Abstract
Seeking for effective and easy–to–implement tactics to mitigate the negative impacts on crop growth as a result of water shortage will remain the main objective in irrigation water rationalization programs. Therefore, along two growing seasons of 2018/19 and 2019/20, field trial was executed to assess the potentiality of humic acids and chitosan for diminishing the unwanted effects of drought in sugar beet yield and quality. The study involved three irrigation regimes (60, 80, and 100% of actual crop evapotranspiration, denoted ETc60, ETc80, and ETc100, respectively). Three humic acids rates (0, 15, and 30 L ha−1) and two chitosan levels (without and with 200 mg L−1) were applied. The trial implemented in a split–split plot design with three replicates. Enzymes activity, anatomical, agronomic, and quality traits have been estimated. Findings revealed that catalase (CAT) and glutathione peroxidase (GPX) activity substantially increased by increasing water deficit degree. There was insignificant difference between ETc80 and ETc100 in root and sugar yields ha−1 in both seasons. ETc60 recorded the highest values of sucrose %, potassium content, and extracted sugar % in both seasons, in addition to α–amino nitrogen in the first season and sugar lost to molasses in the second one. Humic acids markedly increased CAT activity in both seasons and GPX activity in the first one. Application of humic acids at a rate of 30 L ha−1 resulted in the maximum increases in root length, root and top fresh weights plant−1, top/root ratio, leaf area, and root and sugar yields ha−1. Except for sodium content, all other sugar quality traits showed the maximum increases with application of 30 L ha−1 humic acids. Chitosan-treated plants had higher activity of CAT and GPX and produced increases of 1.8, 4.2, 11.7, 7.5, 3.5, and 4.2% in root length, root fresh weight, top fresh weight, top/root ratio, leaf area, and root yield ha−1, respectively, compared to the untreated plants. Also, sucrose %, extracted sugar %, and sugar yield ha−1 showed significant increases with chitosan-treated plants higher than that of untreated ones. Application of humic acids (30 L ha−1) + chitosan (200 mg L−1), compared to no application, under ETc80, reduced the stomatal closure % from 48.86 to 31.06% with promising improvement in root and sugar yields and quality. In conclusion, the interactive effect of humic acids and chitosan exhibited favorable changes in antioxidant defense and stomata performance causing improvements in yield and sugar quality traits under low water supply. Thus, the moderate drought could be managed well with saving 20% of irrigation water by applying 30 L ha−1 humic acids plus 200 mg L−1 chitosan in sugar beet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Sugar beet (Beta vulgaris var saccharifera, L.) is considered the second source of sugar industry in the world, providing approximately 38% of sugar production (SCC 2020). Globally, the cultivated area of sugar beet reached ~ 5 million hectares in 2019 with production of ~ 309.6 million ton beet roots and an average productivity of 61.9 ton ha−1. In Egypt, according to the statistics for the year 2020 (SCC 2020), sugar beet crop contributed to 1.42 million ton of sugar, representing 62.1% of the total sugar production. Increasing sugar beet area along with raising the productivity per land unit is the main objective of the Egyptian government’s policy to increase sugar production in order to shrink the sugar gap between consumption and production which amounted to 0.968 million ton of sugar. However, sugar beet can be exposed to a variety of abiotic stresses such as drought, salinity, and extreme heat and cold, which have a significant impact on crop production (Hossain et al. 2022). Rationalizing water use means using irrigation water in appropriate manner and reducing losses, which is considered one of the most important solutions to face the water shortage issues. In several field crops, water deficit pattern is one of the practical strategies in crop irrigation programs to save water (El–Bially et al. 2018; Abd El-Mageed et al. 2019; Rady et al. 2020; Saudy et al. 2020; Abdou et al. 2021; El–Metwally et al. 2021a; b). However, sugar beet is a sensitive crop to water deficit, particularly in the early growing stages, and there is a positive relationship between water use and root yield production. Water stress is the major cause of sugar beet yield and quality depression, especially in the newly reclaimed lands. Water scarcity or deficit irrigation reduces plant growth and yield (Abd El–Mageed et al. 2021; El–Metwally et al. 2021a, b; Salem et al. 2021) due to production of reactive oxygen species (ROS), causing lipid peroxidation of membrane and interaction with other macromolecules (Yang et al. 2009; Bistgani et al. 2017). Under moderate or severe drought stress, plants close stomata and leaf pigments reduced causing reduction in photosynthesis rate (Yan et al. 2016; Saudy et al. 2021). Owing to drought, canopy expansion is reduced, leading to a drastic reduction of shoot/root ratios (Mohammadian et al. 2005). Accordingly, sugar beet irrigated with 80% of irrigation water requirement recorded the highest significant leaf area index, sucrose %, and extracted sugar %; however, the maximum root yield was gained with 100% of irrigation water requirement (EL–Darder et al. 2017). Abdel Fatah and Khalil (2020) revealed that shortening irrigation interval from 7 up to 3 days significantly increased stomatal pore area and root yield, while stomatal closure %, catalase enzyme activity, root length, α–amino nitrogen (N) content, and potassium (K) content and sugar lost to molasses were sharply declined. Limited irrigation reduced root length and sugar yield, whereas K, α–amino N, and sugar content increased (Ghaffari et al. 2021). The highest rates of photosynthesis and stomata conductance were obtained when sugar beet plants were irrigated with 100% of full irrigation (Khozaei et al. 2021). Therefore, it is necessary to devote more efforts to manage drought stress with economic solutions and eco-friendly tactics.
Several researchers highlighted the advantages of humus substances (humic and fulvic acids) application in improving crop growth and productivity, retaining moisture, and mitigating drought effects. Organic products such as humic acids are regarded as one of the natural antioxidants, which enhance total phenols, total flavonoids, antioxidant activity, and nutrient content in leaves (Bayat et al. 2021). The tolerance improvement of sugar beet plants to drought stress by using humic extract may be due to the balance of quinones present in the extract that can positively modulate ROS level (Monda et al. 2021). Furthermore, using humin materials affects plant cell membranes, leading to enhanced transport of minerals, promoted photosynthesis, modified enzyme activities, solubilization of elements, and enhanced water use efficiency (Bagheri 2010; Rady et al. 2016;). Enan et al. (2016) showed that soil drench with 36 L ha−1 humic acid significantly increased root fresh weight plant−1, leaf area index, K content, gross sugar %, and root and sugar yields. Mohamed et al. (2017) clarified the favorable effect of humic acid application on root dimensions, K content, α–amino N content, sucrose %, and root and sugar yields. Wilczewski et al. (2018) stated that humistar (12% humic acid) applied to the soil improved sugar beet yield, thereby increasing the yield of sugar. Also, Abd El–Haleim (2020) pointed out that increasing level of K humate significantly increased juice K content, root length, root fresh weight plant−1, and root and sugar yields of sugar beet, while Na and α–amino N content were decreased. Mekdad et al. (2021) reported that the addition of 48 kg humic acid ha−1 positively affected sunflower plant growth, biochemistry, and productivity.
There are many reports suggested that chitosan and its oligomers triggered several defense responses in plants and increased drought tolerance. Chitosan is a deacetylated chitin derivative structurally similar to glycosamine and N–acetylglucosamine units. Chitosan enhances antioxidant enzymes and hydrogen peroxide (H2O2) signaling pathways, thereby contributing to enhance scavenging activities of ROS (Guan et al. 2009). Moreover, chitosan stimulates plant growth and development by increasing the availability of water uptake and essential nutrients (Hidangmayum et al. 2019; Bibi et al. 2021). Foliar spray with chitosan (200 mg L−1) improved shoot and root length of seedling, number of leaves, and chlorophyll content in cucumber under abiotic stress (Ali et al. 2020). Marzouk et al. (2022) showed that plant length, leaf fresh weight and its dry matter, and total yield of cauliflower were positively affected by foliar spraying of 250 mg L−1 chitosan, compared to untreated plants, under abiotic stress.
Nevertheless, little knowledge is available about the complementary protective effect of humic and chitosan for enhancing the defense mode of sugar beet under different drought degrees. Accordingly, based on their useful mechanisms, we hypothesized that the combined effects of humic and chitosan could have the potentiality to reduce the hazard impacts of drought. Therefore, the current study was conducted to find out the optimal levels of humic acids and chitosan under different irrigation water regimes to attain the maximum productivity and the best quality of sugar beet.
2 Materials and Methods
2.1 Study Area Attributes
Along two growing seasons of 2018/19 and 2019/20, field experiment was performed at a private farm located at 64th km, Cairo–Alexandria Desert Road (31.14° N, 31.39° E), Giza Province, Egypt. The aim was to investigate the combined effect of humic acids, as an organic soil amendment, chitosan and irrigation regime on growth, physicochemical characteristics, yield and quality of sugar beet grown in a sandy soil. Soil samples were collected at depths of 0–50 cm to determine the initial physical and chemical properties and water status of the experimental soil. Soil analysis was done according to the methods of Jackson (1973) and Black et al. (1981), and the obtained values are presented in Table 1. According to soil taxonomy (IUSS Working Group WRB 2015), the soil is order Entisols and suborder psamments.
2.2 Experimental Design and Treatments
In a split–split plot design with three replicates, 18 combinations of three irrigation regimes, three levels of humic acids, and two levels of chitosan were implemented. In the main plots, irrigation regimes were applied as ratio of actual crop evapotranspiration at 60% (severe stress), 80% (moderate stress), and 100% (well-watered), denoted ETc60, ETc80, and ETc100, respectively. Humic acids levels (0, 15 and 30 L ha−1) were distributed in the subplots, meanwhile the sub–sub plots were assigned to chitosan concentrations (without and with 200 mg L−1, using water as a carrier/solvent, 720 L ha−1). Each level of humic acids was added as soil addition through drip irrigation system in 3 equal portions, after thinning, 4–6 true leaf stage, i.e., 30 days after sowing (DAS), 60, and 90 DAS. Humic acid product had 26.1% humic substances (involving 15.0% humic acid and 10% fulvic acid), 3.3% nitrogen, 1.2% phosphorous, and 4.1% potassium and was obtained from the Microbiology Research Department, Soil, Water and Environment Research Institute, Agricultural Research Center (ARC), Giza, Egypt. Chitosan was sprayed on sugar beet foliage in two equal doses, 45 and 60 DAS. Owing to the low solubility of chitosan in water, it was dissolved in 100 ml of water with the addition of some drops of acetic acid as a weak acid, and then, the volume was completed to 1000 ml to obtain the required concentration. Chitosan (prepared from shrimp exoskeletons) was obtained from the Agricultural Microbiology Department, Agriculture and Biology Institute, National Research Center (NRC), Giza, Egypt.
2.3 Calculations Related to Irrigation
The values of reference evapotranspiration (ETo) were calculated using average of the previous 7 years of weather data obtained from Central Laboratory for Agricultural Climate, ARC, Egypt, using FAO Penman–Monteith equation (Allen et al. 1998). The crop evapotranspiration (ETc) values were calculated according to Eq. (1):
where:
ETc: crop evapotranspiration (mm day−1).
ETo: reference evapotranspiration (mm day−1).
Kc: crop coefficient values (Doorenbos and Kassam 1979). Kc values were 0.40, 0.80, 1.05, and 0.70, in the initial (35 days), development (60 days), mid-season (70 days), and late-season (40 days) crop stages, respectively.
The depth of applied irrigation water was calculated according to the Eq. (2) given by Vermeirer and Jopling (1984) as follows:
where:
AIW: depth of applied irrigation water (mm),
ETc: crop evapotranspiration (mm day−1),
I: irrigation intervals (day),
Ea: irrigation system efficiency, 0.90,
LR: leaching water requirements (since electrical conductivity of soil solution is low, LR was neglected)
Due to the nature of sugar beet seeds as hard and poor in endosperm, beet plants are generally sensitive to water stress during early stage of germination and emergence. Therefore, the studied irrigation water regimes started after 30 DAS. The total seasonal amounts of the applied irrigation water were 3866.4, 4978.3, and 6090.2 m3 ha−1 in the 1st season, and 4084.2, 5250.5, and 6416.9 m3 ha−1 in the 2nd season, for ETc60, ETc80, and ETc100 treatments, respectively.
2.4 Crop Husbandry
During seed bed preparation, single super phosphate (15% P2O5) at a rate of 31.4 kg P ha−1 was added. Field was divided into experimental plots with an area of 24 m2, including 4 ridges of 60 cm in width and 10 m in length. In the 1st week of October, seeds of multi-germ sugar beet variety, namely “Hamza” were sown in hills, 25 cm distance. Plants were thinned 30 DAS to secure one plant per hill. Nitrogen fertilizer was applied at a rate of 288 kg N ha−1 as ammonium nitrate (33.5% N) in 5 equal portions, at 30, 45, 60, 75, and 90 DAS. Potassium fertilizer was added at a rate of 95.5 kg K ha−1 as potassium sulfate (48% K2O) in 3 equal portions, at 60, 75, and 90 DAS. Other field practices were done as recommended by Sugar Crops Research Institute, ARC, Egypt.
2.5 Measurements
2.5.1 Enzymes Activity
A representative sample of ten plants was randomly taken from each experimental plot 110 DAS (initiation of storage root development stage) to measure catalase enzyme (CAT) activity (Aebi 1984) and glutathione peroxidase enzyme (GPX) activity (Flohé and Günzler 1984) in leaves.
2.5.2 Anatomical Study
At 110 DAS, stomatal closure % and stomatal pore area (µm) for adaxial (upper) surface of fully expanded leaves were measured through scanning electron microscope (SEM), Model Quanta 250 FEG (Field Emission Gun) attached with EDX Unit (energy dispersive x-ray analyses), with accelerating voltage 30 k.v., magnification 14 × up to 1,000,000 and resolution for Gun. 1 n), at the Egyptian Mineral Resources Authority, Central Laboratories Sector, Egypt. For deducing the digital reading, the obtained SEM images were processed using ImageJ software (version 1.53a, National Institute Health, USA).
2.5.3 Growth Criteria and Root Yield
At 110 DAS, leaf area was measured using a Li–Cor area meter LI–3000 (Li–Cor., Inc., Lincoln, Nebraska, USA). At harvest (210 DAS), a sample of ten plants was randomly taken from the middle ridges of each experimental plot to determine root length, root fresh weight plant−1, top fresh weight plant−1, and top/root ratio. Moreover, root yield ha−1 was estimated based on the whole plants of the experimental unit.
2.5.4 Quality Parameters and Sugar Yield (at Harvest)
At the Laboratory of El–Nubaria Sugar Factory, El–Beheira Governorate, Egypt, sucrose % and impurities content were determined in fresh sugar beet roots. Sucrose % was determined using saccharometer according to the method described in AOAC (2012). Impurities content, i.e. potassium (K), sodium (Na) and α–amino N (meq100g−1 beet) in roots were estimated (Cooke and Scott 1993). Sugar lost to molasses % was calculated using Eq. (3) (Deviller 1988). Extracted sugar % was computed by Eq. (4) (Dexter et al. 1967). After that, sugar yield ha−1 was calculated by multiplying root yield ha−1 by extracted sugar %.
2.6 Statistical Analysis
The recorded data of the two seasons were statistically analyzed according to Casella (2008), using MSTAT–C computer software package (developed by the Crop and Soil Sciences Department, Michigan State University, USA). Duncan’s multiple range test was used for separating the means only when the F–test indicated significant (p ≤ 0.05) differences among the means.
3 Results
3.1 Main Effects
3.1.1 Antioxidant Enzymes Activity
The antioxidant enzymes activity, i.e., catalase (CAT) and glutathione peroxidase (GPX), substantially increased by increasing water deficit degree, since ETc60 possessed the maximum values. However, insignificant difference between ETc60 and ETc80 for GPX in both seasons was found (Table 2). Regarding the used organic soil amendments, Table 2 points out that soil drench with humic acids markedly increased CAT activity in both seasons and GPX activity in the 1st one. No significant variances were noticed between humic acid levels of zero and 15 L ha−1 as well as between 15 and 30 L ha−1 in GPX enzyme activity in the 1st season, while significant increase was recorded due to 30 L ha−1 than zero. Moreover, antioxidant enzyme activity was considerably increased, when sugar beet foliage was sprayed with chitosan at the rate of 200 mg L−1, in both seasons (Table 2).
3.1.2 Stomatal Parameters
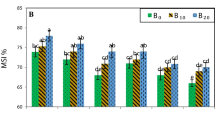
Scanning electron microscopic images (Fig. 1) illustrated that stomatal closure % progressively increased and pore area decreased with decreasing water supply. The reductions in stomata opening were 2.77 and 1.87 times with ETc60 and ETc80 compared to ETc100, respectively. Moreover, the reduction in stomatal pore area due to ETc60 was 49.3% compared to ETc100. Under no humic acid addition, ETc60 without chitosan showed the highest value of stomatal closure % and the lowest stomatal pore area, while application of humic acids (30 L ha−1) plus chitosan (200 mg L−1), compared to no application, under ETc80 reduced the stomatal closure % from 48.86 to 31.06% (Fig. 2).
Scanning electron microscopic of adaxial (upper) surface stomata of sugar beet leaf under ETc100 (a), ETc80 (b), and ETc60 (c). The images clarify the stomatal closure % is 26.02, 48.86 and 72.12% as well as the stomatal pore area is 33.78, 33.77 and 17.11 µm with ETc100, ETc80, and ETc60, respectively. ETc100, ETc80, and ETc60: Irrigation by 100, 80 and 60% of crop evapotranspiration, respectively
Stomatal closure % and pore area (µm) for adaxial (upper) surface of sugar beet leaf as affected by different combinations of irrigation regime, humic acids and chitosan (a), and scanning electron microscopic image of adaxial (upper) surface stomata of sugar beet leaf under ETc80 x Hum3 x Chit2 (b); the image clarifies that the stomatal closure % is 31.06% and the stomatal pore area is 43.94 µm. ETc100, ETc80, and ETc60: Irrigation by 100, 80 and 60% of crop evapotranspiration; Hum1, Hum2, and Hum3: 0, 15, and 30 L ha−1 humic acids; Chit1 and Chit2: 0 and 200 mg L−1 chitosan, respectively
3.1.3 Growth Criteria and Root Yield
Data in Table 3 disclose that root fresh weight plant−1, top fresh weigh plant−1, leaf area, and root yield ha−1, in both seasons, in addition to top/root ratio in the 1st one, were drastically decreased by decreasing irrigation water level from ETc80 to ETc60. While root length was sharply increased as water supply decreased, except for root length in both seasons, root fresh weight plant−1 (in the 1st season) and leaf area (in the 2nd one), there were insignificant differences between ETc80 and ETc100 in their influence on the other growth traits and root yield in both seasons. Meanwhile, decreasing irrigation water quantity from ETc80 to ETc60 led to a considerable reduction in root yield ha–1 amounted to 9.98% in the 1st season and 10.91% in the 2nd one.
Application of humic acids at a rate of 30 L ha−1 resulted in the maximum increases in all growth traits and root yield, in both seasons. However, the differences between 15 L ha−1 and 30 L ha−1 of humic acids did not reach the level of significance in top/root ratio, in the 2nd season (Table 3). Soil drench with 30 L humic acids ha−1 had a statistical increment in root yield ha−1 amounted to 9.7% and 6.6%, in the 1st and 2nd season, consecutively, as compared to no application.
Regarding chitosan effect, the results in Table 3 clarify that foliar spray with 200 mg L−1 on sugar beet foliage significantly increased all the previously mentioned traits in both seasons, compared to untreated plants. In this respect, chitosan-treated plants produced statistical increments (average of the two seasons) of approximately 1.8, 4.2, 11.7, 7.5, 3.5, and 4.2% in root length, root fresh weight plant−1, top fresh weigh plant−1, top/root ratio, leaf area, and root yield, successively, greater than the untreated ones.
3.1.4 Quality Parameters and Sugar Yield
Results in Table 4 prove that the examined water regimes had substantial effects on sugar yield and sugar quality parameters, in both seasons, except Na content in both seasons, α–amino N in the 2nd season, and sugar lost to molasses in the 1st one. ETc60 recorded the highest and significant values of sucrose %, K content, and extracted sugar % in both seasons, in addition to α–amino N in the 1st season and sugar lost to molasses in the 2nd one, compared to ETc100. Sucrose % and extracted sugar % in both seasons and α–amino N in the 1st season produced with ETc80 significantly equaled that of ETc60. On the contrary, sugar yield showed higher increase with ETc80, but statistically leveled ETc100 in both seasons. Statistical increases in sugar yield ha−1 were 8.77 and 8.63% in the 1st and 2nd season, consecutively, owing to raising the amount of irrigation water from ETc60 to ETc80.
Except for Na content, all other sugar quality traits showed the maximum increases with application of 30 L ha−1 humic acids, but without significant differences with 15 L ha−1 humic acids for sucrose % and extracted sugar % in the 1st season as well as α–amino N and sugar lost to molasses in both seasons (Table 4). Humic-untreated plots recorded the highest values of Na content in both seasons. Soil drench with humic acids at 30 L ha−1 recorded an appreciable increase of 9.82% and 9.12% in sugar yield ha−1, in the 1st and 2nd season, respectively, relative to that given by 15 L ha−1.
Sucrose %, extracted sugar %, and sugar yield possessed significant increases with chitosan-treated plants higher than that of untreated ones in both seasons (Table 4), while chitosan-untreated plants produced the highest K content and sugar lost to molasses in both seasons, as well as Na and α–amino N contents, in the 1st and 2nd season, respectively.
3.2 Significant Interaction Effects
3.2.1 First-Order Interactions
The combinations of irrigation regime and humic acids showed that ETc60 × humic acids (30 L ha−1) application recorded the maximum root length and K content in the 1st season (Table 5), while ETc80 × humic acids (30 L ha−1) in both seasons and ETc80 × humic acids (15 L ha−1) in the 1st season possessed the maximum root fresh weight plant−1. In the 2nd season, ETc100 × any humic acids treatment and ETc80 × humic acids 15 or 30 L ha−1 were the effective interactions for enhancing root yield ha−1.
The maximum increase in CAT activity was more pronounced under ETc60 with chitosan supply (200 mg L−1) in the 1st and 2nd seasons (Table 6). ETc100 × chitosan (200 mg L−1) was the efficient combination for increasing top fresh weigh plant−1 and top/root ratio in the 1st season as well as leaf area in the 2nd season. In the 1st season, irrigating sugar beet by ETc80 or ETc100 × with or without chitosan (for K content) and ETc100 × 200 mg L−1 chitosan (for α–amino N content) recorded the lowest values.
Regarding the interaction between humic acids and chitosan, findings in Table 7 generally clarify that application of humic acids (30 L ha−1) plus chitosan (200 mg L−1) practice had the potential to increase top fresh weigh plant−1, root yield ha−1, sucrose %, and extracted sugar % and sugar yield ha−1 in the 1st season as well as GPX, root length, root fresh weigh plant−1, and leaf area in the 2nd season.
3.2.2 Second-Order Interaction
The results in Table 8 reveal a statistical and positive response in sugar and root yields ha−1 by increasing organic acids level from 15 to 30 L ha−1 associated with foliar application of 200 mg L−1 chitosan, when beets were irrigated with ETc60 and/or ETc80, in the 1st season. Likewise, in the 2nd one, a positive response was observed in sucrose% and sugar yield under ETc60 and/or ETc100 with the same raising levels of organic acids and chitosan, with no significant influence under ETc80. Under different water regimes, increasing organic acids level from 15 to 30 L ha−1 + 200 mg L−1 chitosan considerably increased the enzyme activities of CAT (in the 1st season) and GPX (in the 2nd one), as well as CAT enzyme under severe water stress and/or well-watered, in the 2nd one.
The combination of ETc80 + 30 L ha−1 humic acids + 200 mg L−1 chitosan was more distinct, as it achieved a sharp increment reached 12.55% in root yield ha−1, corresponding to 9.26% in sugar yield ha−1, in the 1st season, as compared to that obtained with the same levels of humic acids and chitosan, under ETc60.
4 Discussion
Though the drought generally affects the plant growth and physiology, its influences are relaying on the intensity of the drought (severe or moderate). In this situation, the current study showed that irrigating sugar beet with 80% of crop evapotranspiration (ETc80) gave close responses to that of full irrigation (ETc100). This was obviously found when plants produced similar values of antioxidant enzymes, especially GPX, (Table 2), root yield and its parameters (Table 3), stomatal pore area (Fig. 1), and sugar yield (Table 4) under ETc80 as ETc100. Contrariwise, reducing water supply up to 60% of crop evapotranspiration (ETc60) caused severe depression in sugar beet physiological, anatomical, and agronomic traits. In this regard, higher increase in antioxidant activity and higher reductions in stomatal parameters and yield traits were obtained owing to ETc60. The increase in activity of enzymes under drought conditions might be attributed to low-molecular weight antioxidants (like carotenoids, tocopherols, GPX, and ascorbic acid); in this respect, production of ROS is stimulated in plants as a response to drought stress (Bistgani et al. 2017). Moreover, accumulation of CAT and GPX in sugar beet leaves could protect the plant from oxidative damage such as lipid peroxidation and protein oxidation (Sayfzadeh and Rashidi 2011). Herein, Monda et al. (2021) stated that the level of GPXs gave a power indicator about the oxidative stress impacts, especially inhibition of protein, which induced by severe drought. Stomatal movement regulation is another most aspect of plant response to water shortage condition. SEM image analysis of fully extended leaves indicated the level of drought that the beet crop could tolerate as slight differences in stomata pore area were found between beets irrigated with ETc80 and ETc100 (Fig. 1). In the stressed plants, accumulation of abscisic acid is stimulated in leaves, which sets up ionic imbalance that compels potassium ion (K+) to leak out from guard cells and loss of guard cell turgor pressure. Thus, narrowing the aperture mostly would be due to reduced leaf relative water content and increased stomatal closure (Fig. 1). Also, the adverse impact of drought on sugar beet might be attributed to a decrease in the activity of meristemic tissues responsible for elongation and cell division under insufficient water condition. Thus, higher root/shoot ratio is one of the processes that plants resort to produce more developed root systems for cope with drought conditions, (Du et al. 2020), which matches with our results. Mohammadian et al. (2005) reported that since limited shoot growth occurred with severe drought stress, the ratio of shoot to root dry weight in sugar beet was severely reduced. The root yield and its parameters as root dimensions, weights, and biomass were served as an evidence of morphological changes under water deficit (Meng et al. 2018). Moreover, results of Wang et al. (2017) proved that proteins involved in CO2 fixation or in energy metabolism are profoundly affected by drought. Accordingly, the decrement in root yield with drought could be attributed to morphological mechanisms, performed by plants to be able to withstand drought conditions, where roots tend to grow downwards in the soil searching for water. Significant reduction in sugar beet yield owing to low water supply was reported (Abd El–All and Makhlouf 2017; EL–Darder et al. 2017; Abdel Fatah and Khalil (2020). Low water supply not only influences growth and yield but also sugar quality. Utilization of nutrients uptake and soil organic matter and activity significantly affected by irrigation regime (Saudy and El–Metwally 2019; Mubarak et al. 2021). Herein, both ETc60 (severe stress) and ETc80 (moderate stress) outperformed ETc100 (well-watered) in sucrose % and extracted sugar % (Table 4). Unlike, increases in sugar impurities, i.e., K, α–amino N, and sugar lost in molasses, were sharply increased due irrigation by ETc60. Since root of sugar beet has low moisture content under low water supply, increase in sugar content is expected. In addition, sucrose and hexoses sugars have dual functions by regulating the expression of stress-related genes involved in photosynthesis, osmolyte synthesis, and sucrose metabolism (Khan et al. 2020), which lead to an increase in extracted sugar % despite of increases in the proportion of impurities by moderate drought stress (Makhlouf and Abd El–All 2017; Abdel Fatah and Khalil 2020). Moreover, α–amino N group, represents 85% of the overall amount of the nitrogenous osmolytes, plays a vital role in free amino acid synthesis such as glycine betaine and proline which cause diverse roles in increasing the ability of cells to retain water without affecting normal metabolism (Clarke et al. 1996), and subsequently leads to an increase in sugar lost in molasses.
The synergistic effect of humic application toward antioxidant enzymes (Table 2) could be attributed to the fact that humic substances are powerful antioxidants having ROS scavenging properties (Wang et al. 1996; Avvakumova et al. 2011). Moreover, Bayat et al. (2021) cleared that application of humic and fulvic acids mitigated the adverse effects of drought by increase total phenols, total flavonoids, and antioxidant activity of the leaves. Also, soil addition of humic acids at the rate of 30 L ha−1 recorded appreciable increases in sugar beet yield parameter greater than zero or 15 L ha−1 (Table 3). Since humic substances enhance the soil ability to retain nutrients with reducing soil pH and improve growth parameters, photosynthetic pigments and antioxidants, stimulation of beneficial microbial activity, and improvement in root productivity were achieved (Kabeel et al. 2008; Kandil et al. 2020; Bayat et al. 2021). These results also agree with Ibrahim et al. (2019) and Alotaibi et al. (2021). Since humic acids serve as catalystic for the formation of osmolytic solutes, soil N and K content could increase with humic application, resulting in increases of K and α–amino N content in leaves (Bayat et al. 2021). Additionally, the increases in sugar yield (Table 4) at higher level of humic substances (30 L ha−1) may be due to achieving the increase in root yield ha−1 (Table 3) along with sucrose % increase (Table 4). Alotaibi et al. (2021) stated that soil drench with 10 L ha−1 of humic acid statistically increased sugar and root yields t ha−1 of sugar beet compared to the check treatment. Also, Ibrahim et al. (2019) revealed that increasing humic acid considerably increased sugar and root yields, K, and white sugar % and markedly decreased extraction %, α–amino N, and Na content. El–Hassanin et al. (2016) noticed improvements in sucrose, extractable sugar, purity, sugar lost to molasses, extractability percentages, and yield of sugar beet with humic acids treatment.
Chitosan plays a numerous tasks defense response related to diverse stresses, such as drought stress which is the focus of our research. Being the values of catalase and glutathione peroxidase were higher in chitosan-treated plants than the untreated ones (Table 2), it looks like that chitosan has a distinctive role for motivating the antioxidants activity in sugar beet, since it has hydroxylated amino group which offers an effective scavenger of ROS (Sun et al. 2008). The enhancement in production of antioxidant enzymes owing to chitosan application has been reported (Yin et al. 2008). The promotive effect of chitosan on growth and yield parameters (Table 3) may be ascribed its potentiality to enhance the availability and uptake of water and major nutrients through adjusting cell osmotic pressure and improving enzyme activities (Guan et al. 2009; Yang et al. 2009; Martins et al. 2018). The beneficial impact of chitosan foliar spray could be attributed to its direct antitranspirant coating, induction of stomatal closure through ABA synthesis, and accumulation of stress protective enzymes (Hidangmayum et al. 2019). Since chitosan enhanced root growth, reinforcement the efficiency of water absorption (Zeng and Luo 2012), and increased nutrients uptake (Dzung 2007) were achieved, thus sugar beet yield and its attributes improved. Additionally, our results pointed to a positive effect of chitosan in reducing root impurities and sugar lost in molasses, while improving sucrose %, extracted sugar %, and sugar yield (Table 4). It has been proved that chitosan can enhance the metabolism of sugars (Sun et al. 2019). Foliar application of chitosan enhanced leaf membrane stability and increased antioxidant enzymes in apple (Yang et al. 2009).
The significant variance between 15 and 30 L ha−1 of humic acids under moderate and severe drought stress in root fresh weight plant−1 and root yield (Table 5) referred to the beneficial role that organic acids play in improving soil holding capacity of water and nutrients, and raising plant growth and utilizing them, in addition to enhancing antioxidant defense system (Khodadadi et al. 2020).
The increases in K and α–amino N contents achieved when plants were sprayed with 200 mg L−1 chitosan, under ETc60 (Table 6), reflected the importance function of amino groups in chitosan structure which plays a main role in free amino acids synthesis to support the osmolytes solution under abiotic stress (Guan et al. 2009). In addition, the observed improvement in CAT with spraying chitosan under different water regimes could be attributed to that the antioxidant system creates protection versus oxidative harm, which has been found to increase the lifetime of active oxygen species within the cellular environment (Hidangmayum et al. 2019). Also, chitosan can reduce the inhibition of roots under drought stress and improve growth which implies its ability to promote root system and to absorb more water and to keep the moisture stable (Li et al. 2017).
The significant interaction between humic acids and chitosan (Table 7) which was observed in results of CAT, GPX, growth criteria, sucrose%, extracted sugar%, and root and sugar yields ha−1 confirmed that they played a major role in intensive canopy development in relation to the root growth by enhance nutrient uptake, increase in H2O2 accumulation and induction of stomatal closure, ROS enzyme activities (Martins et al. 2018; Hidangmayum et al. 2019; Bayat et al. 2021; Khozaei et al. 2021), thus improved carbohydrates metabolism and productivity of sugar beet crop.
It is worthy to note that under different water regimes, positive effects were observed with the gradual increase of humic acids up 30 L ha−1 and spraying of chitosan (200 mg L−1), which reduced stomatal closure and improved stomatal pore area, as compared to untreated plants (Fig. 1). ETc80 × 30 L ha−1 humic acids × 200 mg L−1 chitosan was the most effective combination for adjusting the stomata opening by improving the stomatal pore area (Fig. 2). Application of ETc80 × 30 L ha−1 humic acids × 200 mg L−1 chitosan changed the stomatal closure from 48.86 to 31.06% and stomatal pore area from 33.77 to 43.94 µm, compared to ETc80 alone. Also, such promising combination exceeded ETc100 in enhancing stomatal pore area by about 30.1%. Due to their potential act for soil water-holding capacity, ensuring enough water in the root zone of plants for a longer time, by enhancing the plants to uptake more water, humic acids mitigate the damage of water deficit (Cordeiro et al. 2011). Besides, chitosan reduced transpiration rate and enhanced stomatal conductance (Hidangmayum et al. 2019). Moreover, Martins et al. (2018) and Sun et al. (2019) stated that the stimulatory effect of chitosan improved plants growth and performance under drought stress. Chitosan treatment induces production of organic acids, sugars, amino acids, and other metabolites activities required for the osmotic adjustment, stress signaling, and energy metabolism under stresses (Guan et al. 2009). Li et al. (2017) exhibited that foliar application of chitosan led to an accumulation of stress protective metabolites in white clover grown under drought stress. Furthermore, the increase in water use efficiency under moderate and severe drought stress was noted in plants treated with chitosan (Farouk and Metwally 2019). Accordingly, the interactional effect of humic acids plus chitosan should be exploited under low water supply.
5 Conclusion
It seems that sugar beet could be regarded as a moderately tolerant to water stress, since slight discrepancies were obtained between well-watered (ETc100) and moderate drought (ETc80) for agronomic traits and sugar yield. However, sucrose % increased with lowering water supply. Since humic acids and chitosan are potential to adjust the balance between yield and quality, deficit water could be alleviated completely under moderate drought and relatively under severe drought using humic acids and chitosan. Herein, in practice, the tolerance of sugar beet plants to severe or moderate drought could be raised by the application of combination of humic acids (30 L ha−1) and chitosan (200 mg L−1).
References
Abd El–All AEA, Makhlouf BSI (2017) Response of sugar beet to continuous deficit irrigation and foliar application of some micronutrients under sandy soil conditions. J Soil Sci and Agric Eng Mansoura Univ 8:749–760. https://doi.org/10.21608/jssae.2017.38250
Abd El–Haleim MS (2020) Effect of irrigation intervals and potassium humate on sugar beet productivity. J Plant Prod Mansoura Univ 11:1239–1243. https://doi.org/10.21608/jpp.2020.149793
Abd El–Mageed TA, Belal EE, Rady MOA, Abd El–Mageed SA, Mansour E, Awad MF, Semida WM (2021) Acidified biochar as a soil amendment to drought stressed (Vicia faba L) plants: influences on growth and productivity, nutrient status, and water use efficiency. Agron 11:1290. https://doi.org/10.3390/agronomy1107129
Abd El-Mageed TA, El-Sherif AMA, Abd El-Mageed SA, Abdou NM (2019) A novel compost alleviate drought stress for sugar beet production grown in Cd-contaminated saline soil. Agric Water Manage 226:105831. https://doi.org/10.1016/j.agwat.2019.105831
Abdel Fatah EM, Khalil SRA (2020) Effect of zeolite, potassium fertilizer and irrigation interval on yield and quality of sugar beet in sandy soil. J Plant Prod Mansoura Univ 11:1569–1579
Abdou NM, Abdel–Razek MA, Abd El-Mageed SA, Semida WM, Leilah AAA, Abd El-Mageed TA, Ali EF, Majrashi A, Rady MOA (2021) High nitrogen fertilization modulates morpho–physiological responses, yield, and water productivity of low land rice under deficit irrigation. Agron 11:1291. https://doi.org/10.3390/agronomy11071291
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Ali M, Ayyub CM, Hussain Z, Hussain R, Rashid S (2020) Optimization of chitosan level to alleviate the drastic effects of heat stress in cucumber (Cucumis sativus L.). J Pure Appl Agri 5:30–38
Allen RB, Pereira LS, Raes D, Smith M (1998) Crop evapotranspiration. Irrig. Drain. Paper 56, Food and Agric. Org. UN, Rome, Italy
Alotaibi F, Bamagoos AA, Ismaeil FM, Zhang W, Abou-Elwafa SF (2021) Application of beet sugar byproducts improves sugar beet biofortification in saline soils and reduces sugar losses in beet sugar processing. Environ Sci Pollut Res 28:30303–30311. https://doi.org/10.1007/s11356-021-12935-5
AOAC (2012) Association of Official Agriculture Chemists, Official Method of Analysis: association of analytical chemists. 19th Edn, Washington DC, USA
Avvakumova NP, Gerchikov AY, Khairullina VR, Zhdanova AV (2011) Antioxidant properties of humic substances isolated from peloids. Pharm Chem J 45:192–193. https://doi.org/10.1007/s11094-011-0590-2
Bagheri SMM (2010) Influence of humic products on soil health and potato production. Potato Res 53:341–349. https://doi.org/10.1007/s11540-010-9177-7
Bayat H, Shafie F, Aminifard MH, Daghighi S (2021) Comparative effects of humic and fulvic acids as biostimulants on growth, antioxidant activity and nutrient content of yarrow (Achillea millefolium L.). Sci Hortic 279:109912. https://doi.org/10.1016/j.scienta.2021.109912
Bibi A, Ibrar M, Shalmani A, Rehan T, Quratulain, (2021) A review on recent advances in chitosan applications. Pure Appl Biol 10:1217–1229. https://doi.org/10.19045/bspab.2021.100128
Bistgani ZE, Siadat SA, Bakhshandeh A, Pirbalouti AG, Hashemi M (2017) Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensis Celak. Crop J 5:407–415. https://doi.org/10.1016/j.cj.2017.04.003
Black CA, Evans DD, Ensminger LE, White GL, Clark FE (1981) Methods of soil analysis. Part 2. Pp. 1–100. Agron. Inc. Madison. WI., USA
Casella G (2008) Statistical Design. 1st ed. Springer, Gainesville 32611–8545, USA
Clarke NA, Hetschkun HM, Thomas TH (1996) Stress mechanisms in sugar beet. In: Fenwick GR, Hedley C, Richards RL, Khokhar S (eds) Agri-Food Quality. An Interdisciplinary Approach. The Royal Soc. Chem, Cambridge, pp 75–78
Cooke DA, Scott RK (1993) The sugar beet crop. Science Practice. Puplished by Chapman and Hall, London. Pp: 595–605
Cordeiro FC, Santa-Catarina C, Silveira V, Souza SR (2011) Humic acid effect on catalase activity and the generation of reactive oxygen species in corn (Zea mays). J Biosci Biotech Bioch 75:70–74. https://doi.org/10.1271/bbb.100553
Deviller P (1988) Prevision du sucre melasse sucrerie feanases.129:190–200. [C.F. Cooke, D.A. and R.K. Scott (1993) The Sugar Beet Crop Book].
Dexter ST, Frankes MG, Snyder FW (1967) A rapid and practical method of determining extractable white sugar as may be applied to the evaluation of agronomic practices and grower deliveries in the sugar beet industry. J Am Soc Sugar Beet Technol 14:433–454
Doorenbos J and Kassam AH (1979) “Yield response to water”. FAO Irrigation and Drainage Paper No. 33, Rome
Du Y, Zhao Q, Chen L, Yao X, Zhang W, Zhang B, Xie F (2020) Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol Biochem 146:1–12. https://doi.org/10.1016/j.plaphy.2019.11.003
Dzung NA (2007) Chitosan and their derivatives as prospective biosubstances for developing sustainable eco–agriculture. In Senel S, Varum KM, Sumnu MM, Hincal AA (eds) Advances in chitin science X, pp 453–459
El-Bially MA, Saudy HS, El-Metwally IM, Shahin MG (2018) Efficacy of ascorbic acid as a cofactor for alleviating water deficit impacts and enhancing sunflower yield and irrigation water–use efficiency. Agric Water Manage 208(132):139. https://doi.org/10.1016/j.agwat.2018.06.016
El-Darder AMA, Gamaa MA, Sayed MA, Kame MZ (2017) Water stress effects on yield and quality of sugar beet crop in sandy soils. Alex Sci Exch J 38(828):836. https://doi.org/10.21608/asejaiqjsae.2017.4591
El-Hassanin AS, Samak MR, Moustafa SN, Khalifa AM, Ibrahim IM (2016) Effect of foliar application with humic acid substances under nitrogen fertilization levels on quality and yields of sugar beet plant. Int J Curr Microbiol App Sci 5:668–680. https://doi.org/10.20546/ijcmas.2016.511.078
El-Metwally IM, Saudy HS (2021) Interactional impacts of drought and weed stresses on nutritional status of seeds and water use efficiency of peanut plants grown in arid conditions. Gesunde Pflanzen 73:407–416. https://doi.org/10.1007/s10343-021-00557-3
El-Metwally IM, Geries L, Saudy HS (2021a) Interactive effect of soil mulching and irrigation regime on yield, irrigation water use efficiency and weeds of trickle–irrigated onion. Arch Agron Soil Sci. https://doi.org/10.1080/03650340.2020.1869723
El-Metwally IM, Saudy HS, Abdelhamid MT (2021b) Efficacy of benzyladenine for compensating the reduction in soybean productivity under low water supply. Ital J Agrometeorol 2:81–90
Enan SAAM, Aly EFA, Badr AI (2016) Effect of humic acid and potassium on yield and quality of some sugar beet varieties in sandy soil. J Plant Prod Mansoura Univ 7:289–297. https://doi.org/10.21608/jpp.2016.45342
FaroukEl-Metwally SIM (2019) Synergistic responses of drip–irrigated wheat crop to chitosan and/or silicon under different irrigation regimes. Agric Water Manage 226:105807. https://doi.org/10.1016/j.agwat.2019.105807
Flohé L, Günzler WA (1984) Assays of Glutathione Peroxidase. Meth Enzymol 105:114–120. https://doi.org/10.1016/s0076-6879(84)05015-1
Ghaffari H, Tadayon MR, Bahador M, Razmjoo J (2021) Investigation of the proline role in controlling traits related to sugar and root yield of sugar beet under water deficit conditions. Agri Water Manag 243:106448. https://doi.org/10.1016/j.agwat.2020.106448
Guan YJ, Hu J, Wang X, Shao C (2009) Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J Zhejiang Univ Sci 10:427–433. https://doi.org/10.1631/jzus.b0820373
Hidangmayum A, Dwivedi P, Katiyar D, Hemantaranjan A (2019) Application of chitosan on plant responses with special reference to abiotic stress. Physiol Mol Biol Plant 25:313–326. https://doi.org/10.1007/s12298-018-0633-1
Hossain A, Rahman MdME, Ali S, Islam T, Abu Syed M, Syed T, Zafar SA, Behera L, Skalicky M, Brestic M, Islam T (2022) CRISPR–Cas9–mediated genome editing technology for abiotic stress tolerance in crop plant. Plant Perspect to Global Climate Changes, Acad Press, Chap 16:331–354. https://doi.org/10.1016/B978-0-323-85665-2.00008-X
Ibrahim FR, El–Maghraby SS, Kandil EE, Ibrahim NY (2019) Productivity and quality of sugar beet in relation to humic acid and boron fertilization under nubaria conditions. Alex Sci Exch J 40(115):126. https://doi.org/10.21608/asejaiqjsae.2019.29029
IUSS Working Group WRB (2015) World Reference Base for Soil Resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. FAO, Rome. 192 p
Jackson ML (1973) Soil chemical analysis, prentice–hall India private limited, New Delhi, 498p
Kabeel H, Abd El–atif FM, Baza MSM, (2008) Growth, fruiting and nutritional status of “Le–Conte” pear trees in response to mineral and humate fertilizers. Ann of Agric Sci Moshtohor 46:139–215
Kandil EE, Abdelsalam NR, Abd EL–Aziz AA, Ali HM, Siddiqui MH (2020) Efficacy of nanofertilizer, fulvic acid and boron fertilizer on sugar beet (Beta vulgaris L.) yield and quality. Sugar Tech 22:782–791. https://doi.org/10.1007/s12355-020-00837-8
Khan N, Ali S, Zandi P, Mehmood A, Ullah S, Ikram M, Mohammad I, Shahid A, Babar M (2020) Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak J Bot 521–9. https://doi.org/10.30848/PJB20200-2(24))
Khodadadi S, Chegini MA, SoltaniNorouzi AHA, Hemayati SS (2020) Influence of foliar–applied humic acid and some key growth regulators on sugar beet (Beta vulgaris L.) under drought stress: antioxidant defense system, photosynthetic characteristics and sugar yield. Sugar Tech 22:765–772. https://doi.org/10.1007/s12355-020-00839-6
Khozaei M, Haghighi AAK, Parsa SZ, Sepaskhah AR, Razzaghi F, Yousefabadi V, Emam Y (2021) Effects of plant densities and irrigation regimes on yield, physiological parameters and gas exchange of sugar beet under transplanting and direct seeding methods. Int J Plant Prod. https://doi.org/10.1007/s42106-021-00147-3
Li Z, Zhang Y, Zhang X, Merewitz E, Peng Y, Ma X, Yan Y (2017) Metabolic pathways regulated by chitosan contributing to drought resistance in white clover. J Proteome Re 16:3039–3052. https://doi.org/10.1021/acs.jproteome.7b00334
Makhlouf BSI, Abd El–All AEA (2017) Effect of deficit irrigation, nitrogen and potassium fertilization on sugar beet productivity in sandy soils. Menoufia J Plant Prod 2:325–346. https://doi.org/10.21608/mjppf.2017.125867
Martins M, Veroneze-Junior V, Carvalho M, Carvalho DT, Barbosa S, Doriguetto AC, Magalhaes PC, Ribeiro C, Santos MH, Souza TC (2018) Physicochemical characterization of chitosan and its effects on early growth, cell cycle and root anatomy of transgenic and non–transgenic maize hybrids. Aust J Crop Sci 12:56–66. https://doi.org/10.21475/ajcs.18.12.01.pne649
Marzouk NM, Abd-Alrahman HA, El–Sawy SMM (2022) Amino acids sources and chitosan enhance cauliflower yield and quality under heat stress. Asian J Plant Sci 21:9–23. https://doi.org/10.3923/ajps.2022.9.23
Mekdad AAA, Ahmed MAE, Mostafa MR, Ahmed S (2021) Culture management and application of humic acid in favor of Helianthus annuus L. oil yield and nutritional homeostasis in a dry environment. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-021-00636-4
Meng HL, Zhang W, Zhang GH, Wang JJ, Meng ZG, Long GQ, Yang SC (2018) Unigene–based RNA–seqprovides insights on drought stress responses in Marsdenia tenacissima. PLoS ONE 13:e0202848. https://doi.org/10.1371/journal.pone.0202848
Mohamed HY, Bassiony NA, Mashhour AMA (2017) Response of some sugar beet varieties to deficit irrigation and humic acid in a newly reclaimed soil. J Biol Chem Environ Sci 12:537–562
Mohammadian R, Moghaddam M, Rahimian H, Sadeghian SY (2005) Effect of early season drought stress on growth characteristics of sugar beet genotypes. Turk J Agric Forest 29:357–368
Monda H, McKenna AM, Fountain R, Lamar RT (2021) Bioactivity of humic acids extracted from shale ore: molecular characterization and structure–activity relationship with tomato plant yield under nutritional stress. Front Plant Sci 12:1–17. https://doi.org/10.3389/fpls.2021.660224
Mubarak M, Salem EMM, Kenawey MKM, Saudy HS (2021) Changes in calcareous soil activity, nutrient availability, and corn productivity due to the integrated effect of straw mulch and irrigation regimes. J Soil Sci Plant Nutr 21:2020–2031. https://doi.org/10.1007/s42729-021-00498-w
Rady MM, Abd El-Mageed TA, AbdurrahmanMahdi HAAH (2016) Humic acid application improves field performance of cotton (Gossypium barbadense L.) under saline conditions. J Anim Plant Sci 26:487–493
Rady MOA, Semida WM, El-Mageed TAA, Howladar SM, Shaaban A (2020) Foliage applied selenium improves photosynthetic efficiency, antioxidant potential and wheat productivity under drought stress. Intl J Agric Biol 24:1293–1300. https://doi.org/10.17957/IJAB/15.1562
Salem EMM, Kenawey MKM, Saudy HS, Mubarak M (2021) Soil mulching and deficit irrigation effect on sustainability of nutrients availability and uptake, and productivity of maize grown in calcareous soils. Comm Soil Sci Plant Anal 52:1745–1761. https://doi.org/10.1080/00103624.2021.1892733
Saudy HS, El–Bially M, El–Metwally IM, Shahin MG (2021) Physio–biochemical and agronomic response of ascorbic acid treated sunflower (Helianthus Annuus) grown at different sowing dates and under various irrigation regimes. Gesunde Pflanzen 73(169):179. https://doi.org/10.1007/s10343-020-00535-1
SaudyEl–Metwally HSIM (2019) Nutrient utilization indices of NPK and drought management in groundnut under sandy soil conditions. Comm Soil Sci Plant Anal 50:1821–1828. https://doi.org/10.1080/00103624.2019.1635147
Saudy HS, El–Metwally IM, Abd El-Samad GA (2020) Physio–biochemical and nutrient constituents of peanut plants under bentazone herbicide for broad–leaved weed control and water regimes in dry land areas. J Arid Land 12:630–639. https://doi.org/10.1007/s40333-020-0020-y
Sayfzadeh S, Rashidi M (2011) Response of antioxidant enzymes activities of sugar beet to drought stress. J Agric Biol Sci 6:27–33
SCC (2020) Sugar Crops Council, MALR (Ministry of Agriculture and Land Reclamation), Dokki, Giza, Egypt
Sun J, A Li, Jing Q, Huang Y, Han J, Lin L (2019) Effects of chitosan on soluble sugar content in Prunus davidiana seedlings. E3S Web of Conf. 136, 06001, ICBTE. https://doi.org/10.1051/e3sconf/201913606001
Sun T, Yao Q, Zhou D, Mao F (2008) Antioxidant activity of Ncarboxymethyl chitosan oligosaccharides. Bioorg Med Chem Lett 18:5774–5776. https://doi.org/10.1016/j.bmcl.2008.09.072
Vermeirer L, Jopling GA (1984) Localized irrigation FAO. Irrigation paper No.36. Rome, Italy
Wang CX, Wang ZJ, Peng A, Hou JW, Xin WJ (1996) Interaction between fulvic acids of different origins and active oxygen radicals. Sci China Ser C 39:267–275
Wang Y, Peng C, Zhan Y, Yu L, Li M, Li J, Geng G (2017) Comparative proteomic analysis of two sugar beet cultivars with contrasting drought tolerance. J Plant Growth Regul 36:537–549. https://doi.org/10.1007/s00344-017-9703-9
Wilczewski E, Szczepanek M, Wenda-Piesik A (2018) Response of sugar beet to humic substances and foliar fertilization with potassium. J Central Eur Agric 19:153–165. https://doi.org/10.5513/JCEA01/19.1.2033
Yan W, Zhong Y, Shangguan Z (2016) A meta–analysis of leaf gas exchange and water status responses to drought. Sci Rep 6:20917. https://doi.org/10.1038/srep20917
Yang F, Hu J, Li J, Wu X, Qian Y (2009) Chitosan enhances leaf membrane stability and antioxidant enzyme activities in apple seedlings under drought stress. Plant Growth Regul 58:131–136. https://doi.org/10.1007/s10725-009-9361-4
Yin H, Bai XF, Du YG (2008) The primary study of oligochitosan inducing resistance to Sclerotinia scleraotiorum on B. napus. J Biotechnol 136:600–601. https://doi.org/10.1016/J.JBIOTEC.2008.07.1217
Zeng D, Luo X (2012) Physiological effects of chitosan coating on wheat growth and activities of protective enzyme with drought tolerance. Open J Soil Sci 2:282–288. https://doi.org/10.4236/ojss.2012.23034
Acknowledgements
The authors acknowledge the technical support provided by Sugar Crops Research Institute, Agricultural Research Center (ARC), Giza, Egypt.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Makhlouf, B.S.I., Khalil, S.R.A.E. & Saudy, H.S. Efficacy of Humic Acids and Chitosan for Enhancing Yield and Sugar Quality of Sugar Beet Under Moderate and Severe Drought. J Soil Sci Plant Nutr 22, 1676–1691 (2022). https://doi.org/10.1007/s42729-022-00762-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-00762-7