Abstract
Micronized sulfur (S) in urea may reduce nitrogen (N) loss by ammoniacal N (NH3–N) volatilization and provide a better use for S from oil refineries, thereby improving plant nutrition by providing N and S. The objective of this study was to evaluate the use of urea micronized with S (urea+S) to mitigate NH3–N volatilization and the efficiency of the urea+S as source of N and S for maize (Zea mays L.). Three field experiments were implemented, with maize grown in coarse-, medium-, and fine-textured soils and the following treatments: urea, ammonium nitrate, ammonium sulfate, urea+S enhanced with 13% elemental S (urea+SA), urea+S with 4% elemental S and 9% S in sulfate form (urea+SB), and a control without N application. The leaf N concentrations increased similarly for all the N sources. The leaf S concentrations were greater when the ammonium sulfate and urea+S were applied. The use of micronized S does not decrease the NH3–N volatilization from urea side-dress applied to maize. However, in comparison with ammonium sulfate, the urea+S are a viable alternative for supplying N and S to maize because of their greater operational efficiencies and due to their high N concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Urea is the main nitrogen (N) source for agriculture and is used commercially worldwide (Trenkel 2010). When urea is applied on the soil surface without being incorporated, it is hydrolyzed to ammonia and can be lost to the atmosphere through ammoniacal N (NH3–N) volatilization (Azeem et al. 2014). In acidic soil, depending on the soil conditions during surface application, N losses can exceed 70% of the applied N (Lara Cabezas et al. 1997). According to Yamada and Abdalla (2000), most N losses occur due to the volatilization of NH3. The volatilization of NH3–N is the result of alkalization close to the urea granules during their hydrolysis (Raun and Johnson 1999). In turn, the hydrolysis of urea is the result of the action of urease, which is an extracellular enzyme produced by microorganisms or originating from plant residues (Frankenberger and Tabatabai 1982). According to Overrein and Moe (1967), the pH may increase from 6.5 to 8.8 adjacent to urea granules. This increase in pH is proportional to a decrease in H+ concentration, which impedes the conversion of ammonia (NH3) into ammonium (NH4+) and favors NH3–N volatilization (Overrein and Moe 1967). Under no-till conditions, the soil surfaces covered with straw provide an environment favorable for the intensification of the microbial NH3 volatilization process (Rochette et al. 2009).
Preventing N losses is essential to increase the efficiency of N fertilization, which in turn depends on several factors, including soil texture, previously grown crops, climatic conditions, N source, N application rate, and N application method (Al-Kanani et al. 1991; Cantarella 2007; Rodrigues and Kiehl 1986). The methods that have been investigated to mitigate NH3–N loss through the use of urea in agriculture include pre-treatment with urea granules with polymers that have different principles of action, urease and nitrification inhibitors (Alfaro et al. 2018; Cantarella et al. 2008), rock dusts, organic compounds (Jadon et al. 2018), micronutrients (Grohs et al. 2011), and sulfur (S) (Nascimento et al. 2013; Trenkel 2010). Among the possible alternatives, the use of S to reduce N losses through urea has been observed to significantly reduce NH3–N volatilization (Nascimento et al. 2013; Rech et al. 2017). The acidifying effect of elemental S oxidation in soil may neutralize alkalization during urea hydrolysis, minimizing N loss through NH3–N volatilization (Trenkel 2010). Other N sources have been used to avoid N loss through NH3–N volatilization, such as ammonium sulfate and ammonium nitrate (Cantarella 1998). However, while urea contains 45% N and ammonium nitrate contains 34% N, ammonium sulfate contains only 20% N and 22–24% S. The low N concentration in ammonium nitrate and ammonium sulfate results in a greater application and transport cost per unit of N applied (Franco and Saraiva Neto 2007).
In addition to the advantage of reducing NH3–N volatilization, N sources containing S provide a nutrient that is limiting for agricultural production across more than 100 million ha worldwide (Bettany and Stewart 1982). Soils deficient in S are increasingly being reported due to the replacement of soil amendments containing S with soil amendments containing little or no S, such as urea and triple superphosphate (Boswell and Friesen 1993). The reduction in S soil stocks from crop uptake and loss of S through leaching and erosion is also a reasons that S is a limiting factor (Kim et al. 2013). Additionally, the application of S together with N yields has not only agronomic advantages but also ecological advantages because it can provide a suitable use for the excess of S from oil refineries as a result of recent environmental regulations in many countries that require oil refineries to reduce the amount of S in fuel (Babich and Moulijn 2003).
The objectives of this study were to verify whether NH3–N volatilization decreases when urea micronized with S (urea+S) is used rather than conventional nitrogen fertilizers and to evaluate the efficiency of N and S sources in terms of plant nutrition, yield components, and grain yield (GY) for maize grown in three different types of soil.
2 Materials and Methods
2.1 Characterization of the Experimental Sites
Field experiments with maize crops grown under no-till farming were conducted at three sites with sandy loam (coarse-textured soil), sandy clay loam (medium-textured soil), and clay (fine-textured soil) soil (Table 1). The coarse-, medium-, and fine-textured soils at the experimental sites were classified as Typic Hapludox, Rhodic Hapludox, and Typic Rhodudalf (Soil Survey Staff, 2014), respectively. The experiment conducted in a coarse-textured soil was located at latitude 22°77′S and longitude 48°57′W in São Manuel, São Paulo State, Brazil. At Botucatu, the experiments were conducted in medium- and fine-textured soils. The site with the medium-textured soil was located at latitude 22°83′S and longitude 48°42′W, and the site with the fine-textured soil was located at latitude 22°84′S and longitude 48°42′W. All experimental sites were located at altitudes of approximately 800 m.
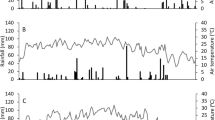
The climate is humid mesothermal and is considered Cwa according to the Köppen classification with mean annual rainfalls of approximately 1450 mm and mean temperatures of 24 °C during the warmest month and 13 °C during the coldest month (Miranda et al. 2015). The temperature and rainfall data for the period of this study were collected from meteorological stations located in São Manuel and Botucatu (Fig. 1).
2.2 Conducting the Experiments
For the initial chemical characterization of the experimental sites, the soil was sampled at two depths, 0–0.20 and 0.20–0.40 m (Table 2). The soil pH was determined in a 0.01 mol L−1 CaCl2 suspension (1:2.5 soil/solution), and the phosphorus (P) and exchangeable calcium (Ca), magnesium (Mg), and potassium (K) concentrations were determined after extraction with ion exchange resin (Raij et al. 1986). The cation exchange capacity (CEC) and base saturation (BS) values were calculated using the exchangeable bases, and the total acidity (H + Al) was determined at pH 7.0 with calcium acetate, according to Raij et al. (2001). The soil organic matter (SOM) concentration was determined according to Nelson and Sommers (1996).
Each plot consisted of six rows that were 10 m long and 0.45 m apart. Maize was sown at the end of October 2014 beneath millet [Pennisetum americanum (L.) K. Schum.] straw in a no-till system. A simple maize hybrid (DKB 390 PRO) was used. In addition to the side-dressing treatments, 24 kg ha−1 N as urea, 37 kg ha−1 P as triple superphosphate, and 40 kg ha−1 K as potassium chloride were applied just below and to the side of the seed furrow.
The treatments consisted of the application of different sources of N and/or S, to compare conventional N sources to urea micronized with S (urea+S), in a concentration of 13% elemental S (urea+SA), and with 4% elemental S and 9% S in sulfate form (urea+SB). The treatments are summarized in Table 3. Fertilizer treatments were manually side-dressed in a uniform line next to the maize row at a rate of 180 kg ha−1 N when maize plants reached the V8 growth stage (Ritchie and Hanway 1986).
2.3 N Loss through NH3–N Volatilization
The loss of N through NH3–N volatilization was evaluated using a semi-open static chamber developed by Nômmik (1973), adapted by Lara Cabezas and Trivelin (1990), and calibrated by Lara Cabezas et al. (1999) for estimating actual losses under field conditions. Collection chambers were constructed from clear and flexible polyvinyl chloride tubing that measured 145.6 mm in diameter and 400 mm in height. The chambers were fitted onto rigid polyvinyl chloride bases that measured 152.8 mm in diameter and 100 mm in height and were inserted into the soil to a depth of 30 mm. The bases were installed in the fields before the application of the N sources, which were weighed in accordance with the base areas of the collector chambers. Instead of a single base for each collector chamber, which was the original method, three bases were used in each experimental unit. Because the chambers for collecting NH3–N protect the soil from rain inside the bases, the chambers were switched from base to base in a rotation scheme after each rainfall event and at each experimental site, thereby enabling the collection of more representative samples. Immediately after the application of the N sources, the collector chambers were installed and fitted onto the previously installed bases. Supports were fitted over the tubes, and protective caps were attached to the tubes to allow air but not rain to enter the system.
Two 20-mm-thick polyurethane foam sponge discs (density of 0.028 g cm−3) were placed in each collector chamber. The first foam disc was placed at a height of 0.15 m above the ground to capture the NH3–N volatilized from the soil, and the second disc was placed at a height of 0.30 m to capture NH3–N from the atmosphere, thereby preventing contamination of the lower foam disc. The foam discs were soaked with 20 mL of glycerol-phosphoric acid (5% v/v H3PO4 and 4% v/v glycerol) (Nômmik 1973). The amount of solution applied to the foam discs was sufficient to soak them without causing dripping.
The foam discs were placed in each collector chamber immediately after the application of N and were replaced at 1, 2, 4, 6, 8, 10, 14, 18, 22, 30, and 40 days after side-dressing. Therefore, the NH3–N volatilization data correspond to the total NH3–N volatilized in the period between sampling times. The three sites were always sampled in the morning and on the same day.
The ammonium phosphate was removed from the foam discs by successively washing them with 300 mL of a 1 mol L−1 KCl solution. Then, a 50-mL aliquot was steam distilled, and 10 mL of 10 mol L−1 NaOH was added to each sample. The distillate was collected in a solution of H3BO3 + indicator. The ammonium phosphate concentration was determined by colorimetry according to the method described by Qiu et al. (1987). The amount of NH3–N determined in each sampling corresponded to the NH3–N volatilized according to the area of the chamber base (0,0166 m2), and was converted to NH3–N volatilized per hectare. The total NH3–N volatilized after 40 days of N application was obtained by the sum of each sampling.
2.4 Maize Crop Measurements
The central third of the leaf from the base of the ear was collected from 10 plants per plot at the VT growth stage for foliar diagnosis (Raij et al. 1997). All samples were dried in a forced-air oven at 60 °C and then ground in an electric Wiley mill. The tissue concentrations of N and S were determined following digestion with H2SO4 and nitro-perchloric acid solution, respectively (Malavolta et al. 1997). The concentrations of N and S in the extracted solutions were determined using the semi-micro Kjeldahl method and atomic absorption spectrophotometry, respectively, following the methods described by Malavolta et al. (1997).
Maize was harvested in March 2015, and the following yield components were determined: final plant population (PP, number of plants extrapolated to the number of plants/ha), number of ears per plant (NEP, number of ears/total number of plants), number of grains per ear (NGE, number of grains in 10 ears), and 100-grain weight (W100, average of eight samples of 100 grains). Grain yield (GY), as the yield component, was determined by manually harvesting the plants from 8-m sections of two central rows. The water content of the grains was determined by oven drying the grains at 105 ± 3 °C for 24 h, and yields were calculated in kg ha−1 based on a moisture content of 130 g kg−1. The N-use efficiency (NUE) was calculated according to the equation proposed by Fageria and Baligar (2005): NUE = kg ha−1 yield increase relative to the control/kg ha−1 N applied.
Grain samples were ground in an electric Wiley mill to determine their N and S concentrations, as described in Malavolta et al. (1997), and the total accumulated N and S contents of the grain were determined by multiplying the N and S concentrations by the GY.
2.5 Statistical Analysis
Each experimental site was established using a randomized complete block design with four replicates. The data from each experimental site were analyzed separately and subjected to analysis of variance at 5% probability, and the means were compared using Fisher’s least significant difference (LSD) test at 5% probability. All of the statistical analyses were performed using SISVAR 5.3 (Ferreira 2010).
3 Results
3.1 NH3–N Volatilization
At all sites and for all treatments, 90% of the NH3–N was volatilized during the first 10 days of the 40-day period after side-dressing (Fig. 2). In the coarse-textured soil, the highest rates of NH3–N occurred until 4 days after N application, resulting in an average total volatilization of 26% of each N source between 1 and 4 days after N application. Between the fourth and sixth days after N application, volatilization was significantly reduced, particularly in the plots that received ammonium nitrate, ammonium sulfate, and urea+SA. This reduction in volatilization occurred due to the rainfall (23.6 mm) that occurred between the third and fourth collection dates (Fig. 2a). The last NH3–N volatilization peak occurred 8 days after the application of urea+SA. However, 10 days after N application, the volatilization decreased for all of the treatments except the urea treatment, possibly due to the occurrence of heavy rainfall (50 mm of rainfall occurred between the fifth and sixth collections) (Fig. 2a).
NH3–N volatilization also increased for up to 4 days after N application in the medium- (Fig. 2b) and fine-textured soils (Fig. 2c), with NH3–N volatilization peaking in the urea+SB and common urea treatments, respectively. Because 33.8 mm of rainfall occurred from 4 to 6 days after N application, NH3–N volatilization decreased in both experiments, especially under the urea alone and urea+SB treatments. Although this rainfall occurred before the third collection (4 days after application), the amount of N volatilized increased at the third collection due to the warm and dry conditions that began several days before the treatment application and persisted for much of the period between the second and third collections. While the treatments with urea and urea+SB decreased NH3–N volatilization by 6 days after N application, the NH3–N volatilization remained high after the application of urea+SA and only decreased 6 to 8 days after N application in both the medium- and fine-textured soils.
In the coarse-textured soil, the sources that resulted in the greatest NH3–N volatilization 40 days after N application were urea+SA and urea+SB, with total respective NH3–N volatilization values of approximately 2.6 and 2.7 kg ha−1 greater than the volatilization observed after urea application (Fig. 3). In the medium-textured soil, urea+SB resulted in the greatest total volatilization, followed by urea+SA, with 3.04 kg ha−1 less NH3–N volatilization than that for urea+SB. The application of urea resulted in approximately 3.94 kg ha−1 less NH3–N volatilization than that for urea+SB. In the fine-textured soil, the application of urea resulted in more total volatilization than that for urea+S.
Total ammoniacal nitrogen (NH3–N) volatilization, as affected by side-dressing nitrogen sources, in coarse-textured soil in São Manuel and medium- and fine-textured soil in Botucatu, São Paulo State, Brazil. Values in the columns followed by the same letter are not significantly different at p ≤ 0.05 according to the Fisher’s LSD test. Error bars represent the standard error
3.2 Concentration of N and S in Maize Leaves
Regardless of the N source, N application increased the concentrations of N in the leaves of plants grown in the coarse-textured soil, but no difference was observed among the treatments for the medium-textured soil, not even for the control. The leaf N concentrations increased relative to the control only when urea+SB was applied to the fine-textured soil (Table 4).
In the coarse-textured soil, the leaf S concentrations were greater only when ammonium sulfate and urea+SA were applied (Table 4). In the medium-textured soil, the treatments with ammonium sulfate also resulted in greater S concentrations in the maize leaves, and the application of urea+S resulted in greater S concentrations than those in the control, urea, and ammonium nitrate treatments (Table 4). In the fine-textured soil, the S concentrations in the maize leaves were similar in all the treatments (Table 4).
3.3 Maize Yield Components
The final plant population was not affected by any of the treatments, and the average plant population in all experimental sites was 58,160 plants ha−1. In the coarse-textured soil, the NEP was lower in the control and ammonium sulfate treatments. The highest NGE was observed when ammonium sulfate was applied, and the lowest NGE was observed in the control treatment. The W100, GY, and NUE were the same in all the treatments in the coarse-textured soil that received a N source side-dressing (Table 5).
The application of common urea and urea+S provided the highest NEP in relation to the other treatments in the medium-textured soil (Table 5). However, the NGE was the highest for the urea+SA application, which resulted in consecutively higher GY than the other treatments even when the W100 showed no differences among the treatments (Table 5). The NUE was higher when prototype A was applied than urea, providing 10 kg of grain per kg of N more in comparison with the urea treatment (Table 5).
In the fine-textured soil, the NEP was the same for all the treatments, including the control, whereas the NGE, W100, and GY were lower only in the control treatment. Therefore, there were no differences in GY and NUE regardless of the N sources (Table 5).
3.4 Concentrations and Total Accumulation of N and S in Maize Grains
The treatments had no effect on the concentrations of N and S in the maize grains in the coarse- and medium-textured soils (Table 4). In the fine-textured soil, a higher N concentration was measured in response to applications of ammonium sulfate and urea+S. However, there was no effect on the S concentration in the grains (Table 4).
Although the grain N contents were not affected by the treatments, the total accumulation of N in the grains was greater in the treatments than in the control for the coarse- and medium-textured soils. However, no differences in the N accumulated in grains were observed in the fine-textured soil (Table 4). The total accumulation of S in the grains was greatest for the ammonium sulfate treatment on the coarse-textured soil. The control and ammonium nitrate treatment resulted in the lowest S accumulation in the grains in the medium-textured soil, and no differences were observed in the fine-textured soil, not even in the control (Table 4).
4 Discussion
Peaks and valleys in the NH3–N volatilization data were observed, possibly in response to the climatic conditions. The movement of N into deeper soil layers can be stimulated by rain, which would reduce volatilization. In contrast, when little or no rainfall occurs, intense hydrolysis of urea occurs via urease, which increases the potential for losses of NH3–N. To avoid great losses of NH3–N after side-dressing N fertilizer, appropriate irrigation management is recommended among other solutions, such as the incorporation of fertilizer by applying water immediately after the application of N sources that have a high potential for losses through volatilization (e.g., urea) (Wang et al. 2004). When urea is transported into deeper soil layers, gaseous NH3 molecules migrate from below the soil to the atmosphere. During this process, these molecules may encounter areas of soil with low pH, which results in the transformation of NH3 into NH4+ upon the receipt of a proton. These molecules subsequently remain in the soil solution or are electrostatically adsorbed into negative charges. In addition to dry periods, periods of high temperatures favor increased NH3–N loss through volatilization due to an increased NH3/NH4+ ratio in the soil solution, reduced NH3 solubility, increased urease activity, and increased evaporation rate (Trivelin et al. 1994). Generally, temperatures are milder during and shortly after rainfall events. Therefore, the peaks and valleys observed in the NH3–N volatilization data may be strongly related to climatic conditions, especially with the application of conventional or S-treated urea.
The similarity between urea alone and urea+S regarding NH3–N volatilization may be related to the form of S and its concentration in the urea+S. Urea+SA contains only elemental S, urea+SB contains only 9% S as sulfate, while ammonium sulfate contains one molecule of sulfate for every two molecules of NH4+ [(NH4)2SO42−]. The presence of the sulfate anion leads to an acidic reaction in the soil, and the sulfate acts as an accompanying ion for NH4+, favoring the vertical displacement of NH4+ in the soil (Lara Cabezas et al. 1997). The presence of S in urea granule was not enough to reduce the NH3–N volatilization losses in the coarse- and medium-textured soil; however, the higher initial amount of SO42−–S in the fine-texture soil may have allowed a bigger interaction between sulfate and NH4+ reducing the volatilization compared with regular urea, once the soil chargers were filled with SO42−–S more than the other two sites. Further studies are necessary to verify the ability of higher concentrations of micronized S in sulfate urea granules to prevent NH3–N loss through volatilization in soils with different SO42−–S concentrations.
In the three studied soils, no single N source resulted in a greater total volatilization of N for any of the soil types. These differences occurred due to the characteristics of the soils in each experiment. According to Bayer and Mielniczuk (1997), an increase in the SOM concentration is related to an increase in the microbial population and CEC. With a larger microbial population, the urease activity is also higher, thus favoring the hydrolysis and consequent loss of NH3–N. In no-till systems, the presence of plant residues is ideal for increasing the SOM concentration and microbial population, and plant residues decrease the contact of urea with the soil, thereby decreasing the adsorption of NH4+ into the soil and favoring N loss through the volatilization of NH3–N (Rochette et al. 2009).
Although the experiments in the different textural classes of soil were not statistically designed to have their results compared with one another, the total amount of volatilized NH3–N did differ according to each N source and soil type. Due to strong interactions between the factors that determine NH3–N volatilization, it is very difficult to compare the results of experiments in different soil types. It is even more difficult to compare the results obtained in coarse-textured soil with those obtained in the other soils because the distance between the sites results in climatic differences. The climatic differences between the medium- and fine-textured soils were less, which allowed for a fair comparison.
The total amount of NH3–N was low for the ammonium sulfate and ammonium nitrate as expected, but the total volatilization from urea alone and urea+S was also low, resulting in average less than 5% of the total N side-dress applied. According to Lara Cabezas et al. (1997), N losses can exceed 70% of the applied N from urea, being common to observe losses of 15–30% of the total N applied from urea (Cantarella et al. 2008; Otto et al. 2017; Viero et al. 2014). Once there was a rain only 4 days after N application, the N released from urea was carried into the soil, avoiding high volatilization. Similar results were observed by Nascimento et al. (2013), due to rainfall precipitation few days after urea application in the soil surface. The total NH3–N volatilized after the application of urea was greater in the fine-textured soil than in the medium-textured soil. The fine-textured soil had a greater CEC and a similar SOM value than the medium-textured soil based on the initial analysis. However, there was typically more plant residue coverage for the fine-textured soil, which could have resulted in reduced contact of the NH4+ with the soil and favored more NH3–N volatilization.
In the coarse-textured soil, the ammonium sulfate treatment produced the lowest NEP, but the compensatory effect resulted in a larger NGE. Thus, there was no difference in productivity. Haag et al. (2017) reported that the NGE benefits from the compensatory effect mainly due to decreased PP. In the medium-textured soil, although there was no difference in the NEP, the urea+SA treatment produced the largest NGE, resulting in a higher GY, while urea+SB resulted in a small NGE. Soils with high P concentrations after liming favors the movement of sulfate to deeper layers (Rajan 1978), as in the medium-textured soil. The large amount of elemental S in urea+SA may have been oxidized and released gradually, while the sulfate from urea+SB and ammonium sulfate was probably transported to the deep soil layers. Nevertheless, the oxidation of elemental S is slow, about 20 days to start (Horowitz and Meurer 2006), while the definition phase of maximum number of rows per ear is on V8 growth stage, when the treatments were applied, and the maximum potential of ovule formation is established on V12 (Abendroth et al. 2011).
The greater N concentrations in urea+S resulted in lower transportation costs, improved operational performance, and avoided frequent fertilizer shipments (Franco and Saraiva Neto 2007). Because S is supplied with the applications of urea+S, the S concentrations in the maize leaves were high, and this result was even more pronounced with application of urea+SA in the coarse-textured soil, possibly due to the S in its elemental form. In addition, the soils with high P concentrations tended to leach sulfate (Rajan 1978), and the slowly oxidized elemental S results in the advantage of avoiding the loss of S to deep soil layers, favoring S uptake by plants during plant cycles and for the next season crop. It is worth mentioning that the oxidation of elemental S is inversely proportional to the clay content in the soil (Germida and Janzen 1993).
The S deficiency in the medium-textured soil may be fundamental to the differences observed in the yield components and GY. When this deficiency was prevented by the urea+S but not by the urea, the NGE and GY with the urea+SA treatment were higher than that with the urea treatment. The fine-textured soil, which had a higher SOM concentration compared with the other soils, had a very high initial concentration of S (Raij et al. 1997), which may have eliminated the effect of S supply on the leaf S concentrations.
Because S and N are common elements in the composition of many proteins (Dijkshoorn and van Wijk 1967), it is expected that supplying these elements could result in maize grains with a greater nutritional value. In this case, when urea+S was applied to the fine-textured soil, a high concentration and accumulation of N in the grains occurred, which was similar to the effect of ammonium sulfate. Nevertheless, no differences were observed in the N accumulated in the grains from the coarse- and medium-textured soil.
5 Conclusions
The micronized sulfur in the urea granule is not able to reduce losses through NH3–N volatilization on maize crop top-dressing and the amount of NH3–N losses is directly related to characteristic of each soil type. Despite the N losses due to NH3–N volatilization, the application of urea+S provided adequate N nutrition to the maize plants, resulting in GYs similar to those observed following the application of other N sources with low NH3–N volatilization, such as ammonium nitrate and ammonium sulfate. The urea+S provided S in a form available to the plants. Maize growing in coarse-textured soils, such as sandy and sandy clay loam soils, appeared to be more responsive to urea+S since the S concentration in these soils was low.
References
Abendroth LJ, Elmore RW, Boyer MJ, Marlay SK (2011) Corn growth and development. Iowa State University Extension, Ames
Al-Kanani T, MacKenzie AF, Barthakur NN (1991) Soil water and ammonia volatilization relationships with surface-applied nitrogen fertilizer solutions. Soil Sci Soc Am J 55:1761–1766. https://doi.org/10.2136/sssaj1991.03615995005500060043x
Alfaro M, Salazar F, Hube S, Ramírez L, Mora MS (2018) Ammonia and nitrous oxide emissions as affected by nitrification and urease inhibitors. J Soil Sci Plant Nutr 18:479–486. https://doi.org/10.4067/S0718-95162018005001501
Azeem B, KuShaari K, Man ZB, Basit A, Thanh TH (2014) Review on materials and methods to produce controlled release coated urea fertilizer. J Control Release 181:11–21. https://doi.org/10.1016/j.jconrel.2014.02.020
Babich IV, Moulijn JA (2003) Science and technology of novel processes for deep desulfurization of oil refinery streams: a review. Fuel 82:607–631. https://doi.org/10.1016/S0016-2361(02)00324-1
Bayer C, Mielniczuk J (1997) Características químicas do solo afetadas por métodos de preparo e sistemas de cultura. Rev Bras Cienc Solo 21:105–112. https://doi.org/10.1590/S0100-06831999000300023
Bettany JR, Stewart JWB (1982) Sulphur cycling in soils. Paper presented at The proceedings of international Sulphur'82 conference, London
Boswell CC, Friesen DK (1993) Elemental sulfur fertilizers and their use on crops and pastures. Fert Res 35:127–149. https://doi.org/10.1007/bf00750226
Cantarella H (1998) Adubação nitrogenada em sistema de cana crua. STAB - Açúcar. Álcool e Subprodutos 16:21–22
Cantarella H (2007) Nitrogênio. In: Novais R, Alvarez V, Barros N, Fontes R, Cantarutti R, Neves J (eds) Fertilidade do solo, vol 2. Sociedade Brasileira de Ciência do Solo, Viçosa, pp 375–470
Cantarella H, Trivelin PCO, Contin TLM, Dias FLF, Rossetto R, Marcelino R, Coimbra RB, Quaggio JA (2008) Ammonia volatilization from urease inhibitor-treated urea applied to sugarcane trash blankets. Sci Agric 65:397–401. https://doi.org/10.1590/S0103-90162008000400011
Dijkshoorn W, van Wijk AL (1967) The sulphur requirements of plants as evidenced by the sulphur-nitrogen ratio in the organic matter a review of published data. Plant Soil 26:129–157. https://doi.org/10.1007/bf01978680
Fageria NK, Baligar VC (2005) Enhancing nitrogen use efficiency in crop plants. Adv Agron 88:97–185. https://doi.org/10.1016/S0065-2113(05)88004-6
Ferreira DF (2010) SISVAR - programa estatístico, 5.3 edn. Universidade Federal de Lavras, Lavras
Franco JAM, Saraiva Neto A (2007) Produção de fertilizantes nitrogenados e suprimento de matéria seca. In: Yamada T, Abdalla SRS, Vitti GC (eds) Nitrogênio e enxofre na agricultura brasileira. IPNI, Piracicaba, pp 73–108
Frankenberger WT, Tabatabai MA (1982) Amidase and urease activities in plants. Plant Soil 64:153–166. https://doi.org/10.1007/bf02184247
Germida JJ, Janzen HH (1993) Factors affecting the oxidation of elemental sulfur in soils. Fert Res 35:101–114. https://doi.org/10.1007/bf00750224
Grohs M, Marchesan E, Santos DS, Massoni PFS, Sartori GMS, Ferreira RB (2011) Resposta do arroz irrigado ao uso de inibidor de urease em plantio direto e convencional. Cienc Agrotec 35:336–345. https://doi.org/10.1590/S1413-70542011000200015
Haag LA, Holman JD, Ransom J, Roberts T, Maxwell S, Zarnstorff ME, Murray L (2017) Compensation of corn yield components to late-season stand reductions in the central and northern great plains. Agron J 109:524–531. https://doi.org/10.2134/agronj2015.0523
Horowitz N, Meurer EJ (2006) Oxidação do enxofre elementar em solos tropicais. Cienc Rural 36:822–828. https://doi.org/10.1590/S0103-84782006000300015
Jadon P, Selladurai R, Yadav SS, Coumar MV, Dotaniya ML, Singh AK, Bhadouriya J, Kundu S (2018) Volatilization and leaching losses of nitrogen from different coated urea fertilizers. J Soil Sci Plant Nutr. https://doi.org/10.4067/S0718-95162018005002903
Kim KI, Kaiser DE, Lamb J (2013) Corn response to starter fertilizer and broadcast sulfur evaluated using strip trials. Agron J 105:401–411. https://doi.org/10.2134/agronj2012.0299
Lara Cabezas WAR, Korndorfer GH, Motta SA (1997) Volatilização de N-NH3 na cultura de milho: II. Avaliação de fontes sólidas e fluidas em sistema de plantio direto e convencional. Rev Bras Cienc Solo 21:489–496. https://doi.org/10.1590/S0100-06831997000300019
Lara Cabezas WAR, Trivelin PCO (1990) Eficiência de um coletor semi-aberto estático na quantificação de N-NH3 volatilizado da uréia aplicada ao solo. Rev Bras Cienc Solo 14:345–352
Lara Cabezas WAR, Trivelin PCO, Bendassolli JA, de Santana DG, Gascho GJ (1999) Calibration of a semi-open static collector for determination of ammonia volatilization from nitrogen fertilizers. Commun Soil Sci Plant Anal 30:389–406. https://doi.org/10.1080/00103629909370211
Malavolta E, Vitti GC, Oliveira AS (1997) Avaliação do estado nutricional das plantas: Princípios e aplicações, 2nd edn. Potafós, Piracicaba
Miranda MJ, Pinto HS, Júnior JZ, Fagundes RM, Fonsechi DB, Calve L, Pelllegrino GQ (2015) Climate of the Paulista Municipalities. Cepagri 1–4. http://www.cpa.unicamp.br/outras-informacoes/clima_muni_086.html. Accessed 20 Sep 2015
Nascimento CAC, Vitti GC, Faria LA, Luz PHC, Mendes FL (2013) Ammonia volatilization from coated urea forms. Rev Bras Cienc Solo 37:1057–1063. https://doi.org/10.1590/S0100-06832013000400022
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Sparks DL (ed) Methods of soil analysis. SSSA and ASA, Madison, pp 961–1010. https://doi.org/10.2136/sssabookser5.3.c34
Nômmik H (1973) The effect of pellet size on the ammonia loss from urea applied to forest soil. Plant Soil 39:309–318. https://doi.org/10.1007/bf00014798
Otto R, Zavaschi E, Souza Netto GJM, Machado BA, Mira AB (2017) Ammonia volatilization from nitrogen fertilizers applied to sugarcane straw. Rev Ciênc Agron 48:413–418. https://doi.org/10.5935/1806-6690.20170048
Overrein LN, Moe PG (1967) Factors affecting urea hydrolysis and ammonia volatilization in soil. Soil Sci Soc Am J 31:57–61. https://doi.org/10.2136/sssaj1967.03615995003100010018x
Qiu XC, Liu GP, Zhu YQ (1987) Determination of water-soluble ammonium ion in soil by spectrophotometry. Analyst 112:909–911. https://doi.org/10.1039/AN9871200909
Raij B, Andrade JC, Cantarella H, Quaggio JA (2001) Análise química para avaliação da fertilidade de solos tropicais. Instituto Agronômico, Campinas
Raij B, Cantarella H, Quaggio JA, Furlani AMC (1997) Recomendações de adubação e calagem para o Estado de São Paulo (Boletim Técnico, 100)., 2nd edn. Instituto Agronômico/Fundação IAC, Campinas
Raij B, Quaggio JA, Da Silva NM (1986) Extraction of phosphorus, potassium, calcium, and magnesium from soils by an ion-exchange resin procedure. Commun Soil Sci Plant Anal 17:547–566. https://doi.org/10.1080/00103628609367733
Rajan SSS (1978) Sulfate adsorbed on hydrous alumina, ligands displaced, and changes in surface charge. Soil Sci Soc Am J 42:39–44. https://doi.org/10.2136/sssaj1978.03615995004200010009x
Raun WR, Johnson GV (1999) Improving nitrogen use efficiency for cereal production. Agron J 91:357–363. https://doi.org/10.2134/agronj1999.00021962009100030001x
Rech I, Polidoro JC, Pavinato PS (2017) Additives incorporated into urea to reduce nitrogen losses after application to the soil. Pesqui Agropecu Bras 52:194–204. https://doi.org/10.1590/s0100-204x2017000300007
Ritchie SW, Hanway JJ (1986) How a corn plant develops. Iowa State University of Science and Technology, Ames
Rochette P, Angers DA, Chantigny MH, MacDonald JD, Bissonnette N, Bertrand N (2009) Ammonia volatilization following surface application of urea to tilled and no-till soils: a laboratory comparison. Soil Tillage Res 103:310–315. https://doi.org/10.1016/j.still.2008.10.028
Rodrigues MB, Kiehl JC (1986) Volatilização de amônia após emprego de uréia em diferentes doses e modos de aplicação. Rev Bras Cienc Solo 10:37–43
Soil Survey Staff (2014) Keys to soil taxonomy, 12th edn. USDA-Natural Resources Conservation Service, Washington, DC
Trenkel ME (2010) Slow-and controlled-release and stabilized fertilizers: an option for enhancing nutrient use efficiency in agriculture. International fertilizer industry association, Paris
Trivelin PCO, Lara Cabezas WAR, Boareto AE (1994) Dinâmica do nitrogênio de fertilizantes fluidos no sistema solo-planta. In: Vitti G, Boareto A (eds) Fertilizantes fluídos. Potafos, Piracicaba, pp 314–330
Viero F, Bayer C, Fontoura SMV, RPd M (2014) Ammonia volatilization from nitrogen fertilizers in no-till wheat and maize in southern Brazil. Rev Bras Cienc Solo 38:1515–1525. https://doi.org/10.1590/S0100-06832014000500017
Wang ZH, Liu XJ, Ju XT, Zhang FS, Malhi SS (2004) Ammonia volatilization loss from surface-broadcast urea: comparison of vented- and closed-chamber methods and loss in winter wheat–summer maize rotation in North China plain. Commun Soil Sci Plant Anal 35:2917–2939. https://doi.org/10.1081/CSS-200036499
Yamada T, Abdalla S (2000) Como melhorar a eficiência da adubação nitrogenada do milho. Informações Agronômicas 91. Potafos, Piracicaba, pp 1–5
Acknowledgments
We would like to thank the National Council for Scientific and Technological Development (CNPq) for an award for excellence in research to C.A.C. Crusciol.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Crusciol, C.A.C., Almeida, D.S., Alves, C.J. et al. Can Micronized Sulfur in Urea Reduce Ammoniacal Nitrogen Volatilization and Improve Maize Grain Yield?. J Soil Sci Plant Nutr 19, 701–711 (2019). https://doi.org/10.1007/s42729-019-00070-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-019-00070-7