Abstract
Large-sized biopores in the subsoil can provide rapid access to deeper soil layers and potentially enhance nutrient acquisition. Besides site conditions, the extent of these effects depends on crop root architecture. The aim of this study was to compare barley (Hordeum vulgare L.), wheat (Triticum aestivum L.), oilseed rape (Brassica napus L.) and faba bean (Vicia faba L.) with respect to time of macropore entry, root soil contact and root hair formation. Crops were grown in columns with condensed subsoil and an artificial macropore. Root growth dynamics in the macropore were monitored with in situ endoscopy. After harvest, the share of roots in macropores was quantified. Oilseed rape had many vertical roots with root hairs contacting the pore wall and was the only crop with preferential root growth in macropores. Cereal roots were mostly crossing the pore horizontally, partially with root hairs and partially contacting the pore wall. Nearly all faba bean roots were crossing the pore without root-soil contact. Our results support the notion that the importance of biopores for crop root growth and resource acquisition depends on root architecture. Under the conditions of the study with sufficient water supply and therefore comparatively low penetration resistance, macropores were only marginally used by cereals and faba bean. Oilseed rape roots were apparently much more sensitive to mechanical impedance, and the pores were used more intensively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cylindrically shaped, continuous vertical biopores in arable fields are typically created either by taprooted crops or by anecic earthworms (Kautz et al. 2014). In the subsoil below the plough layer, they may be stable for decades (Hagedorn and Bundt 2002) and may play a key role for active nutrient mobilization (Kautz et al. 2013a). In contrast to the surrounding bulk soil, which is characterized by high penetration resistance and low nutrient concentrations, in biopores no mechanical impedance hinders vertical root growth. Here, high aeration, high microbial activity (Tiunov and Scheu 1999; Jégou et al. 2001) and high nutrient contents (Edwards and Lofty 1980) are provided, especially after passage of anecic earthworms (Athmann et al. 2017a). Thus, biopores may both facilitate better access to deeper soil layers and the nutrient and water reserves stored therein and serve as hotspots for plant nutrient acquisition from the subsoil (Kuzyakov and Blagodatskaya, 2015). Consequently, crop roots have been reported to preferentially follow such pores (Ehlers et al. 1983, Stewart et al. 1999, Kautz et al. 2013b).
There are conflicting results regarding the implications of this preferential root growth in macropores for crop performance, both from pot and field experiments: Plain additional artificial macropores in pots with tightly packed soil resulted in smaller leaf area of barley (Passioura 2002). A similar experimental arrangement, but with earthworm incubation into macropores preceding crop cultivation and with a water source in the 70-cm soil depth, resulted in higher shoot dry matter and N uptake of winter wheat (Dresemann et al. 2018). In the field on a Red Kandosol in Australia, White and Kirkegaard (2010) found 85–100% of wheat roots in the subsoil clumping in biopores, establishing contact only via root hairs, without access to the surrounding bulk soil. Ehlers et al. (1983) studied root growth of oats with and without ploughing. They also found virtually all subsoil roots growing in biopores, but here resulting in higher overall root length densities in the subsoil in the treatment without tillage—i.e. biopores counteracted the possible root restricting effect of increased soil strength, which was observed in the zero tillage system. On a Haplic Luvisol, Perkons et al. (2014) and Han et al. (2015) detected preferential root growth in biopores for spring wheat, winter barley and winter oilseed rape, with only up to 20% of subsoil roots in biopores. However, this was accompanied by higher root length densities in treatments with more biopores also in the bulk soil, and, as more recent results from the same field trial indicate, in a dry year, also by higher crop yield of spring barley (Athmann et al. 2017b). At the same site, root-soil contact in biopores was studied with in situ endoscopy (Athmann et al. 2013a, b), revealing that irrespective of crop species, between 80 and 90% of all vertical roots did establish contact to the pore wall, facilitating water and nutrient uptake—i.e. clumping of roots in biopores was not observed.
Whether or not biopores are beneficial for crop growth depends on numerous factors. Colonization by anecic earthworms considerably increases microbial biomass, enzyme activities and contents of plant available N and P in biopore walls, reinforcing biopores as hotspots for the nutrient acquisition from the subsoil (Athmann et al. 2017a). Furthermore, the effect of biopores on root elongation rate depends on physical characteristics of the surrounding bulk soil, namely humidity and soil strength (Gaiser et al. 2013). Finally, root architecture also has a strong impact on biopore use: In our own long-term studies on a Haplic Luvisol, the share of roots in biopores was higher for allorhizous than for homorhizous species (Perkons 2018). Consequently, the share of nitrogen taken up from biopores was higher for allorhizous mallow than for spring wheat (Athmann et al. 2016). Extent of root-soil contact quantified with in situ endoscopy varied between species: Spring wheat and winter barley with fibrous root systems were mainly directly in contact to the pore wall, while allorhizous mallow and winter oilseed rape established contact predominantly via lateral roots (Athmann et al. 2013a, b). Further parameters potentially affecting nutrient acquisition from the drilosphere that were endoscopically assessed included root status (active, fresh, or old) and share of roots with root hairs. For these two parameters, no differences were detected between species: For all species, many root hairs were observed in biopores, and the share of active roots (i.e. roots with turgescent, glossy aspect and/or many root hairs present) increased with soil depth. However, these studies were conducted after flowering i.e. quite late in the vegetation period. Information on root growth dynamics into biopores is still missing. Consequently, in the presented study, we aimed to study time of pore entrance, root-soil contact, share of roots with root hairs and root status dynamically during the first 33 days of crop growth. In a microcosm approach enabling continuous monitoring of root growth in macropores over several weeks, four species with contrasting root systems were studied, namely wheat and barley with fibrous root systems and oilseed rape and faba bean with allorhizous root systems. As in former field studies (Kautz and Köpke 2010, Athmann et al. 2013a, b), we used in situ endoscopy. This non-destructive technique allows quantification of root-soil contact, root status and root hair formation as aspects of root growth relevant for nutrient acquisition in biopores and can thus complement analysis of root length density.
2 Material and Methods
2.1 Experimental Setup
Root growth of the four crop species spring wheat (Triticum aestivum L.), spring barley (Hordeum vulgare L.), spring oilseed rape (Brassica napus L.) and faba bean (Vicia faba L.) was studied in a greenhouse microcosm experiment. Soil columns with 10-cm diameter and a total length of 60 cm were used, containing a subsoil section of 40-cm length with a central artificial macropore underneath a topsoil section of 20-cm length. Before crop cultivation, these artificial macropores were colonized by anecic earthworms (Lumbricus terrestris L.) for 3 weeks, with the aim to provide coverage of macropore walls with nutrient-rich earthworm cast as in the field situation. After earthworm removal, crops were cultivated for 48 days. During 33 days of cultivation, root growth in these macropores covered with earthworm cast was regularly monitored with in situ endoscopy.
Subsoil mainly from the Bt horizon (42–86-cm soil depth) was collected from a well-described Haplic Luvisol (WRB) derived from loess (loamy silt) with about 30% clay in the Bt horizon, a pH of 6.9 and a subsoil bulk density of about 1.6 g cm−3 (see Vetterlein et al. 2013). The site of soil origin is situated in Klein-Altendorf near Bonn, Germany (50° 37′ 9″ N 6° 59′ 29″ E, 9.6 °C mean annual temperature, 625 mm mean annual rainfall). The soil was sieved (2 mm), filled into 16 columns (subsoil sections) to a height of 38 cm and recompacted with a hydraulic device and a die to a bulk density of 1.6 g cm−3 in two steps (2 × 19 cm), corresponding to the situation in the field. As in a study by Perreault and Whalen (2006), high soil moisture content was found to intensify feeding and decrease burrowing of anecic earthworms; the soil was moistened to a water content of 20%. This is also within the moisture range preferred by Lumbricus terrestris in an experiment by Daugbjerg (1988). One artificial vertical pore with 6-mm diameter, i.e. well within the range of natural biopores colonized by earthworms (see Athmann et al. 2013a), was formed in the centre of each column using an electric drill.
In order to enrich pore walls with nutrients and provide surface properties similar to natural biopores, earthworms were introduced into the artificially created pores before cultivation (Fig. 1a). Twenty-eight adult individuals were rinsed with cold water and kept in boxes with moist paper towels to defecate for 2 days. Afterwards, each pore was incubated with one individual for 3 weeks. Worms were equally distributed over the repetitions of the four crop species according to their body mass. Dried ryegrass (0.6 g) cut to 2 mm was placed around each pore as food for the earthworms. Dried ryegrass (0.3 g) was added when approximately half of the material was removed. During earthworm incubation, columns were kept at 14 °C. After 3 weeks, all worms were removed from the pores with a stick.
Experimental setup with earthworm incubation into artificial pore in subsoil segment covered with lucerne as a food source for the earthworm (a) and cultivation of barley in composite columns after earthworm removal, with the topsoil segment mounted on the subsoil segment and the pipe collar filled with vermiculite for introducing the endoscope up to the depth where the deepest root appeared (b). With the endoscope, pictures of the roots in the macropore were taken every 2 days until roots had reached the bottom of the column
After earthworm removal, the subsoil sections were placed into a matching pipe collar (10 cm height) with a lid at the bottom that was filled with water-saturated vermiculite which was then condensed by placing the subsoil sections inside, thus ensuring good contact between vermiculite and soil. The vermiculite, a swelling clay mineral, was used due to its capacity for rapid water uptake, to enable watering of the columns from below to simulate water supply from the subsoil in the field during the vegetation period. As a guide rail for the endoscope, a flexible tube with 11-mm diameter and 9.5-cm length with a plastic pipe with 8-mm diameter inside was introduced into a hole in the centre of the lid, ensuring smooth transition from the plastic pipe to the macropore. An opening for watering the columns was drilled laterally into the pipe collar, also equipped with a flexible tube that entered the sections with the vermiculite. A topsoil section of 20-cm height was then placed on top of the subsoil sections. Sieved topsoil (4 mm) from the same site was filled to a height of 18 cm into the topsoil sections and condensed to a bulk density of 1.3 g cm−3. Transitions of all sections were secured with duct tape (Fig. 1b).
2.2 Cultivation
Columns were set up in a greenhouse. On April 12, 2017, five seeds of spring wheat cv. Sonett, spring barley cv. Sydney or spring oilseed rape cv. Makro, or four seeds of faba bean cv. Alexia were sown into four columns each, representing sowing densities typical for the field situation. Thus, the experiment consisted of four replications per crop with one central macropore each. After germination, plants were reduced to four plants per column for the first three crops, and two plants per column for faba bean, corresponding to a seeding density of 509 and 255 grains m−2 respectively. The soil was kept close to field capacity by watering regularly, applying 200 ml water to each column after sowing and afterwards 100 ml water through the watering pipe in the pipe collar below the subsoil section every 2 days. After 7 days, all crops had germinated completely except for oilseed rape, which in three columns was supplemented with two to three grains. After 12 days, also oilseed rape had reached the targeted seeding density. Crops were cultivated for 33 days. The air temperature amplitude during plant growth was about 20–35 °C.
2.3 In Situ Endoscopy and Root Analysis
For following root growth in macropores, we used a videoscope (Karl Storz GmbH, Tuttlingen, Germany, outer diameter of 3.8 mm, with a 0° direction of view and a flare angle of 80°). The otherwise flexible shaft has a rigid end that can be deflected in two directions by 180°/190°. The shaft tip contains a CCD colour camera chip. Illumination was provided by a 150-W cold light projector (Karl Storz GmbH, Tuttlingen, Germany). Real-time pictures were transmitted via a “WinTV-PVR-USB2” video recorder (Hauppauge Computer Works Inc., New York City, USA). The endoscope was introduced through the plastic tube into the pore of each column and moved upwards until the first root was spotted. All visible root segments were photographed and saved in jpeg format with a resolution of 500 × 582 pixels (PAL) for later analysis. Only undisturbed pore areas not touched by the endoscope were photographed. The depth in which the youngest roots were spotted was recorded.
The images gained were evaluated visually with respect to the number of roots per soil depth class, root orientation, root-soil contact, root hairs and root status. The categories used are given in Table 1.
Starting 9 days after sowing, root growth was studied with in situ endoscopy about every 2 days. Endoscopy was stopped when crop roots had reached the bottom of the subsoil segments 33 days after sowing. At this time, wheat had reached EC 29–30 with 8.1–9.0 g shoot dry matter per column, barley EC 37–43 with 10.5–12.6 g, oilseed rape EC 13–22 with 11.1–15.0 g and faba bean EC 59–61 with 16.1–19.9 g.
After harvesting the shoot, topsoil segments were removed. Subsoil segments were horizontally dissected into four slices, representing subsoil depths of 0–10, 10–20, 20–30 and 30–40 cm. Each slice was then vertically dissected so that pores could be laterally opened using sharp knives. Roots growing inside the pore lumen were collected with tweezers. The remaining bulk soil samples were soaked in tap water and washed over a sieve (mesh size 0.5 mm) in order to separate roots from soil and organic debris. Immersed roots were placed on trays, with care taken to avoid overlapping, and scanned (Perfection V700 Photo Scanner, Epson Corp.). Root length density (RLD, cm roots cm−3 soil) was determined with the software package WinRHIZO Pro Version 2016.
Where normal distribution was given, ANOVA followed by Tukey test at α = 0.05 was used for the comparison of soil layers and crop species. Otherwise, data were evaluated with the parameter free Kruskal-Wallis test followed by Bonferroni-Dunn correction at α = 0.05. All tests were performed with SPSS statistics (IBM, version 24.0).
3 Results
Observations with in situ endoscopy revealed considerable differences with respect to root growth in macropores between the four crop species. The first spring wheat root appeared in the 10–20-cm-depth layer; for the other three crops, the first root was spotted in the 0–10-cm-depth layer (Fig. 2). Homorhizous spring barley and spring wheat roots entered the pore early (9 days after seeding), while oilseed rape and faba bean roots appeared in the pore much later (17 and 20 days after seeding). Consequently, wheat and barley also reached the lowest soil layer earlier than oilseed rape and faba bean (Fig. 2). At the last observation date, spring wheat, barley and oilseed rape had on average 5–6 roots in the pore in the lowest soil layer, while faba bean had only two.
Besides the time of entering the pore shown in Fig. 2, there were also differences with respect to orientation, root-soil contact and root hair formation between the species: Oilseed rape had a large share of vertical roots with root hairs in direct contact to the pore wall (Fig. 3a–c). The share of vertical roots was smaller for homorhizous barley and wheat, especially during the last four observation days (Fig. 3a). Most roots had root hairs, but were only partially in direct contact to the pore wall (Fig. 3b, c). Faba bean had no vertical roots at all (Fig. 3a), and roots crossed the pore wall almost without establishing direct contact (Fig. 3b). The share of roots with hairs was small, but increased strongly at the last day of the observation period (Fig. 3c).
Share of vertical roots (a), share of roots with contact to the pore wall (b) and share of roots with root hairs (c) 20–33 days after seeding as determined based on endoscopy images. The error bars indicate the standard deviation. Different letters indicate significant differences between the crop species (Kruskal-Wallis test followed by Bonferroni-Dunn correction, α = 0.05)
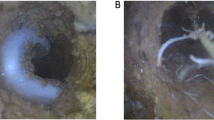
No differences were detected with respect to root status (data not shown). The different types of root growth are visualized in Fig. 4 (during crop development) and Fig. 5 (after harvest). These images show that especially in later growth stages and after harvest, oilseed rape roots fill the pore lumen most completely due to many roots and many long root hairs, while faba bean has only few and very thick roots in the pore lumen.
Visualization of roots in macropores during crop development of a spring barley, b spring wheat, c spring oilseed rape and d faba bean. From left to right: spring barley: 15, 23, 28 and 33 days after seeding (DAS), in 0–10, 30–40, 30–40 and 30–40-cm soil depths. Spring wheat: 17, 26, 30 and 33 DAS, in 10–20, 30–40, 30–40 and 30–40-cm soil depths. Spring oilseed rape: 20, 23, 30 and 33 DAS, in 0–10, 10–20, 30–40 and 30–40-cm soil depths. Faba bean: 20, 26, 28 and 33 DAS, in 0–10, 0–10, 20–30 and 30–40-cm soil depths. All images of one crop species are from the same column. Images were captured in colour and afterwards converted to black and white
From left to right: roots of spring barley, spring wheat, spring oilseed rape and faba bean in macropore after harvest. Images are from the same columns as in Fig. 3. Images were captured in colour and afterwards converted to black and white
Root growth into the upper subsoil layers continued after harvest (Fig. 5). All species had about equal root numbers in all four soil depth levels: barley, wheat and oilseed rape between 3.5 and 7 and faba bean between 1.5 and 2 (Fig. 6a). All four crops had a large share of active roots after harvest, but oilseed rape was the only crop with only active roots over all soil depths (Fig. 6b). Root length density (RLD) in bulk soil was much smaller for faba bean than for the other three species (Fig. 7a), and RLD in the pore (Fig. 7b) and the share of roots in the pore was significantly higher for oilseed rape with 3% as compared to the other three crop species (1%) (Fig. 7c). For all four crop species, the average root diameter in the bulk soil was larger than in the pore; root diameter both in bulk soil and in the pore was much larger for faba bean as compared to the other three species (Fig. 7 d and e).
Mean number of roots (a) and root status (b) of spring barley, spring wheat, spring oilseed rape and faba bean in macropore after harvest as determined based on endoscopy images. The error bars indicate the standard deviation. Different letters indicate significant differences between the crop species (Kruskal-Wallis test followed by Bonferroni-Dunn)
Root length density (RLD) in bulk soil (a), RLD in macropore (b), share of RLD in macropore (c), root diameter in bulk soil (d) and root diameter in macropore (e) of spring barley, spring wheat, spring oilseed rape and faba bean after harvest. The error bars indicate the standard deviation. Different letters indicate significant differences between the crop species (Kruskal-Wallis test followed by Bonferroni-Dunn, α = 0.05)
At harvest, oilseed rape had the largest percentage of roots with direct contact to the pore wall (68%) as compared to the other three crop species (35–43%). For vertical roots with direct contact, the difference between oilseed rape and faba bean was significant (Fig. 8). While for barley, wheat and faba bean the largest portion of roots crossed the pore without establishing direct contact, this portion was rather small for oilseed rape (Fig. 8).
Distribution of roots in different classes of orientation and contact after harvest as determined based on endoscopy images averaged over the 0–40-cm subsoil depth in percentage. The different soil depth steps did not differ significantly (Kruskal-Wallis test, α = 0.05). The error bars indicate the standard deviation. Different letters indicate significant differences between the crop species (Kruskal-Wallis test followed by Bonferroni-Dunn, α = 0.05)
Also, the percentage of roots with root hairs was highest for oilseed rape (99%), followed by faba bean (60%), barley (39%) and wheat (22%) (Fig. 9).
Share of roots with hairs after harvest averaged over the 0–40-cm subsoil depth as determined based on endoscopy images. The different soil depth steps did not differ significantly (Kruskal-Wallis test, α = 0.05). The error bars indicate the standard deviation. Different letters indicate significant differences between the crop species (Kruskal-Wallis test followed by Bonferroni-Dunn, α = 0.05)
4 Discussion
Close contact to the pore wall over long distances of the root length is an essential prerequisite for the uptake of nutrients from the nutrient-rich pore wall in later growth stages. Out of the four crops studied, oilseed rape fulfilled this requirement most completely. The large share of vertical roots in close contact to the pore wall forms a contrast to faba bean, which displays only roots crossing the pore horizontally, often without establishing contact to the pore wall, and barley and wheat with intermediate characteristics. The fact that virtually all oilseed rape roots, including the presumably older roots in the upper soil layers, had root hairs is a second aspect pointing to the capability of oilseed rape roots to extensively explore nutrient-rich biopores. Root hairs can greatly elevate the root surface area, and thus increase the acquisition of plant nutrients from soil (Jungk 2001, Yan et al. 2004). Furthermore, Yan et al. (2004) found rhizodeposition to be positively correlated with root hair number and density. Ma et al. (2018) found broader zones of elevated enzyme activities reflecting microbial and root activities and faster substrate turnover around root regions with root hairs as compared to hairless regions in soil. Based on microscopic investigation of different Sorghum grasses, Czarnota et al. (2003) concluded that exudates were produced solely by the root hairs. This stimulation of microbial activity by rhizodeposits is a plausible explanation for the fact that elevated microbial abundance and activity in biopores colonized by plant roots persisted even after roots had decayed (Athmann et al. 2017a). Potentially, root hairs may also mechanically anchor root tips in biopores, facilitating reentry to the bulk soil (Bengough et al. 2011). Especially on the vertically oriented oilseed rape roots, root hairs were observed over long distances, and across all soil depths and accordingly root ages. It is known that environmental conditions may have a strong influence on the life span of root hairs (Jungk 2001), and the notion that elevated concentrations of plant available N and P decrease root hair formation (Föhse and Jungk 1983, Hoffmann and Jungk 1995, Bates and Lynch 1996) is widely accepted (Datta et al. 2011). However, facing the high root hair density across all soil depths in nutrient-rich macropores at least for oilseed rape, it is worthwhile researching if this observation is also true for the biopore environment.
For oilseed rape, after harvest the share of vertical roots in close contact to the pore wall was still larger than for the other three species. Additionally, a considerable share of vertical roots had developed lateral roots leaving the pore, while the vertical root had detached from the pore wall. This type of root growth in biopores was characteristic for oilseed rape in a former field study, where oilseed rape was studied in a later development stage (Athmann et al. 2013a). In the same study, an equal share of barley and oilseed rape roots had root hairs, which could, however, in the field situation not be distinguished from mycorrhizal hyphae in the case of barley. As oilseed rape is non-mycorrhizal, disruption of hyphae networks through soil sieving and recompaction in the current study may be the reason for these differences.
The share of roots in the macropore was small for all four crop species in our study as compared to field observations (Ehlers et al. 1983, White and Kirkegaard 2010: 85–100%, Perkons et al. 2014, Han et al. 2015: 20%). In our study, 2.4% of the total subsoil volume comprised the macropore, which is slightly higher than that under field conditions (Perkons et al. 2014). Therefore, here only oilseed rape showed preferential root growth in the macropore. However, both Kautz et al. (2013b) and Han et al. (2015) reported values below 5% in early development stages. The higher share of oilseed rape roots in macropores observed in the current study is in line with a higher share of oilseed rape roots in biopores as compared to barley in later development stages (Perkons 2018, Huang unpublished). A higher share of roots in biopores (Huang unpublished) and higher N uptake from biopore walls for allorhizous mallow as compared to fibrous wheat (Athmann et al. 2016) indicates that biopores may be especially beneficial for taprooted crops.
Faba bean is characterized by thick roots that concentrate mostly in the topsoil (Li et al. 2006; Schröder and Köpke 2012). Athmann et al. (2013a, b) suggested that root growth in biopores depends mainly on the root system (taproot vs. fibrous). In this respect, the fact that faba bean has neither fibrous roots nor a pronounced taproot may explain why, in our study, faba bean roots hardly used the macropores in terms of time of pore entry, root hair formation, root orientation and especially root-soil contact. Another aspect is that, as a legume, faba bean may not profit from the nutrient-rich pore wall as much as non-leguminous crop species. Finally, the large root diameter of faba bean roots implies that they are presumably less affected by high penetration resistance in the bulk soil than the other three crop species: According to Lynch et al. (2012), roots are able to counteract soil bulk density by increasing their diameter.
Furthermore, efficient utilization of biopore resources requires pronounced plasticity of root characteristics like root orientation, branching and root hair formation and maintenance. It is well established that with decreased penetration resistance, roots elongate faster (Bengough et al. 2006), while locally enriched nutrients, especially N and plant available P, promote the formation of lateral roots (Smith and De Smet 2012). In this regard, the small number of faba bean roots observed in macropores which started to establish contact only at the very end of the experiment may indicate a low plasticity of the root system in response to nutrient supply and favourable physical conditions for root growth. This assumption is supported by other studies: Koebernick et al. (2015) reported that the root-shoot ratio of faba bean did not change under dry conditions. Furthermore, in a study by Li et al. (2014), faba bean, in contrast to the graminaceous species maize and wheat, was attested to have poor morphological plasticity in response to variation in nutrient availability, because it did not show a significant growth response to localized nutrient supply. Rather, in a study by Materechera et al. (1991), faba bean reacted with stronger root thickening to high penetration resistances than other crop species.
As the plasticity of root architecture and consequently the ability to explore biopores may also depend on the genotype (Arredondo and Johnson 1999; Bingham and Bengough 2003), other faba bean genotypes may have greater capacity to adapt their morphology to the conditions of the biopore. Finally, since after harvest the share of roots in macropores was not smaller for faba bean than for the two cereals and more than half of all roots had root hairs, faba bean may simply start biopore exploration in later development stages.
Consequently, future studies should be extended to later phases in crop development. Since it is likely that besides root architecture, soil physical parameters like penetration resistance (Bengough et al. 2006, Materechera et al. 1991), the surrounding pore network (Pagenkemper et al. 2015, Zhang et al. 2018) and soil biological parameters like mycorrhizal networks affect root growth and nutrient acquisition in biopores, dynamic root growth monitoring in undisturbed soils in the field is necessary. Furthermore, investigations e.g. by tracing the pore wall with isotopes will show the implications of root growth dynamics, share of roots in biopores, extent of root-soil contact and root hair formation and maintenance for nutrient uptake from biopores.
5 Conclusion
In this first investigation of root growth dynamics in macropores in an early stage of crop growth, the four species under study differed considerably in terms of time of pore entry, root orientation, root-soil contact, root hairs and share of roots in macropores.
-
(1)
Oilseed rape as a taprooted species entered the pore rather late with many thin laterals, and had the largest share of vertical roots with root-soil contact over the whole root length. Virtually all roots had root hairs. At the end of the experiment, oilseed rape had the largest share of roots in macropores.
-
(2)
Fibrous barley and wheat entered the pore early with many branched roots, had intermediate shares of vertical and ingrowing roots with root-soil contact over the whole root length and intermediate shares of roots with hairs. The share of roots in macropores was low.
-
(3)
Taprooted faba bean entered the pore late with only a few thick laterals, almost exclusively crossing the pore without establishing contact. Root hairs and root-soil contact were only observed at the end of the experiment. The share of roots in macropores was low.
These results indicate that during early crop development and under conditions of sufficient water supply and therefore comparably low penetration resistance, nutrient-enriched macropores were only marginally used by cereals and faba bean. In contrast, oilseed rape roots were apparently much more sensitive to mechanical impedance and the pores were used more intensively. Extending these investigations to later stages of crop development, similar studies under field conditions and combination of monitoring root growth in macropores with isotopic labelling of pore walls will show the implications of the different strategies identified for nutrient acquisition.
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
- cv:
-
cultivar
- DAS:
-
days after seeding
- RLD:
-
root length density
References
Arredondo JT, Johnson DA (1999) Root architecture and biomass allocation of three range grasses in response to non uniform supply of nutrients and shoot defoliation. New Phytol 143:373–385
Athmann M, Kautz T, Pude R, Köpke U (2013a) Root growth in biopores - evaluation with in situ endoscopy. Plant Soil 371:179–190
Athmann M, Kautz T, Köpke U (2013b) Charakterisierung des Wurzelwachstums in Bioporen mit in situ Endoskopie. Beiträge zur 12. Wissenschaftstagung Ökologischer Landbau, Bonn, 5.-8. März 2013:202–205
Athmann M, Beuters P, Küpper PM, Kautz T, Köpke U (2016) Quantifying N uptake from the subsoil: a biopore labelling field approach. Vienna, Geophysical Research Abstracts 18, EGU2016-6186. EGU General Assembly, 17–22 April, Vienna, p 2016
Athmann M, Kautz T, Banfield C, Bauke S, Hoang DTT, Lüsebrink M, Pausch J, Amelung W, Kuzyakov Y, Köpke U (2017a) Six months of L. terrestris L. activity in root-formed biopores increases nutrient availability, microbial biomass and enzyme activity. Appl Soil Ecol 120:135–142
Athmann M, Kautz T, Herkenhöner A, Köpke U (2017b) Bioporing: yield insurance during dry spells? In: Rahmann et al. (ed) Innovative research for organic 3.0 - Proceedings of the Science Track at the Organic World Congress, November 9–11, 2017 in Delhi, India, pp 370–373
Bates TR, Lynch JP (1996) Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ 19:529–538
Bengough AG, Bransby MF, Hans J, McKenna SJ, Robert TJ, Valentine TA (2006) Root responses to soil physical conditions; growth dynamics from field to cell. J Exp Bot 57:437–447
Bengough AG, McKenzie BM, Hallett PD, Valentine TA (2011) Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. J Exp Bot 62:59–68
Bingham IJ, Bengough AG (2003) Morphological plasticity of wheat and barley roots in response to spatial variation in soil strength. Plant Soil 250:273–282
Czarnota MA, Paul RN, Weston LA, Duke SO (2003) Anatomy of sorgoleone-secreting root hairs of Sorghum species. Int J Plant Sci 164:861–866
Datta S, Kim CM, Pernas M, Pires ND, Proust H, Tan T, Vijayakumar P, Dolan L (2011) Root hairs: development, growth and evolution at the plant-soil interface. Plant Soil 346:1–14
Daugbjerg P (1988) Temperature and moisture preferences of three earthworm species (Oligochaeta, Lumbricidae). Pedobiologia 32:57–64
Dresemann T, Athmann M, Heringer L, Kautz T (2018) Effects of continuous vertical soil pores on root and shoot growth of winter wheat: a microcosm study. Agric Sci 9:750–764
Edwards CA, Lofty JR (1980) Effects of earthworm inoculation upon the root growth of direct drilled cereals. J Appl Ecol 17:533–543
Ehlers W, Köpke U, Hesse F, Böhm W (1983) Penetration resistance and root growth of oats in tilled and untilled loess soil. Soil Till Res 3:261–275
Föhse D, Jungk A (1983) Influence of phosphate and nitrate supply on root hair formation of rape, spinach and tomato plants. Plant Soil 74:359–368
Gaiser T, Perkons U, Kupper PM, Kautz T, Uteau-Puschmann D, Ewert F, Enders A, Krauss G (2013) Modeling biopore effects on root growth and biomass production on soils with pronounced sub-soil clay accumulation. Ecol Model 256:6–15
Hagedorn F, Bundt M (2002) The age of preferential flow paths. Geoderma 108:119–132
Han E, Kautz T, Perkons U, Uteau D, Peth S, Huang N, Horn R, Köpke U (2015) Root growth dynamics inside and outside of soil biopores as affected by crop sequence determined with the profile wall method. Biol Fertil Soils 51:847–856
Hoffmann C, Jungk A (1995) Growth and phosphorus supply of sugar beet as affected by soil compation and water tension. Plant Soil 176:15–25
Jégou D, Schrader S, Diestel H, Cluzeau D (2001) Morphological, physical and biochemical characteristics of burrow walls formed by earthworms. Appl Soil Ecol 17:165–174
Jungk A (2001) Root hairs and the acquisition of plant nutrients from soil. J Plant Nutr Soil Sci 164:121–129
Kautz T, Köpke U (2010) In situ endoscopy: new insights to root growth in biopores. Plant Biosyst 144:440–442
Kautz T, Amelung W, Ewert F, Gaiser T, Horn R, Jahn R, Javaux M, Kemna A, Kuzyakov Y, Munch JC, Pätzold S, Peth S, Scherer HW, Schloter M, Schneider H, Vanderborght J, Vetterlein D, Walter A, Wiesenberg GLB, Köpke U (2013a) Nutrient acquisition from arable subsoils in temperate climates: a review. Soil Biol Biochem 57:1003–1022
Kautz T, Perkons U, Athmann M, Pude R, Köpke U (2013b) Barley roots are not constrained to biopores in the subsoil of a deep Haplic luvisol. Biol Fertil Soils 49:959–963
Kautz T, Lüsebrink M, Pätzold S, Vetterlein D, Pude R, Athmann M, Küpper PM, Perkons U, Köpke U (2014) Contribution of anecic earthworms to biopore formation during cultivation of perennial ley crops. Pedobiologia 57:47–52
Koebernick N, Huber K, Kerkhofs E, Vanderborght J, Javaux M, Vereecken H, Vetterlein D (2015) Unraveling the hydroponics of split root water uptake experiments using CT scanned root architectures and three dimensional flow simulations. Front Plant Sci 6:370
Kuzyakov Y, Blagodatskaya E (2015) Microbial hotspots and hot moments in soil: concept & review. Soil Biol Biochem 83:184–199
Li L, Sun J, Zhang F, Guo T, Bao X, Smith FA, Smith SE (2006) Root distribution and interactions between intercropped species. Oecol 147:280–290
Li HB, Ma QH, Li HG, Zhan FS, Rengel Z, Shen JB (2014) Root morphological responses to localized nutrient supply differ among crop species with contrasting root traits. Plant Soil 376:151–163
Lynch J, Marschner P, Rengel Z (2012) Effect of internal and external factors on root growth and development. In P Marschner (Ed.), Marschner’s mineral nutrition of higher plants (3rd ed., pp. 331–346). Amsterdam, Boston: Elsevier/Academic Press. https://doi.org/10.1016/B978-0-12-384905-2.00013-3
Ma X, Zarebanadkouki M, Kuzyakov Y, Blagodatskaya E, Pausch J, Razavi BS (2018) Spatial patterns of enzyme activities in the rhizosphere: effects of root hairs and root radius. Soil Biol Biochem 118:69–78
Materechera SA, Dexter AR, Alston AM (1991) Penetration of very strong soils by seedling roots of different plant species. Plant Soil 135:31–41
Pagenkemper SK, Athmann M, Uteau D, Kautz T, Peth S, Horn R (2015) The effect of earthworm activity on soil bioporosity – investigated with X-ray computed tomography and endoscopy. Soil Till Res 146:79–88
Passioura JB (2002) Soil conditions and plant growth. Plant Cell Environ 25:311–318
Perkons U, Kautz T, Uteau D, Peth S, Geier V, Thomas K, Lütke Holz K, Athmann M, Pude R, Köpke U (2014) Root-length densities of various annual crops following crops with contrasting root systems. Soil Till Res 137:50–57
Perkons U (2018) Bioporengenese durch homo- und allorhize Kulturpflanzen: Einfluss auf das Wurzelwachstum der Nachfrüchte [biopore genesis through fibrous and tap root systems: influence on root growth of subsequent crops]. Diss University of Bonn, 150 pp
Perreault JM, Whalen JK (2006) Earthworm burrowing in laboratory microcosms as influenced by soil temperature and moisture. Pedobiologia 50:397–403
Schröder D, Köpke U (2012) Faba bean (Vicia faba L.) intercropped with oil crops – a strategy to enhance rooting density and to optimize nitrogen use and grain production? Field Crops Res 135:74–81
Smith S, De Smet I (2012) Root system architecture: insights from Arabidopsis and cereal crops. Philos Trans R Soc Lond Ser B Biol Sci 367:1441–1452
Stewart JB, Moran CJ, Wood JT (1999) Macropore sheath: quantification of plant root and soil macropore association. Plant Soil 211:59–67
Tiunov AV, Scheu S (1999) Microbial respiration, biomass, biovolume and nutrient status in burrow walls of Lumbricus terrestris L. (Lumbricidae). Soil Biol Biochem 31:2039–2048
Vetterlein D, Kühn T, Kaiser K, Jahn R (2013) Illite transformation and potassium release upon changes in composition of the rhizosphere soil solution. Plant Soil 371:267–279
White RG, Kirkegaard JA (2010) The distribution and abundance of wheat roots in a dense, structured subsoil – implications for water uptake. Plant Cell Environ 33:133–148
Yan X, Liao H, Beebe SE, Blair MW, Lynch JP (2004) QTL mapping of root hair and acid exudation traits and their relationship to phosphorus uptake in common bean. Plant Soil 265:17–29
Zhang Z, Liu K, Zhou H, Lin H, Li D, Peng X (2018) Three dimensional characteristics of biopores and non-biopores in the subsoil respond differently to land use and fertilization. Plant Soil 428:453–467
Funding
This study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft—DFG) within the DFG project AT 171/1-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Athmann, M., Sondermann, J., Kautz, T. et al. Comparing Macropore Exploration by Faba Bean, Wheat, Barley and Oilseed Rape Roots Using In Situ Endoscopy. J Soil Sci Plant Nutr 19, 689–700 (2019). https://doi.org/10.1007/s42729-019-00069-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-019-00069-0