Abstract
Molybdenum (Mo), an essential microelement, may enhance the oxidative stress tolerance in plants. However, the efficacy of Mo might be variable with different forms of nitrogen (N) fertilizer. The present study was conducted to investigate the role of Mo application in regulating oxidative stress tolerance in winter wheat under different N sources. A hydroponic study was carried out comprising of two winter wheat cultivars ‘97003’ and ‘97014’ as Mo efficient and Mo inefficient, respectively, under two Mo levels (0 and 1 μM) and three different N sources (NO3̶, NH4NO3, or NH4+). Winter wheat plants supplied with different N sources accumulated superoxide anions (O2−), and malonaldehyde (MDA) contents in the order of NH4+ > NO3̶ > NH4NO3, suggesting that sole application of either N sources, especially sole NH4+ source, may induce oxidative stress in winter wheat. However, Mo application decreased the MDA contents by 20.02%, 15.11%, and 25.89% in Mo-efficient cultivar and 30.75%, 23.79%, and 37.76% in Mo-inefficient cultivar under NO3̶, NH4NO3, and NH4+ sources, respectively, while increased antioxidant enzyme activities and carotenoids and abscisic acid (ABA) contents up-regulated the expressions of TaAO and TaAba3 genes. Mo application regulated oxidative stress tolerance in winter wheat under different N sources through enhancing ABA production and ROS-scavenging enzymes. Mo-efficient ‘97003’ winter wheat cultivar possesses a wider range of adaptability to withstand Mo-deficient conditions than Mo-inefficient ‘97014’ cultivar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nitrogen (N) is available and absorbed in different forms from soil colloids, especially inorganic ions as nitrate (NO3−) and ammonium (NH4+). Most of the growth and developmental processes in higher plants have long been known to benefit from NO3− fertilizers (Marschner 2012; Coruzzi and Bush 2001). Nevertheless, despite the fact that more energy is consumed during NO3− assimilation pathways than NH4+ form of N, a few species have been investigated to perform well under sole NH4+ source (Britto and Kronzucker 2013; Raven 2010). Indeed, most of the plant species start showing toxicity symptoms under higher NH4+ concentrations, which are normally undetectable in plants grown under same NO3− concentration or in mixed N nutrition (Britto and Kronzucker 2002).
However, a single mechanism cannot explain the sole NH4+ toxicity effects on plant growth and developmental processes. Previous studies have reported that NH4+ toxicity indeed impairs the photosynthetic apparatus, i.e., damaged chloroplast ultrastructure, reduced Rubisco activity (Britto and Kronzucker 2002; Lu and Zhang 2000), and severe impairment in the photosystem I and II functions (Asada 2006; Drath et al. 2008), and this malfunctioning triggers the overproduction of reactive oxygen species (ROS) (Wang et al. 2018). However, in contrast, some studies have highlighted that long-term NH4+ nutrition in Arabidopsis thaliana did not impair photosynthetic capacity but increased the leaf levels of mitochondrial ROS (Podgórska et al. 2013). Similarly, some reports indicated that functional impairments due to NH4+ toxicity are associated with greater ROS production due to increased lipid peroxidation, enhanced glutathione-ascorbate cycle enzyme activity, or severe alterations in the redox state (Patterson et al. 2010). Therefore, ROS-scavenging or mitigating approaches may represent a strategy to alleviate NH4+ toxicity effects on NH4+-sensitive plants.
In the scenario of NH4+ toxicity mitigation, most of the previous studies have focused on the co-application with NO3− (Britto and Kronzucker 2002), which counterbalances the NH4+ toxicity through alkalinization (Britto and Kronzucker 2013). Similarly, abscisic acid (ABA) treatment has also been reported to alleviate the NH4+ toxicity effects on Arabidopsis by regulating the expressions of NH4+-stress-responsive genes (Meng et al. 2012; Li et al. 2014). However, Mo-induced mitigation of NH4+ toxicity in winter wheat plants has not been investigated.
Molybdenum (Mo), an essential microelement, improved the plant growth and oxidative tolerance through increased antioxidant enzyme activities in Chinese cabbage (Zhang et al. 2012), ABA productions in wheat (Wu et al. 2018; Sun et al. 2009), nitrogen assimilation in sugarcane (Santos et al. 2018), and iron nutrition in peanut (Su et al. 2019). Oxidative tolerance is reflected by the increased activities of antioxidant enzymes which play effective roles to maintain the ROS homeostasis and alleviate the oxidative injuries in plant cells (Hussain et al. 2016, 2020).
Aldehyde oxidase (AO), responsible for the oxidization of abscisic aldehyde to ABA synthesis, requires sulfurylated form of molybdenum cofactor (MoCo) for its activity (Bittner 2014). However, Mo is a vital element for MoCo biosynthesis (Nie et al. 2016), and Mo-deficient plants severely inhibited the AO activities and concomitantly ABA contents in winter wheat plants (Sun et al. 2009; Wu et al. 2018). ABA, as one of the phytohormones, plays fundamental roles in regulating the expressions of stress-induced genes and multiple signal transduction pathways that are essential for plant survival against oxidative stresses (Mccourt and Creelman 2008; Meng et al. 2012).
Although several studies have reported that the NH4+ toxicity in model plant Arabidopsis thaliana was alleviated by co-application with NO3− (Britto and Kronzucker 2013), exogenous ABA (Meng et al. 2012; Li et al. 2012), and auxin treatments (Zou et al. 2012), however, if NH4+ toxicity in winter wheat could be alleviated by Mo application with different N sources is not clear. The main objective of this experiment was to investigate the role of Mo application in regulating the oxidative stress tolerance in two winter wheat cultivars with contrasting Mo efficiencies under different N sources.
2 Materials and Methods
2.1 Plant Material and Growth Conditions
Seeds of two winter wheat cultivars, viz., ‘97003’ and ‘97014’, were obtained from the Laboratory of Trace Elements, Huazhong Agricultural University, China. Seeds were surface sterilized with 0.5% sodium hypochlorite (NaOCl) solution and rinsed with sterile distilled water. Seeds were allowed to germinate at 25 °C in deionized water for 5 days. The environmental conditions in the controlled growth chamber were maintained according to our previous study (Imran et al. 2019a). Uniform-sized wheat seedlings were transplanted to plastic containers. For the first and second 2-day intervals, one fourth and one half strengths of Hoagland solution were applied respectively, and subsequently, whole strength was applied until 30-day-old wheat seedlings were harvested for measurement of required parameters. To prevent NH4+ oxidation in the Hoagland solution, dicyandiamide (8.0 μM) was added as a nitrification inhibitor. Compositions of the modified Hoagland nutrient solution were as follows: macronutrients, 15 mM NH4+-N as 7.5 mM (NH4)2SO4; 15 mM NO3̶ -N as 5 mM Ca (NO3)2.4H2O, 5 mM KNO3; 15 mM NH4NO3 as 3.75 mM (NH4)2SO4 and 3.75 mM Ca (NO3)2.4H2O; 6 mM K as 1 mM KH2PO4 and 2.5 mM K2SO4 (NH4+-N and NH4NO3); 1 mM P as KH2PO4; 5 mM Ca as CaCl2.2H2O; and 2 mM Mg as MgSO4.7H2O; and micronutrients, 0.1 mM Fe as Fe-Na2-EDTA, 0.3 μM Cu as CuSO4.5H2O, 0.8 μM Zn as ZnSO4.7H2O, 9.1 μM Mn as MnCl2.4H2O, and 46.2 μM B as H3BO3. Na2MoO4.2H2O was used as Mo fertilizer.
2.2 Treatments and Experimental Design
The treatments were comprised of two Mo levels (0 and 1 μM) with three different N sources (NO3̶, NH4NO3, or NH4+) in two winter wheat cultivars (97003 and 97014). Plastic pots (30 cm × 20 cm × 15 cm) containing 4 L of the respective solution and a perforated floating board on the Hoagland solution surface with two rows for each cultivar were used, and each treatment was replicated four times. The pH of the nutrient solution was maintained at 6.5 ± 0.05 by adding 0.1 mM HCl or NaOH to the solutions every day. The nutrient solutions were renewed after every 2 days during the course of study. The experiment was laid out in a completely randomized design with factorial arrangement.
2.3 Determination of AO Activities
Aldehyde oxidase activities were measured according to the protocol described by Sun et al. (2009). The fresh wheat leaf samples were ground in cold Tris–HCl buffer (pH 8.5) containing dithiothreitol (DTT), ethylene diamine tetraacetic acid (EDTA), reduced glutathione (GSH), and polyvinylpolypyrrolidone (PVPP). The crude extracts were separated by centrifuging at 4 °C for 20 min at 12,000 rpm. The reaction mixture was comprised of supernatant and active solution containing phenazine methosulphate (PMS), 2, 6-dichloroindorphenol (DCIP), and indole-3-aldehyde. Absorbance was measured at 600 nm by spectrophotometer.
2.4 Measurement of ABA Contents
In the fresh leaf samples, ABA contents were measured using ELISA kits following the operational instruction manual.
2.5 Total RNA Extraction and qRT-PCR
Frozen wheat samples were subjected to evaluate the regulation pattern of target genes by following the protocol mentioned in our previous study (Imran et al. 2019b). Briefly, total RNA was extracted from leaf tissues, liquefied with 30 μL DEPC·H2O, and the quantity was measured with Nano Drop 2000 UV-VIS spectrophotometer (Thermo Fisher, Waltham, MA, USA). Then cDNA was synthesized from the quantified RNA using Oligo (dT18) primers through reverse transcriptase, dNTP, M-MLVRTase, and a detection system of IQ5 Real-Time PCR (Bio-Rad, USA). The reaction mixture, comprised of SYBR Green mix (Bio-Rad, USA), gene-specific primers, and cDNA templates, was assorted in a 96-well plate for subsequent measurements. The cycling program was as follows: 30-s denaturation at 95 °C and 44 cycles of 10 s at 95 °C, 20 s at annealing temperatures of the specific primers (Table 1), followed by 30 s at 72 °C. The primers of TaAO and TaAba3 genes were used from our previous lab study (Nie et al. 2016). The detailed information regarding primer sequences, gene index number, and annealing temperature are available in Table 1.
2.6 Carotenoid Contents
Fresh wheat samples were soaked in 95% ethanol under darkness until the green leaves were decolorized. Spectrophotometer was used to measure the absorbance at 665, 649, and 470 nm, and carotenoid contents were measured according to the method described by (Wu et al. 2014).
2.7 Measurement of Antioxidant Enzyme Activities
Superoxide dismutase (SOD; EC 1.15.1.1) activities were measured by following the method of Wu et al. (2014). Fresh leaf samples were homogenized with cold sodium phosphate buffer (pH 7.8), and supernatant was separated from the crude fibers by centrifuging at 4 °C for 10 min at 4000 rpm. Reaction mixture was comprised of sodium phosphate (pH 7.8), riboflavin, nitro blue tetrazolium (NBT), EDTA-Na2, methionine, enzyme extract, and distilled water. Spectrophotometer was used to measure the absorbance at 560 nm.
Peroxidase (POD; EC 1.11.1.7) activity was determined by following the method as described by Wu et al. (2014). Fresh plant samples were ground with ice-cold 50 mM sodium phosphate (pH 5.5) extraction buffer, and the supernatant was separated after centrifugation at 3000 rpm for 10 min at 4 °C. The reaction mixture was comprised of 50 mM sodium phosphate (pH 5.5), 0.3% H2O2, 0.2% guaiacol, and the enzyme extract. The absorbance values were measured by spectrophotometer at 470 nm for POD activity.
Catalase (CAT; EC 1.11.1.6) activity was measured by following the protocol of Wu et al. (2014). Fresh plant samples were ground with cold 50 mM sodium phosphate (pH 7.8) extraction buffer, and the supernatant was separated after centrifugation at 4000 rpm for 15 min at 4 °C. The reaction mixture was comprised of 50 mM sodium phosphate (pH 7.8), 0.1 M H2O2, distilled water, and the enzyme extract. The absorbance values were measured at 240 nm by spectrophotometer to determine the CAT enzyme activities.
For the ascorbate peroxidase (APX; EC 1.11.1.11) activity determination, fresh wheat leaf samples were homogenized with cold 50 mM sodium phosphate (pH 5.5) buffer containing 0.2 mM EDTANa2, and the homogenate was centrifuged at 4 °C for 15 min at 10000 rpm. The supernatant was collected to determine APX activity by following the method of Mohanty (2004).
2.8 Analysis of Superoxide Anion (O2−) and Membrane Damage
Superoxide anion (O2−) staining was executed in fresh wheat samples following the method of Wu et al. (2017). Briefly, top fully expanded winter wheat leaves were detached (5 cm) from the leaf tip, and 2-cm leaf tip was cut. Then, remaining 3-cm leaf segments were subjected to staining procedure for O2− analysis. The measurement of O2− was visually detected using nitro blue tetrazolium solution (0.5 mg mL−1) for 8 h and decolorized in boiling ethanol.
The malonaldehyde (MDA) contents were measured according to the method used in our previous study (Wu et al. 2017). Fresh wheat samples were thoroughly homogenized with cold TCA (0.1%), and the crude extracts were centrifuged at 4 °C for 20 min at 12000 rpm. The supernatant was used to measure MDA contents.
2.9 Statistical Analysis
Two-way analysis of variance (ANOVA) was followed to statistically analyze the data using Statistix 8.1 software (Analytical Software, Tallahassee, FL, USA). Least significant difference (LSD) test was used at P < 0.05 to analyze the mean variances of the data. Sigmaplot 10.0 was used to plot the graphs.
3 Results
3.1 Effects of Mo Application on Winter Wheat Growth Under Different N Sources
Total plant dry weight under different N sources followed the order of NH4NO3 > NO3− > NH4+ in both wheat cultivars (Fig. 1), signifying that sole NH4+ nutrition intensely hampered wheat plant growth and dry matter accumulation as compared with the NO3− source, and combined supply of both sources as NH4NO3 provided complementary environment for optimum growth and development of winter wheat plants. However, Mo application increased plant dry matter by 24.15%, 30.28%, and 12.33% in Mo-efficient cultivars while by 36.16%, 47.75%, and 17.59% in Mo-inefficient cultivars as compared with –Mo-treated plants under NO3̶, NH4NO3, and NH4+ sources, respectively (Fig. 1a and b), suggesting that Mo has complementary effects to all N sources. Noticeably, between the two winter wheat cultivars, Mo-inefficient cultivar recorded more prominent enhancement in plant dry weight accumulation than Mo-efficient cultivar under +Mo conditions, indicating that Mo-inefficient cultivar is more dependent on Mo supply (Fig. 1).
Influence of molybdenum (Mo) and different nitrogen (N) sources on total plant dry matter accumulations of two winter wheat cultivars, i.e., Mo-efficient ‘97003’ (a) and Mo-inefficient ‘97014’ cultivar (b). Both wheat cultivars were exposed to molybdenum treatments: –Mo and + Mo as 0 and 1 μM Mo [Na2MoO4.2H2O] concentrations, respectively, against three different N sources: NO3̶, NH4NO3, and NH4+ sources in modified Hoagland solution. The LSD test was used to determine significant differences (P < 0.05, n = 4)
3.2 Mo Supply Enhanced AO Activities and Endogenous ABA Contents Under Different N Sources
Aldehyde oxidase (AO) is a crucial Mo enzyme involved in the endogenous ABA biosynthesis process. Thus, the effects of Mo supply on AO activities and concomitantly ABA contents under different N sources were measured in the leaves of winter wheat cultivars (Fig. 2). Among different N sources, AO activities were significantly higher under NH4NO3 source as compared with the sole NH4+ or NO3− sources in both winter wheat cultivars (Fig. 2a and b). Compared with –Mo plants, Mo application increased the AO activities in leaf tissues by 36.10%, 46.93%, and 29.34% in Mo-efficient cultivar while by 64.70%, 88.56%, and 46.87% in Mo-inefficient cultivar under NO3̶, NH4NO3 and NH4+ sources, respectively (Fig. 2a and b).
Effects of molybdenum (Mo) and different nitrogen (N) sources on aldehyde oxidase (AO) activities and abscisic acid (ABA) contents in leaf tissues of two winter wheat cultivars, i.e., Mo-efficient ‘97003’ (a, c) and Mo-inefficient ‘97014’ cultivar (b, d). Both wheat cultivars were exposed to molybdenum treatments: –Mo and + Mo as 0 and 1 μM Mo [Na2MoO4.2H2O] concentrations, respectively, against three different N sources: NO3̶, NH4NO3, and NH4+ in modified Hoagland solution. The LSD test was used to determine significant differences (P < 0.05, n = 4)
Nevertheless, in contrast to AO activities, ABA contents in the leaf tissues followed the order of NH4+ > NO3− > NH4NO3 under different N sources (Fig. 2c and d). However, Mo application increased the ABA contents in leaf tissues by 42.80%, 31.74%, and 58.47% in Mo-efficient cultivar while by 95.52%, 73.26%, and 132.05% in Mo-inefficient winter wheat cultivars under NO3̶, NH4NO3, and NH4+ sources, respectively (Fig. 2c and d), indicating that Mo-induced rises in ABA contents were higher under sole application of either N sources especially sole NH4+ supply.
3.3 Mo Application Regulated the Expressions of TaAO and TaAba3 Genes
The transcript abundance of TaAO and TaAba3 genes under various N forms followed the order of NH4+ > NO3̶ > NH4NO3 in winter wheat (Fig. 3). However, Mo supply significantly up-regulated TaAO and TaAba3 genes expressions in leaf tissues under sole either NO3̶ or NH4+ sources relative to NH4NO3 source as compared to –Mo plants (Fig. 3). Interestingly, the expressions of TaAO and TaAba3 genes under Mo-deficient conditions were higher in Mo-efficient ‘97003’ cultivar compared with those in Mo-inefficient ‘97014’ cultivar (Fig. 3), suggesting that ‘97003’ cultivar is comparatively more resistant to withstand Mo-deficient conditions than Mo-inefficient winter wheat ‘97014’ cultivar.
Effects of molybdenum (Mo) and different nitrogen (N) sources on qRT-PCR analysis of TaAO and TaAba3 gene transcripts in leaf tissues of two winter wheat cultivars, i.e., Mo-efficient ‘97003’ (a, c) and Mo-inefficient ‘97014’ cultivar (b, d). Both wheat cultivars were exposed to molybdenum treatments: –Mo and + Mo as 0 and 1 μM Mo [Na2MoO4.2H2O] concentrations, respectively, against three different N sources: NO3̶, NH4NO3, and NH4+ in modified Hoagland solution. The LSD test was used to determine significant differences (P < 0.05, n = 4)
3.4 ROS and the Activities of ROS-Scavenging Enzymes in Winter Wheat Plants
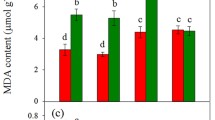
To assess the rate of oxidative damage caused by different N sources and Mo-induced amelioration in winter wheat cultivars, the superoxide anion (O2−) accumulations, MDA contents, and correspondingly ROS-scavenging enzyme activities were measured in two winter wheat leaf tissues (Figs. 4, 5, and 6). The present study revealed that O2− accumulations and MDA contents under different N sources followed the order of NH4+ > NO3̶ > NH4NO3, whereas antioxidant enzyme activities as NH4NO3 > NO3̶ > NH4+ in both cultivars (Figs. 4, 5, and 6), suggesting that sole N sources either NH4+ or NO3− caused more oxidative damage to wheat plants as compared with the mixture supply as NH4NO3 source. Ostensibly, Mo-inefficient ‘97014’ cultivar accumulated more O2− in leaf tissues as compared with Mo-efficient ‘97003’ cultivar under –Mo treatment (Fig. 4), indicating that Mo-efficient ‘97003’ winter wheat cultivar might better adapt to Mo-deficient conditions. However, Mo application diminished the lipid peroxidation rates, as is manifest from the decreased MDA contents, by 20.02%, 15.11%, and 25.89% in Mo-efficient ‘97003’ cultivar while by 30.75%, 23.79%, and 37.76% in Mo-inefficient ‘97014’ cultivar under NO3̶, NH4NO3, and NH4+ sources, respectively (Fig. 5a and b). Moreover, Mo application dramatically enhanced the antioxidant enzymes (SOD, POD, CAT, and APX) activities compared with –Mo plants, under different N sources in both winter wheat cultivars (Fig. 6). These observations conclude that sole application of either N sources (NH4+ or NO3̶) causes more oxidative damage to winter wheat with sole NH4+ source; however, being the more destructive than co-application as NH4NO3 source and Mo supply has significant role in mitigating the different N sources induced oxidative damage to winter wheat.
Impacts of molybdenum (Mo) and different nitrogen (N) sources on visual observation of superoxide anions (O2−) contents in leaf tissues of two winter wheat cultivars, i.e., Mo-efficient ‘97003’ and Mo-inefficient ‘97014’ cultivar. Both wheat cultivars were exposed to molybdenum treatments: –Mo and + Mo as 0 and 1 μM Mo [Na2MoO4.2H2O] concentrations, respectively, against three different N sources: NO3̶, NH4NO3, and NH4+ in modified Hoagland solution. Each treatment has at least 10 similar results for superoxide anions (O2−) contents in both winter wheat leaf tissues
Effects of molybdenum (Mo) and different nitrogen (N) sources on malonaldehyde (MDA) and carotenoid (CAR) contents in leaf tissues of two winter wheat cultivars, i.e., Mo-efficient ‘97003’ (a, c) and Mo-inefficient ‘97014’ cultivar (b, d). Both wheat cultivars were exposed to molybdenum treatments: –Mo and + Mo as 0 and 1 μM Mo [Na2MoO4.2H2O] concentrations, respectively, against three different N sources: NO3̶, NH4NO3, and NH4+ in modified Hoagland solution. The LSD test was used to determine significant differences (P < 0.05, n = 4)
Influence of molybdenum (Mo) and different nitrogen (N) sources on antioxidant enzymes (SOD, POD, CAT, and APX) activities in leaf tissues of two winter wheat cultivars, i.e., Mo-efficient ‘97003’ (a, c, e, g) and Mo-inefficient ‘97014’ cultivar (b, d, f, h). Both wheat cultivars were exposed to molybdenum treatments: –Mo and + Mo as 0 and 1 μM Mo [Na2MoO4.2H2O] concentrations, respectively, against three different N sources: NO3̶, NH4NO3, and NH4+ in modified Hoagland solution. The LSD test was used to determine significant differences (P < 0.05, n = 4)
4 Discussion
Wheat is an NH4+-sensitive crop, and previous studies on model plant Arabidopsis thaliana (NH4+ sensitive plant) have frequently reported that sole NH4+ source causes more severe oxidative damages through ROS production than NO3−-based fertilizers (Britto and Kronzucker 2002; Britto and Kronzucker 2013). Moreover, Mo, a stress-resistant microelement, has been reported to enhance the plant oxidative stress tolerance under drought, salinity, and cold stresses through endogenous ABA production and increased activities of antioxidant enzymes (Sun et al. 2009; Zhang et al. 2012; Wu et al. 2018). However, Mo-induced amelioration in the oxidative damages caused by different N sources in winter wheat plants still remains unclear.
In the present study, a severe reduction in the plant dry matter accumulation was observed under sole NH4+ source as compared with NO3− fertilizers in both winter wheat cultivars (Fig. 1), indicating that sole NH4+ source might have inhibited the plant growth and developmental processes and thereby accumulated less biomass. Similar results were reported in previous studies where wheat plants supplied with sole NH4+ source significantly reduced the plant dry biomass due to NH4+-induced toxicity in the plant growth and developmental processes including damaged photosynthetic apparatus and superfluous ROS production (Wang et al. 2016; Wang et al. 2018). Compared with the –Mo-treated plants, Mo application increased the plant dry weight of both wheat cultivars under different N sources in the order of NH4NO3 > NO3̶ > NH4+ (Fig. 1). These observations suggest that Mo fertilizer has complementary effects to all N sources whether NO3− or NH4+ in winter wheat. Most of the previous studies have repeatedly focused and reported the Mo and NO3− interactions in different crop plants (Kovács et al. 2015; Liu et al. 2017; Wen et al. 2019); however, none of the studies has still explored the role of Mo application in regulating the oxidative stress tolerance in winter wheat cultivars under different N sources.
Abscisic acid is a vital hormone in the defense responses and orchestration of stress signal transductions in different crop plants (Xiong et al. 2002; Hubbard et al. 2010). It regulates the expressions of stress-induced genes and activates signal transduction pathways that are essential for plant survival and productivity (Mccourt and Creelman 2008). Downton et al. (1988) have reported that endogenous ABA production in leaves responds in a similar way to exogenous ABA application. Moreover, previous studies have reported that Mo-enzyme AO is a key regulator for the oxidization of abscisic aldehyde to ABA in Arabidopsis thaliana (Seo et al. 2000), pea (Zdunek-Zastocka 2008), and wheat (Sun et al. 2009; Wu et al. 2018). In the present study, Mo application enhanced the AO activities and ABA contents under all N sources compared with the –Mo-treated plants; however, Mo-induced increases in the AO activities under different N sources followed the order of NH4NO3 > NO3̶ > NH4+ while ABA contents as NH4+ > NO3̶ > NH4NO3 in both wheat cultivars (Fig. 2). However, these higher ABA contents might also be due to concomitantly increased carotenoid contents (Fig. 5c and d) because carotenoids are precursor substances in the process of ABA synthesis (Xiong et al. 2001). Moreover, the present results imply that AO might be a stress-sensitive enzyme whose efficacy decreased under stressed environment (NH4+ toxicity), while ABA synthesis is specifically induced when plants are under stress. These observations coincide with previous reports that Mo-induced AO activities decreased with increasing intensities of drought or cold stresses, while ABA contents continued to increase in winter wheat (Wu et al. 2018; Sun et al. 2009). In the present study, Mo-induced transcript abundance of TaAO and TaAba3 genes and ABA contents under different N sources followed the order of NH4+ > NO3− > NH4NO3 sources in both wheat cultivars (Figs. 2 and 3). These results suggest that wheat plants experience more oxidative stress when grown under sole NH4+ source than NO3−-based fertilizers, and Mo-induced higher ABA production and transcript abundance of TaAba3 gene may represent a strategy of Mo fertilizer to mitigate NH4+ toxicity effects in winter wheat plants. Previous studies also reported that overexpression of LOS5/ABA3 gene led to notable increases in ABA accumulations, expressions of stress-up-regulated genes, drought tolerance, and a series of physiological and biochemical resistant responses in transgenic soybean (Li et al. 2013).
Generally, there exists a natural coordinated balance for ROS production and utilization to run several retrograde signaling pathways, photosynthetic processes, and numerous redox homeostasis under normal conditions (Müge 2014). However, in the NH4+-sensitive plant species, sole NH4+ source implicates ROS overproduction through impairment in the photosystem II functions (Drath et al. 2008), damaged chloroplast ultrastructure, abridged photosynthesis rate (Britto and Kronzucker 2002), and increased lipid peroxidation (Patterson et al. 2010). In the present study, O2− accumulations in the leaves of both winter wheat cultivars under different N sources followed the order of NH4+ > NO3̶ > NH4NO3 (Fig. 4), indicating that sole NH4+ source triggered the ROS production as compared with NO3− fertilizers, and these findings are in line with previous reports on wheat plants (Yang et al. 2016; Wang et al. 2018; Polesskaya et al. 2004). However, Mo application reduced O2− accumulations as evident from Fig. 4 and verified from the MDA contents in winter wheat leaf tissues under different N sources (Fig. 5a and b). The MDA contents indeed represent the lipid peroxidation rate and the oxidative injury mediated by ROS (Moore and Roberts 1998). Therefore, to overcome the cascades of uncontrolled redox reactions and protect the cells from ROS-induced oxidative damages, plants significantly enhance the activities of antioxidant enzymes such as SOD, POD, CAT, and APX (Hussain et al. 2016; Foyer and Noctor 2010; Zou et al. 2012). In the present study, Mo application enhanced the activities of SOD, POD, CAT, and APX under different N sources (Fig. 6), indicating that Mo application strengthened the antioxidant defense system to protect the wheat plants against oxidative injuries induced by sole N sources especially sole NH4+ nutrition relative to mixture supply as NH4NO3. These results are in line with previous studies where Mo application significantly improved the antioxidant enzyme (SOD, POD, CAT, and APX) activities under drought and low-temperature stresses in wheat (Sun et al. 2009; Wu et al. 2018) and salinity stress in Chinese cabbage (Zhang et al. 2012).
Taken together, our results conclude that oxidative damage due to higher ROS production under different N sources followed the order of NH4+ > NO3̶ > NH4NO3, and Mo application mitigated the oxidative stress through concomitantly ABA productions and regulating the ROS-scavenging enzyme activities in winter wheat. This may represent a strategy of Mo fertilizer to mitigate the NH4+ toxicity effects in NH4+-sensitive plants. However, further genetic and molecular studies should be meditated to gain deeper insights for the better understanding of the detailed mechanisms between Mo and different N sources, especially sole NH4+ nutrition.
5 Conclusions
Molybdenum application enabled the winter wheat plants to efficiently detoxify the reactive oxygen species and alleviate the NH4+ toxicity through increasing abscisic acid production, carotenoid contents, and transcript abundance of TaAO and TaAba3 genes and mediating the antioxidant enzyme activities in leaf tissues. Oxidative stress induced under different N sources in winter wheat plants followed the order of NH4+ > NO3̶ > NH4NO3 sources. Moreover, higher abscisic acid contents, antioxidant enzyme activities, and gene expressions in Mo-efficient ‘97003’ cultivar than Mo-inefficient ‘97014’ cultivar under Mo-deprived environment suggested that Mo-efficient winter wheat ‘97003’ cultivar might proficiently adapt to versatile environmental stresses with minimum harms to plant growth and developmental processes under Mo-deficient conditions.
References
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141(2):391–396
Bittner F (2014) Molybdenum metabolism in plants and crosstalk to iron. Front Plant Sci 5(2):28
Britto DT, Kronzucker HJ (2002) NH4+ toxicity in higher plants: a critical review. J Plant Physiol 159(6):567–584
Britto DT, Kronzucker HJ (2013) Ecological significance and complexity of N-source preference in plants. Ann Bot 112(6):957–963
Coruzzi G, Bush DR (2001) Nitrogen and carbon nutrient and metabolite signaling in plants. Plant Physiol 125(1):61–64
Downton W, Loveys B, Grant W (1988) Non-uniform stomatal closure induced by water stress causes putative non-stomatal inhibition of photosynthesis. New Phytol 110(4):503–509
Drath M, Kloft N, Batschauer A, Marin K, Novak J, Forchhammer K (2008) Ammonia triggers photodamage of photosystem II in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Physiol 147(1):206–215
Foyer CH, Noctor G (2010) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28(8):1056–1071
Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI (2010) Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev 24(16):1695–1708
Hussain S, Khan F, Cao W, Wu L, Geng M (2016) Seed priming alters the production and detoxification of reactive oxygen intermediates in rice seedlings grown under sub-optimal temperature and nutrient supply. Front Plant Sci 7(116)
Hussain S, Khaliq A, Noor MA, Tanveer M, Hussain HA, Hussain S, Shah T, Mehmood T (2020) Metal toxicity and nitrogen metabolism in plants: an overview. In: Carbon and Nitrogen Cycling in Soil. Springer, pp 221–248
Imran M, Hu C, Hussain S, Rana MS, Riaz M, Afzal J, Aziz O, Elyamine AM, Ismael MAF, Sun X (2019a) Molybdenum-induced effects on photosynthetic efficacy of winter wheat (Triticum aestivum L.) under different nitrogen sources are associated with nitrogen assimilation. Plant Physiol Biochem 141:154–163
Imran M, Sun X, Hussain S, Ali U, Rana MS, Rasul F, Saleem MH, Moussa MG, Bhantana P, Afzal J (2019b) Molybdenum-induced effects on nitrogen metabolism enzymes and elemental profile of winter wheat (Triticum aestivum L.) under different nitrogen sources. Int J Mol Sci 20(12):3009
Kovács B, Puskás-Preszner A, Huzsvai L, Lévai L, Bódi É (2015) Effect of molybdenum treatment on molybdenum concentration and nitrate reduction in maize seedlings. Plant Physiol Biochem 96:38–44
Li B, Li Q, Xiong L, Kronzucker HJ, Krämer U, Shi W (2012) Arabidopsis plastid AMOS1/EGY1 integrates abscisic acid signaling to regulate global gene expression response to ammonium stress. Plant Physiol 160(4):2040–2051
Li Y, Zhang J, Zhang J, Hao L, Hua J, Duan L, Zhang M, Li Z (2013) Expression of an Arabidopsis molybdenum cofactor sulphurase gene in soybean enhances drought tolerance and increases yield under field conditions. Plant Biotech J 11(6):747–758
Li B, Li G, Kronzucker HJ, Baluška F, Shi W (2014) Ammonium stress in Arabidopsis: signaling, genetic loci, and physiological targets. Trends Plant Sci 19(2):107–114
Liu L, Xiao W, Li L, Li D-M, Gao D-S, Zhu C-y FX-L (2017) Effect of exogenously applied molybdenum on its absorption and nitrate metabolism in strawberry seedlings. Plant Physiol Biochem 115:200–211
Lu C, Zhang J (2000) Photosynthetic CO(2) assimilation, chlorophyll fluorescence and photoinhibition as affected by nitrogen deficiency in maize plants. Plant Sci 151(2):135–143
Marschner H (2012) Mineral nutrition of higher plants. J Ecol 76(4):1250
Mccourt P, Creelman R (2008) The ABA receptors -- we report you decide. Curr Opin Plant Biol 11(5):474–478
Meng ZB, Chen LQ, Suo D, Li GX, Tang CX, Zheng SJ (2012) Nitric oxide is the shared signalling molecule in phosphorus- and iron-deficiency-induced formation of cluster roots in white lupin (Lupinus albus). Ann Bot 109(6):1055–1064
Mohanty P (2004) Defense potentials to NaCl in a mangrove, Bruguiera parviflora: differential changes of isoforms of some antioxidative enzymes. J Plant Physiol 161(5):531–542
Moore K, Roberts LJ (1998) Measurement of lipid peroxidation. Free Radic Res Comm 28(6):659–671
Müge O (2014) Reactive oxygen species: the good, the bad, and the enigma. Mol Cell Oncol 1(3)
Nie Z, Hu C, Tan Q, Sun X (2016) Gene expression related to molybdenum enzyme biosynthesis in response to molybdenum deficiency in winter wheat. J Soil Sci Plant Nutr 16(4):979–990
Patterson K, Cakmak T, Cooper A, Lager I, Rasmusson AG, Escobar MA (2010) Distinct signalling pathways and transcriptome response signatures differentiate ammonium- and nitrate-supplied plants. Plant Cell Environ 33(9):1486–1501
Podgórska A, Gieczewska K, Ł-K K, Rasmusson AG, Gardeström P, Szal B (2013) Long-term ammonium nutrition of Arabidopsis increases the extrachloroplastic NAD(P)H/NAD(P)(+) ratio and mitochondrial reactive oxygen species level in leaves but does not impair photosynthetic capacity. Plant Cell Environ 36(11):2034–2045
Polesskaya OG, Kashirina EI, Alekhina ND (2004) Changes in the activity of antioxidant enzymes in wheat leaves and roots as a function of nitrogen source and supply. Russ J Plant Physiol 51(5):615–620
Raven JA (2010) Tansley review no. 2. Regulation of pH and generation of osmolarity in vascular plants: a cost-benefit analysis in relation to efficiency of use of energy, nitrogen and water. New Phytol 101(1):25–77
Santos RL, Freire FJ, Oliveira ECA, Simões Neto DE, Medeiros MRFA, Bezerra PC, Moura MJA, Barbosa JA, Lopes NRC, Santos NL (2018) Productivity and technological quality of sugarcane under fertilization of nitrogen and molybdenum. J Soil Sci Plant Nutr 18(4):1002–1020
Seo M, Peeters AJ, Koiwai H, Oritani T, Marion-Poll A, Zeevaart JA, Koornneef M, Kamiya Y, Koshiba T (2000) The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc Natl Acad Sci U S A 97(23):12908–12913
Su C-L, Zhang F-M, Sun K, Zhang W, Dai C-C (2019) Fungal endophyte Phomopsis liquidambari improves iron and molybdenum nutrition uptake of peanut in consecutive monoculture soil. J Soil Sci Plant Nutr 19(1):71–80
Sun X, Hu C, Tan Q, Liu J, Liu H (2009) Effects of molybdenum on expression of cold-responsive genes in abscisic acid (ABA)-dependent and ABA-independent pathways in winter wheat under low-temperature stress. Ann Bot 104(2):345–356
Wang F, Gao J, Tian Z, Liu Y, Abid M, Jiang D, Cao W, Dai T (2016) Adaptation to rhizosphere acidification is a necessary prerequisite for wheat (Triticum aestivum L.) seedling resistance to ammonium stress. Plant Physiol Biochem 108:447–455
Wang F, Gao J, Shi S, He X, Dai T (2018) Impaired electron transfer accounts for the photosynthesis inhibition in wheat seedlings (Triticum aestivum L.) subjected to ammonium stress. Physiol Plant 167(2):159–172
Wen X, Hu C, Sun X, Zhao X, Tan Q (2019) Research on the nitrogen transformation in rhizosphere of winter wheat (Triticum aestivum) under molybdenum addition. Environ Sci Pollut Res 26(3):2363–2374
Wu S, Hu C, Tan Q, Nie Z, Sun X (2014) Effects of molybdenum on water utilization, antioxidative defense system and osmotic-adjustment ability in winter wheat (Triticum aestivum L.) under drought stress. Plant Physiol Biochem 83:365–374
Wu S, Hu C, Tan Q, Xu S, Sun X (2017) Nitric oxide mediates molybdenum-induced antioxidant defense in wheat under drought stress. Front Plant Sci 8:1085
Wu S, Hu C, Tan Q, Zhao X, Xu S, Xia Y, Sun X (2018) Nitric oxide acts downstream of abscisic acid in molybdenum-induced oxidative tolerance in wheat. Plant Cell Rep 37(4):599–610
Xiong L, Ishitani M, Lee H, Zhu J-K (2001) The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress–and osmotic stress–responsive gene expression. Plant Cell 13(9):2063–2083
Xiong L, Schumaker KS, Zhu J-K (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14(1):165–183
Yang L, Sun J, Tian Z, Hakeem A, Feng W, Dong J, Cao W, Adkins SW, Dai T (2016) Physiological responses of wheat (Triticum aestivum L.) germination to elevated ammonium concentrations: reserve mobilization, sugar utilization, and antioxidant metabolism. Plant Growth Reg 81(2):1–12
Zdunek-Zastocka E (2008) Molecular cloning, characterization and expression analysis of three aldehyde oxidase genes from Pisum sativum L. Plant Physiol Biochem 46(1):19–28
Zhang M, Hu C, Zhao X, Tan Q, Sun X, Cao A, Cui M, Zhang Y (2012) Molybdenum improves antioxidant and osmotic-adjustment ability against salt stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis). Plant Soil 355(1–2):375–383
Zou N, Li B, Dong G, Kronzucker HJ, Shi W (2012) Ammonium-induced loss of root gravitropism is related to auxin distribution and TRH1 function, and is uncoupled from the inhibition of root elongation in Arabidopsis. J Exp Bot 63(10):3777–3788
Funding
This work was supported by the National Natural Science Foundation of China (Program No. 41771329) and the 948 Project from the Ministry of Agriculture of China (2016-X41).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Imran, M., Sun, X., Hussain, S. et al. Molybdenum Application Regulates Oxidative Stress Tolerance in Winter Wheat Under Different Nitrogen Sources. J Soil Sci Plant Nutr 20, 1827–1837 (2020). https://doi.org/10.1007/s42729-020-00254-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-020-00254-6