, C-, and P-acquiring enzymes in the current study were strongly correlated with soil properties, especially with soil pH, total N, total C, N-NH4, and P-PO4 (Fig. 4). The strong correlations indicated that microbes could obtain relatively scarce resources by optimizing the distribution of C, N, and P during enzyme synthesis (Zhang et al. 2018; Dong et al. 2019; Gai et al. 2021). The RF analysis and RDA indicated that pH was the most important driving factor for soil extracellular enzymes and enzymatic stoichiometry in the rhizosphere soil of A. palmeri (Figs. 5, 6). Considerable investigations have confirmed the role of pH as a key factor influencing soil EEAs (Stone et al. 2014; Peng and Wang 2016; Wei et al. 2017).
The response of the soil N acquisition enzyme (NAG) was most strongly associated with the N-NH4 content in the rhizosphere soil of A. palmeri (Fig. 5). This was likely associated with the difference in nutrient utilization rate between invasive plants and native plants, resulting in a significant decrease in the N-NH4 content of A. palmeri (P < 0.05). Soil total C demonstrated significant or even extremely significant explanation rates for N-, C-, and P-acquiring enzymes in the rhizosphere soil of A. palmeri (Fig. 5). Moreover, RDA also showed that total C had the greatest explanation rate (20.8%) for C-, N-, P-acquiring enzymes and enzymatic stoichiometry (Fig. 5). Because it is a fast-growing plant, A. palmeri tended to allocate more resources for the growth of its aboveground biomass, and the input of available carbon sources (i.e., rhizosphere secretions) to its underground was reduced, which lead to accelerated metabolism of soil microorganisms, release of more extracellular enzymes into the rhizosphere, and provision of more nutrients and resources for the growth of invasive plants (Zhu et al. 2014; Zuo et al. 2018). The soil C:N and C:P ratios of native plants increased considerably, which caused phosphorus limitation in native vegetation (Kitayama et al. 2013; Fan et al. 2015; Zhang et al. 2018). Therefore, the explanation rate of soil P-PO4 for N- and C-acquiring enzyme activities was greater in the native vegetation rhizosphere soil (Fig. 5). The results of Monte Carlo analysis also showed that the EEAs and ES of native plants were largely dependent upon the soil P-PO4 concentrations (Fig. 6). Overall, the P limitation was alleviated, and the carbon limitation intensified in the A. palmeri–invaded ecosystems.
5 Conclusions
Our study found that invasion by A. palmeri was associated with higher soil C- and N-acquiring enzyme activity, and lower P-acquiring enzyme activity than that found in native vegetation soil. The enzymatic C:N ratio decreased significantly (P < 0.05), and the enzymic C:P and N:P ratios were higher in the invasive plots, indicating strengthened carbon limitation and weakened phosphorus limitation due to encroachment by A. palmeri. The alterations in soil extracellular enzyme activities and enzymatic stoichiometry were better explained by the total carbon in A. palmeri soil, whereas pH and available phosphorus were dominant drivers of soil extracellular enzyme activities and enzymatic stoichiometry in native vegetation soil. Our findings will be crucial in understanding the nutrient limitation and nutrient cycles of invasive plant soils.
References
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol and Biochem 37(5):937–944. https://doi.org/10.1016/j.soilbio.2004.09.014
Allison SD, Mcguire KL, Treseder KK (2010) Resistance of microbial and soil properties to warming treatment seven years after boreal fire. Soil Biol and Biochem 42(10):1872–1878. https://doi.org/10.1016/j.soilbio.2010.07.011
Allison SD, Romero-Olivares AL, Lu Y, Taylor JW, Treseder KK (2018) Temperature sensitivities of extracellular enzyme Vmax and Km across thermal environments. Global Change Biol 24(7):2884–2897. https://doi.org/10.1111/gcb.14045
Allison SD, Nielsen C, Hughes RF (2006) Elevated enzyme activities in soils under the invasive nitrogen-fixing tree Falcataria moluccana. Soil Biol and Biochem 38(7):1537–1544. https://doi.org/10.1016/j.soilbio.2005.11.008
Araujo ASF, Bonifacio A, Araujo Pereira APD, Medeiros EV, Araujo FF, Mendes LW (2022) Enzymatic stoichiometry in soils from physiognomies of Brazilian Cerrado. J Soil Sci Plant Nut. https://doi.org/10.1007/s42729-022-00840-w
Dong CC, Wang W, Liu HY, Xu XT, Zeng H (2019) Temperate grassland shifted from nitrogen to phosphorus limitation induced by degradation and nitrogen deposition: evidence from soil extracellular enzyme stoichiometry. Ecol indic 101:453–464. https://doi.org/10.1016/j.ecolind.2019.01.046
Fan HB, Wu JP, Yuan YH, JP, Hu L, Cai QK, (2015) Linkages of plant and soil C:N: P stoichiometry and their relationships to forest growth in subtropical plantations. Plant Soil 392:127–138. https://doi.org/10.1007/s11104-015-2444-2
Fan KK, Cardona C, Li YT, Shi Y, Xiang XJ, Shen CC, Wang HF, Gilbert JA, Chu HY (2017) Rhizosphere-associated bacterial network structure and spatial distribution differ significantly from bulk soil in wheat crop fields. Soil Biol Biochem 113:275–284. https://doi.org/10.1016/j.soilbio.2017.06.020
Fang K, Chen LM, Zhang HB (2021) Evaluation of foliar fungus-mediated interactions with below and aboveground enemies of the invasive plant Ageratina adenophora. Ecol and Evol 11:526–535. https://doi.org/10.1002/ece3.7072
Gai X, Li SC, Zhang XP, Bian FY, Yang CB, Zhong ZK (2021) Effects of chicken farming on soil extracellular enzyme activity and microbial nutrient limitation in Lei bamboo forest (Phyllostachys praecox) in subtropical China. Appl Soil Ecol 168:104106. https://doi.org/10.1016/j.apsoil.2021.104106
Hawes MC, Bengough G, Ponce CG, G, (2002) Root caps and rhizosphere. J Plant Growth Regul 21(4):352–367. https://doi.org/10.1007/s00344-002-0035-y
Hawkes CV, Wren IF, Herman DJ, Firestone MK (2005) Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol Lett 8:976–985. https://doi.org/10.1111/j.1461-0248.2005.00802.x
Hu ZY, Li JT, Shi KW, Ren GQ, Dai ZC, Sun JF, Li GL, Du DL (2021) Effects of Canada Goldenrod invasion on soil extracellular enzyme activities and ecoenzymatic stoichiometry. Sustainability-Basel 13:3768. https://doi.org/10.3390/su13073768
Jian ZJ, Ni YY, Zeng LX, Lei L, Xu J, Xiao WF, Li MH (2021) Latitudinal patterns of soil extracellular enzyme activities and their controlling factors in Pinus massoniana plantations in subtropical China. Forest Ecol Manag 495:119358. https://doi.org/10.1016/j.foreco.2021.119358
Jiao S, Chen WM, Wang JL, Du NN, Li QP, Wei QH (2018) Soil microbiomes with distinct assemblies through vertical soil profiles drive the cycling of multiple nutrients in reforested ecosystems. Microbiome 6:146. https://doi.org/10.1186/s40168-018-0526-0
Kitayama K (2013) The activities of soil and root acid phosphatase in the nine tropical rain forests that differ in phosphorus availability on Mount Kinabalu. Borneo Plant Soil 367(1–2):215–224. https://doi.org/10.1007/s11104-013-1624-1
Kourtev PS, Ehrenfeld JG, Häggblom M (2003) Experimental analysis of the effect of exotic and native plant species on the structure and function of soil microbial communities. Soil Biol and Biochem 35:895–905. https://doi.org/10.1016/S0038-0717(03)00120-2
Lee MR, Flory SL, Phillips RP (2012) Positive feedbacks to growth of an invasive grass through alteration of nitrogen cycling. Oecologia 170(2):457–465. https://doi.org/10.1007/s00442-012-2309-9
Lemanski K, Armbruster M, Bonkowski M (2019) Linking soil microbial nutrient limitation to fertilizer regime and sugar beet yield. Plant Soil 1–7. https://doi.org/10.1007/s11104-019-04114-w
Li QW, Liu Y, Gu YF, Guo L, Huang YY, Zhang J, Xu JF, Tan B, Zhang L, Chen LG, Xiao JJ, Zhu P (2019) Ecoenzymatic stoichiometry and microbial nutrient limitations in rhizosphere soil along the Hailuogou Glacier forefield chronosequence. Sci Total Environ 704:135413. https://doi.org/10.1016/j.scitotenv.2019.135413
McLeod ML, Cleveland CC, Lekberg Y, Maron JL, Philippot L, Bru D, Callaway RM (2016) Exotic invasive plants increase productivity, abundance of ammonia-oxidizing bacteria and nitrogen availability in intermountain grasslands. J Ecol 104:994–1002. https://doi.org/10.1111/1365-2745.12584
Mincheva T, Barni E, Varese GC, Brusa G, Cerabolini B, Siniscalco C (2014) Litter quality, decomposition rates and saprotrophic mycoflora in Fallopia japonica (Houtt.) Ronse Decraene and in adjacent native grassland vegetation. Acta Oecol 54:29–35. https://doi.org/10.1016/j.actao.2013.03.010
Moorhead DL, Sinsabaugh RL, Hill BH, Weintraub MN (2016) Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol Biochem 93:1–7. https://doi.org/10.1016/j.soilbio.2015.10.019
Nardi S, Concheri G, Pizzeghello D, Sturaro A, Rella R, Parvoli G (2000) Soil organic matter mobilization by root exudates. Chemosphere 41:653–658. https://doi.org/10.1016/S0045-6535(99)00488-9
Pandey D, Agrawal M, Bohra JS (2014) Effects of conventional tillage and no tillage permutations on extracellular soil enzyme activities and microbial biomass under rice cultivation. Soil till Res 136:51–60. https://doi.org/10.1016/j.still.2013.09.013
Peng XQ, Wang W (2016) Stoichiometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of northern China. Soil Biol Biochem 98:74–84. https://doi.org/10.1016/j.soilbio.2016.04.008
Richardson DM, Ricciardi A (2013) Misleading criticisms of invasion science: a field guide. Divers and Distrib 19:1461–1467. https://doi.org/10.1111/ddi.12150
Rippel TM, Iosue CL, Succi JP, Wykoff DD, Chapman SK (2020) Comparing the impacts of an invasive grass on nitrogen cycling and ammonia-oxidizing prokaryotes in high-nitrogen forests, open fields, and wetlands. Plant Soil 1-13. https://doi.org/10.1007/s11104-020-04458-8
Rodríguez-Caballero G, Caravaca F, Alguacil MM, Fernández-López M, Fernández-González AJ, Roldán A (2017) Striking alterations in the soil bacterial community structure and functioning of the biological N cycle induced by Pennisetum setaceum invasion in a semiarid environment. Soil Biol Biochem 109:176–187. https://doi.org/10.1016/j.soilbio.2017.02.012
Song MH, Guo Y, Yu FH, Zhang XZ, Cao GM, Cornelissen JHC (2018) Shifts in priming partly explain impacts of long-term nitrogen input in different chemical forms on soil organic carbon storage. Glob Change Biol 24:4160–4172. https://doi.org/10.1111/gcb.14304
Souza-Alonso P, Guisande-Collazo A, González L (2015) Gradualism in Acacia dealbata Link invasion: Impact on soil chemistry and microbial community over a chronological sequence. Soil Biol Biochem 80:315–323. https://doi.org/10.1016/j.soilbio.2014.10.022
Stefanowicz AM, Zubek S, Stanek M, Grześ IM, Rożej-Pabijan E, Błaszkowski J, Woch MW (2019) Invasion of Rosa rugosa induced changes in soil nutrients and microbial communities of coastal sand dunes. Sci Total Environ 677:340–349. https://doi.org/10.1016/j.scitotenv.2019.04.408
Stone MM, DeForest JL, Plante AF (2014) Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol Biochem 75:237–247. https://doi.org/10.1016/j.soilbio.2014.04.017
Tian HQ, Chen GS, Zhang C, Melillo JM, Hall CAS (2010) Pattern and variation of C:N: P ratios in China’s soils: a synthesis of observational data. Biogeochemistry 98:139–151. https://doi.org/10.1007/s10533-009-9382-0
van der Putten WH, Klironomos JN, Wardle DA (2007) Microbial ecology of biological invasions. ISME J 1:28–37. https://doi.org/10.1038/ismej.2007.9
Vilà M, Basnou C, Pyšek P, Josefsson M, Genovesi P, Gollasch S, Nentwig W, Olenin S, Roques A, Roy D, Hulme PE (2010) How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front Ecol Environ 8(3):135–144. https://doi.org/10.1890/080083
Wang YN, Li RY, Zhu XH, Ma DW, Zhang H (2017) Allelochemical stress effects of volatile oils from Chenopodium ambrosioides on extracellular enzyme activities and soil microbial diversity. Acta Ecol Sin 37(13):4318–4326 (In Chinese)
Wang PD, Li JL, Luo XQ, Ahmad M, Duan L, Yin LZ, Fang BZ, Li SH, Yang YC, Jiang L, Li WJ (2022) Biogeographical distributions of nitrogen-cycling functional genes in a subtropical estuary. Funct Ecol 36:187–201. https://doi.org/10.1111/1365-2435.13949
Ward SM, Webster TM, Steckel LE (2013) Palmer amaranth (Amaranthus palmeri): a review. Weed Technol 27(1):12–27. https://doi.org/10.1614/WT-D-12-00113.1
Waring BG, Weintraub SR, Sinsabaugh RL (2014) Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 117(1):101–113. https://doi.org/10.1007/s10533-013-9849-x
Wei H, Yan WB, Quan GM, Zhang JE, Liang KM (2017) Soil microbial carbon utilization, enzyme activities and nutrient availability responses to Bidens pilosa and a non-invasive congener under different irradiances. Sci Rep-UK 7:11309. https://doi.org/10.1038/s41598-017-11707-x
Xiao H, Yang HL, Zhao ML, Monaco TH, Rong YP, Ding HG, Qian SG, Zhao K, Wang DP (2020) Soil extracellular enzyme activities and the abundance of nitrogen-cycling functional genes responded more to N addition than P addition in an Inner Mongolian meadow steppe. Sci Total Environ 759:143541. https://doi.org/10.1016/j.scitotenv.2020.143541
Xie XF, Tao W, Zhu M, Jiang GJ, Xu Y, Wang XH, Pu LJ (2021) Comparison of random forest and multiple linear regression models for estimation of soil extracellular enzyme activities in agricultural reclaimed coastal saline land. Ecol Indicat 120:106925. https://doi.org/10.1016/j.ecolind.2020.106925
Yin LJ, Liu B, Wang HC, Zhang Y,Wang S, Jiang F, Ren YW, Liu H, Liu C, Wan F, Wang H, Qian WQ, Fan (2020) The Rhizosphere microbiome of Mikania micrantha provides insight into adaptation and invasion. Front Microbiol 11:1462. https://doi.org/10.3389/fmicb.2020.01462
Zhang P, Nie M, Li B, Wu JH (2017) The transfer and allocation of newly fixed C by invasive Spartina alterniflora and native Phragmites australis to soil microbiota. Soil Biol Biochem 113:231–239. https://doi.org/10.1016/j.soilbio.2017.06.003
Zhang W, Qiao WJ, Gao DX, Dai YY, Deng J, Yang GH, Han XH, Ren GX (2018) Relationship between soil nutrient properties and biological activities along a restoration chronosequence of Pinus tabulaeformis plantation forests in the Ziwuling Mountains, China. CATENA 161:85–95. https://doi.org/10.1016/j.catena.2017.10.021
Zhang M, Li XY, Shi C, Qiu ZL, Han JH, Wang KF, Zheng PF, Shi FC (2022) Driving factors, co‑occurrence networks, and metabolic profiles of soil bacterial communities within the root proximity of Amaranthus palmeri. J Soil Sci Plant Nut. https://doi.org/10.1007/s42729-022-00783-2
Zhong ZK, Li WJ, Lu XQ, Gu YQ, Wu SJ, Shen ZY, Han XH, Yang GH, Ren CJ (2020) Adaptive pathways of soil microorganisms to stoichiometric imbalances regulate microbial respiration following afforestation in the Loess Plateau. China Soil Biol Biochem 17:108048. https://doi.org/10.1016/j.soilbio.2020.108048
Zhu B, Gutknecht J, Herman DJ, Keck DC, Firestone MK, Cheng WX (2014) Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol Biochem 76:183–192. https://doi.org/10.1016/j.soilbio.2014.04.033
Zuo Y, Li JP, Zeng H, Wang W (2018) Vertical pattern and its driving factors in soil extracellular enzyme activity and stoichiometry along mountain grassland belts. Biogeochemistry 141:23–39. https://doi.org/10.1007/s10533-018-0499-x
Funding
This research was financially supported by the project of the Tianjin Municipal Education Commission, Tianjin, China (No. 2020YJSB121).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, M., Li, X., Qiu, Z. et al. Effects of Amaranthus palmeri Invasion on Soil Extracellular Enzyme Activities and Enzymatic Stoichiometry. J Soil Sci Plant Nutr 22, 5183–5194 (2022). https://doi.org/10.1007/s42729-022-00994-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-00994-7