Abstract
Moringa oleifera (Capparales: Moringaceae) is a plant grown for its roots, stems and leaves. However, the leaves are attacked by insect pests including larvae of Noorda blitealis (Lepidoptera: Crambidae). These larvae cause a significant reduction in leaf yield and loss of income for growers. This study was conducted to determine some morphological and ecological aspects of larvae, using mass-reared larvae. Parameters such as larva length, body coloration, number of instars and their duration, damages caused on Moringa leaves, and larvae survival rate were collected. Results showed that N. blitealis larvae passed into 5 instars before pupating. The first instar larva averaged 2.4 ± 0.8 mm in length and was light green. At the 5th instar, the larvae reach 10.8 ± 0.4 mm and appear reddish. The mean duration of egg incubation was 3.00 ± 0.35 days, that of larval instars and chrysalis were respectively, 10.61 ± 2.28 days and 9.78 ± 0.42 days. The Kruskal-Wallis test showed that the leaf attack rate depends on the larva’s age (p-value < 0.0001). Larvae aged between 4 and 7 days attacked the greatest number of leaflets. Larvae survival rate was 86.67%, for 1st instar and 100% for the other instars. Knowledge of larvae’s biological and morphological characters is necessary to identify the pest in the early stages of infestation and develop control methods based on using bioinsecticides could rapidly be developed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The insect pests are one of the most common constraints for Moringa cultivation (Halilou et al. 2022; Kabré et al. 2020; Kant et al. 2017; Yusuf and Yusif 2014; Ojiako et al. 2012). According to Ke et al. (2016), more than 40 main pest species were found on the Moringa and each species can cause damage to different parts of the plant. Among these insect pests budworm (N. moringae), hairy caterpillar (Eupterote mollifera), bark caterpillar (Indaberla tetraonis), pod fly (Gitona distigma), and leaf caterpillar (N. blitealis) cause major agricultural losses (Suman et al. 2024).
N. blitealis (Lepidoptera: Crambidae) is a small moth whose larvae are phytophagous and feed on Moringa leaves. Generally, the larvae remain on a thin silken web on the underside of the leaf and feed on the leaflets, resulting in the drying of the leaves into a papery structure (Ravindra et al. 2016). In cases of heavy infestation, the larvae can cause 100% defoliation of trees (Bedane et al. 2013; Satti et al. 2013). The larvae can also destroy the apical bud and make a gallery in the plant’s central cylinder, which dries (Kant et al. 2017). The silken webs of larvae asphyxiate the leaves and eventually die (Dao et al. 2015).
N. blitealis is a small moth whose larvae are phytophagous and feed on Moringa leaves. Generally, the larvae remain on a thin silken web on the underside of the leaf and feed on the leaflets, resulting in the drying of the leaves into a papery structure (Ravindra et al. 2016). in cases of heavy infestation, the larvae can cause 100% defoliation of whole trees (Bedane et al. 2013; Satti et al. 2013). The larvae can also destroy the apical bud and make a gallery in the plant’s central cylinder, which dries (Kant et al. 2017). Because of this damage, N. blitealis is an important barrier to Moringa cultivation (Ke et al. 2016).
To control these insect pests, particularly N. blitealis larvae, Moringa growers use bioinsecticides (Kabré et al. 2022), synthetic chemical insecticides (Halilou et al. 2022; Ratnadass et al. 2011), and in some cases silvicultural practices such as pruning infested plants (Kabré et al. 2022). These control methods are used singly or in combination, but the results are below growers’ expectations. Furthermore, synthetic chemical insecticides present health risks for users, consumers, and the environment (Devine and Furlong 2007), and can lead to resistance phenomena in insect pests (Chaturvedi and Chaturvedi 2007; Srinivas et al. 2004).
In Burkina Faso, the extent of leaf damage has forced some growers who earned additional income from this crop to abandon it. However, Moringa is a plant that is being popularized by institutions to fight malnutrition among children and women (PNDES-II 2021) and poverty in rural areas by selling different parts of the tree. Given the socio-economic importance of Moringa for the population (Fadeyi et al. 2023; Tanga 2022), it is important to implement management strategies against N. blitealis.
This study aims to provide a better understanding of the bio-ecology of N. blitealis immature instars for a biological control perspective. Specifically, this involves (i) determine some characteristics of N. blitealis immature instars (larva length, body coloration, number of instars and their duration); (ii) assess damages caused by N. blitealis larvae on Moringa leaves and, (iii) assess larvae survival rate.
Materials and methods
Experimental site
The experiment was conducted in the Central Agricultural Entomology Laboratory (12°27’21,66 N; 1°32’59,46 W) in Ouagadougou (Burkina Faso). During experimentation, the mean laboratory temperature was 26 ± 1 °C, the relative humidity was 62 ± 5% and the photoperiod was 12 h.
Origin of larvae used for rearing
In the absence of a laboratory strain, mass rearing was carried out using N blitealis larvae collected from Moringa plants at the nursery of the Environment and Forestry Department (12°22’49.90 N; 1°30’15.40 W) of the National Centre for Scientific and Technological Research (CNRST). The Moringa plants were less than a year old. The larvae collected were transported to the laboratory in 900 ml glass containers containing sand and fresh Moringa leaves.
Rearing
Rearing was first realized in 900 ml glass containers. Fresh Moringa leaves were served as food for the larvae. The cut ends of these leaves were wrapped in hydrophilic cotton impregnated with water and covered with pieces of polyethylene. This was done to prevent water loss from the leaves and keep them turgid. Each glass jar was covered with a piece of muslin cloth fastened with a rubber band. One-third part of the jar was filled with moist sand, which provides optimal conditions for pupation. The grown-up larvae pupated in the glass containers. The emerged moths were next released in rearing cages measuring 60 cm x 60 cm x 60 cm. They were fed with a 10% sugar solution suspended inside the cage. Fresh Moringa plants were used for egg-laying.
The eggs laid are then collected and stored in petri dishes until they hatch. Newly emerged larvae were used in the present study.
Data collection
Characteristics of N. Blitealis immature instars
Larva length, body coloration and number of larval instars
Larvae length and body color were the morphological characteristics determined in this study. To this end, 15 newly emerged larvae were placed individually in 90 mm diameter Petri dishes and fed daily with fresh Moringa leaves. Food in each petri dish was changed daily by bringing fresh Moringa leaves from the field. The larvae length was recorded on graph paper after immobilization in the cold. The body color was determined visually with the naked eye. the number of larval instars was determined at pupation by counting the number of larval capsules rejected (Tano et al. 2011).
Immature instars duration
The following durations were determined:
-
Egg incubation: leaves containing eggs were removed and placed in 90 mm Petri dishes and covered with muslin cloth fastened with a rubber band. Observations were made daily. A total of 15 eggs were collected and observed until the larvae emerged.
-
Larva instars by observing and recording time elapse between the time between two head capsule discharge. These observations were made on 30 larvae that emerged on the same day, using a binocular magnifier Optika brand, coupled to a monitor. The total duration of larval life was obtained by summing the durations of the five larval instars.
-
pupal instar by recording the time elapsed between imaginal molting until the apparition of the adult moth in 15 last-stage larvae.
Assessment of damage caused by N. Blitealis larvae on Moringa leaves
Type of damage according to larval instars
The type of damage caused by each larval stage of N. blitealis was observed. This observation was made with the naked eye. It was carried out using the same larvae as those on which the measurements were taken.
Quantity of Moringa leaflets consumed according to larva age
This parameter was determined according to the protocol described by Dao et al. (2015). The protocol consisted of depositing a newly emerged larva on a potted Moringa plant in a 60 cm x 60 cm x 60 cm rearing cage. The number of leaflets on the plant was counted and marked at the start of the test. Daily observations were made until larvae were pupated. During these observations, the attacked leaflets were pulled off. A leaflet was considered attacked when it showed visible signs of attack. The seedling was replaced when all its leaves had been consumed by the larva. A total of 15 replicates were used for this determination.
Larvae survival rate
Newly emerged larvae were placed individually in 90 mm-diameter petri dishes. They were fed daily on fresh Moringa leaves. The larvae were observed daily in the event of death. The Optika binocular magnifier coupled to a monitor was used to observe larvae and count the number of cephalic capsules present in the petri dish. The number of cephalic capsules has been used to determine the corresponding larval instar (Tano et al. 2011).
Data analysis
Analyses were performed using R software 4.4.1. The Shapiro test showed that the data did not follow a normal distribution, so the length of the larvae and the number of leaves consumed for each larval stage were compared respectively using the Kruskal-Wallis test In addition, a Khi2 test was used to compare larval survival rates. All statistical tests were performed at the 5% significance level.
Results
Characteristics of N. Blitealis immature instars
Larvae length, body coloration, number of instars
Based on the number of cephalic capsules rejected, 5 larval instars were determined. Each larval instar differs from the others by the length and the color of its body The average length of 1st instar was 2.4 ± 0.8 mm. The 2nd instar larva averaged 4.8 ± 1.2 mm in length, reaching an average of 7.7 ± 0.6 mm in the 3rd instar. At the last instar, larvae averaged 10.8 ± 0.4 mm. The Kruskal-Wallis test showed a statistically significant difference between larvae of different stages (p < 0.05). Body color changes from light green in 1st instar to red in 5th instar (Fig. 1).
Immature instar durations
The incubation period of the eggs was recorded as 3.00 ± 0.35 days. The total larva life was 10.61 ± 2.28 days. The 5th instar was the longest, with an average of 3.12 ± 0.64 days, while the 4th instar was the shortest, with an average duration of 1.62 ± 0.51 days. The chrysalis phase takes on average 9.78 ± 0.42 days. From egg laying to adult emergence was 23.14 ± 1.21 days (Table 1).
Assessment of damage caused by N. Blitealis larvae on Moringa leaves
Type of damage
Observations showed that the type of damage caused to M. oleifera leaflets varied with the larval instars. Larvae of 1st and 2nd instar feed on the underside of Moringa leaves. The other instars (3rd, 4th and 5th) gnaw on leaf blades, causing perforations or total loss of leaves (Fig. 2).
Quantity of Moringa leaflets consumed according to larva age
Results showed that the mean of leaflets attacked varied with larvae age (p < 0.0001). Larvae at 4 and 7 days after emergence attacked more leaflets than younger or older larvae (Table 2).
Survival rate
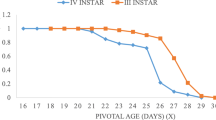
Results on larval survival rates showed that N. blitealis larvae had a survival rate of over 90% in the laboratory. The first larval instar showed a survival rate of 86.67%, while larvae of other instars showed survival rates of 100% (Fig. 3). The Khi2 test showed that there was no statistically significant difference between the survival rates (p = 0.81).
Discussion
Characteristics of N. Blitealis immature instars
The results showed that these larvae, like all holometabolous insects, develop through successive molt. In N. blitealis, five (05) larva instars were identified based on the number of head capsules rejected. Similar results were obtained by Ratnadass et al. (2011) who based their analysis on the observation of larval behavior, and those of Sharjana and Mikunthan (2019) who used the morphometry of the head capsule. Capsule rejection is associated with rejecting the larva’s old integument as an exuvium. This process is responsible for the insect’s growth and passage from one larval stage to another. The length of the larvae increased rapidly. These larvae grew from less than 2 mm at egg eclosion to more than 10.8 mm at the 5th instar. This rapid development is associated with a variation in larval coloration. The rapid growth of the larvae could be explained by the presence of the food substrate represented by the Moringa leaves, which they actively consume. As they grow, N. blitealis larvae change their color from light green to red. The variation in the color could be a homotypy or mimicry mechanism that allows the larvae to be camouflaged from their predators, adapting their coloring to the leaves of the host plant (Vukusic and Chittka 2012). Stage 1–4 larvae are green and resemble the leaves of the host plant, making them easy to camouflage. Stage 5 larvae and pupae are immobile, so red color could be an aposematic color designed to scare off predators. These stages are immobile. Homotypy and mimicry are evolutionary mechanisms that ensure the survival of a species in its battle for existence (Frawal 2018; Shamim et al. 2014). Other authors explain this variation in color for thermoregulatory needs (Umbers et al. 2013), this being controlled by biotic and abiotic factors (Tanaka and Nishide 2012; Verlinden et al. 2009). From the preceding, we can say that in N. blitealis, the most plausible explanation for the change in body color is homotypy. The lifetime observed from 1st instar to pupation was 10.61 ± 2.28 days. This result corroborates those of Subramoniam and Chitra (2019) who had obtained durations varying between 9 and 12 days under laboratory conditions almost similar to those of our study. In addition, the total lifespan of the immature N. blitealis stage is an average of 23.39 ± 3,05 days.
Damage caused on Moringa leaves
The observation showed that 1st and 2nd instar larvae feed on the underside of Moringa leaves. This feeding method makes the leaves chewy and transparent, limiting their photosynthesis ability. The other instars (3rd, 4th and 5th) gnaw on leaf blades, causing perforations or total loss of leaves. Furthermore, observations have shown that larvae of all stages consume Moringa leaves but the type of damage varies according to the larva instar considered. Larvae aged between 4 and 7 days are the most voracious on leaves, compared with larvae from other days. Two hypotheses could explain this situation. The first is based on the fact that the buccal pieces of young larvae are not sufficiently developed to cut and crush leaves. The second is that, according to coevolutionary theory, the nutrition of individuals depends on the enzymes they have at their disposal to ensure their proper digestion (Strebler 1980). In this way, the nutrients contained in Moringa leaves are digested by the larval stages, which can synthesize the enzymes necessary for their digestion. In addition, this could be explained by the fact that larvae from days 4 to 7 need to store sufficient food as an energy source.
Survival rate
In this study, larval survival rates ranged from 86.67% for 1st instar larvae to 100% for larvae of other instars. Umbers et al. (2013) obtained larval survival rates of 98.33% in Spodoptera frugiperda larvae reared at 25 ± 1ºC, 70 ± 10% relative humidity, and a 14-hour photophase. This high survival rate is due to the fact that the larvae were reared individually in Petri dishes, thus preventing competition for food. For their survival and normal development, insects consume specific diets (Behmer 2009) which must contain proteins and carbohydrates. These two nutrients provide them with the essential amino acids and energy they need (Wang et al., 2018). In this way, N. blitealis larvae find all the nutrients they need for survival and growth in Moringa oleifera leaves. The mortality observed in first-day larvae could be explained by the fact that, at this age, larvae are unable to metabolize correctly the toxins or non-nutritive compounds contained in Moringa leaves.
Conclusion
The present study reports on the biological and morphological aspects of N. blitealis larvae on Moringa oleifera trees. The results show that all larva stages feed on Moringa plants, causing significant losses and loss of income for growers. Knowledge of the larvae’s biological and morphological characteristics could help develop biological control methods capable of limiting its impact on Moringa plantations.
References
Bedane MT, Singh SK, Selvaraj T, Mulugeta N (2013) Distribution and damage status of Moringa Moth (Noorda Blitealis Walker) on Moringa Stenopetala Baker (Cufod.) In Southern Rift Valley of Ethiopia. J Agricultural Technol 9(4):963–985. https://doi.org/10.4172/2157-7471.1000166
Behmer ST (2009) Insect herbivore nutrient regulation. Ann Rev Entomol 54:165–187. https://doi.org/10.1146/annurev.ento.54.110807.090537
Chaturvedi I, Chaturvedi I (2007) Status of insecticide resistance in the cotton bollworm, Helicoverpa armigera (HUBNER). 8(2):171–182
Dao MCE, Traore M, Pare S, Ouedraogo DB, Ouedraogo S (2015) Ravageurs des planches maraîchères de Moringa oleifera dans la région du centre (Burkina Faso). J Anim Plant Sci 25(2):3857–3869
Devine GJ, Furlong MJ (2007) Insecticide use: contexts and ecological consequences. Agric Hum Values 24:281–306. https://doi.org/10.1007/s10460-007-9067-z
Fadeyi OJ, Fabunmi TO, Soretire AA, Olowe VIO, Raphael AO (2023) Application of Moringa leaves as soil amendment to tiger-nut for suppressing weeds in the Nigerian Savanna. BMC Plant Biol 23(1):1–5
Frawal A (2018) Couleurs d’insectes. Insectes 188(1):15–21
Halilou MS, Ba MN, Karimoune L, Doumma A (2022) Farmers’ knowledge, perceptions and management of the moringa tree defoliator, Noorda Blitealis Walker (Lepidoptera: Crambidae), in Niger. Int J Trop Insect Sci 42(1):905–915. https://doi.org/10.1007/s42690-021-00617-1
Kabré S, Dao MCE, Bazié BF, Traoré M, Gnankiné O (2020) Diversité des insectes ravageurs foliaires de Moringa oleifera (Moringaceae) dans les zones climatiques Nord et sud soudaniennes du Burkina Faso, vol 08. REV. RAMRES, Science de La Vie, de La Terre et Agronomie, pp 114–120. 2
Kabré S, Dao M, Bazongo J (2022) Pratiques agroécologiques de production et de lutte contre les insectes ravageurs des feuilles de moringa (Moringa oleifera) Au Burkina Faso. Sci et Techniques 41(n–2 3):135–149
Kant R, Josshi RC, Faleono I (2017) Survey of insect pests on Moringa oleifera in Samoa. Acta Hort 1158(April 2017):195–200. https://doi.org/10.17660/ActaHortic.2017.1158.23
Ke R, Hao R, Qian L, Tian Y, Gui F (2016) Review of Moringa oleifera insect pests and control methods. J Yunnan Agricultural Univ 31(4):745–750
Ojiako FO, Okafor OE, Ihejirika G (2012) Nursery insect pests of Moringa oleifera Lam in Owerri Area, Imo State, Nigeria. Int J Agric Rural Dev 15(3):1322–1328. https://www.researchgate.net/publication/266559464
PNDES-II (2021) Plan national de développement économique et social 2021–2025. Burkina Faso. Available: https://www.pndes.gov.bf
Ratnadass A, Zakari-Moussa O, Salha H, Minet J, Seyfoulaye AS (2011) Noorda Blitealis Walker, Un Ravageur Majeur Du Moringa Au Niger (Lepidoptera, Crambidae). Bull De La Société Entomologique De France 116(4):401–404
Ravindra C, Joshi B, Vasantharaj D, Rashmi K (2016) A review of the insect and mite pests of Moringa oleifera Lam. Agric Dev 29:1–5
Satti AA, Nasr OE, Fadelmula A, Ali FE (2013) New record and preliminary bio-ecological studies of the leaf caterpillar, Noorda Blitealis Walker (Lepidoptera: Pyralidae) in Sudan. Int J Sci Nat 4(1):57–62
Shamim G, Ranjan SK, Pandey DM, Ramani R (2014) Biochemistry and biosynthesis of insect pigments. Eur J Entomol 111(2):149–164. https://doi.org/10.14411/eje.2014.021
Sharjana K, Mikunthan G (2019) Biology of Leaf Eating Caterpillar Noorda Blitealis Wlk. On Moringa oleifera Lam. Int J Agric Biol Sci, 64–68
Srinivas R, Udikeri SS, Jayalakshmi SK, Sreeramulu K (2004) Identification of factors responsible for insecticide resistance in Helicoverpa armigera. / Comp Biochem Physiol 137:261–269. https://doi.org/10.1016/j.cca.2004.02.002
Suman S, Kumar N, Janguir N (2024) Insect pests of drumstick and their management. Agri Articles 4(1):265–268
Tanaka S, Nishide Y (2012) Do desert Locust hoppers develop gregarious characteristics by watching a video? J Insect Physiol 58(8):1060–1071. https://doi.org/10.1016/J.JINSPHYS.2012.04.005
Tanga TT (2022) Moringa oleifera as a gift of nature to human beings. Int J Pharm Bio-Medical Sci 02(04):50–56. https://doi.org/10.47191/ijpbms/v2-i4-02. https://doi.org/DOI
Tano B, Aboua L, Seri-Kouassi RN, Ouali-N’Ggoran BP SW, M., Kouassi A (2011) Etude De quelques paramètres biologiques de Pseudotheraptus devastans Distant (Heteroptera: Coreidae) sur les noix de Cocos nucifera L. De La variété PB 121+ à La station Marc Delorme (Côte d’Ivoire). Sci Nat 8(1):13–21
Umbers KDL, Herberstein ME, Madin JS (2013) Color in insect thermoregulation: empirical and theoretical tests in the color-changing grasshopper, Kosciuscola Tristis. J Insect Physiol 59(1):81–90. https://doi.org/10.1016/J.JINSPHYS.2012.10
Verlinden H, Badisco L, Marchal E, Van Wielendaele P, Vanden Broeck J (2009) Endocrinology of reproduction and phase transition in locusts. Gen Comp Endocrinol 162(1):79–92. https://doi.org/10.1016/J.YGCEN.2008.11.016
Vukusic P, Chittka L (2012) Visual signals: color and light production. The Insects: Structure and Function (5th Edition), Ed. S. J. Simpson and A. E. Douglas. Published by Cambridge University Press., 793–823. https://doi.org/10.1017/cbo9781139035460.032
Yusuf SR, Yusif DI (2014) Severe damage of Moringa Oleifera Lam. Leaves by Ulopeza Phaeothoracica Hampson (Lepidoptera: crambidae) in Ungogo local government area, Kano state, Nigeria: a short communication. Bayero J Pure Appl Sci 7(1):127–130
Acknowledgements
The authors are grateful to the technicians who monitored larval rearing and to anyone who contributed to improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kabre, S., Dao, M.C.E., Traore, F. et al. Morphology and biology of Noorda blitealis (Lepidoptera: Crambidae) immature instar for a biological control perspective. Int J Trop Insect Sci 44, 2139–2145 (2024). https://doi.org/10.1007/s42690-024-01310-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-024-01310-9