Abstract
Sitobion avenae presents a major challenge, leading to significant reductions in wheat yield per year. This study aimed to explore the potential of targeting Voltage-gated Sodium Channels (VGSCs) in S. avenae through RNA interference (RNAi) as a means to combat this pest. Two-dimensional polyacrylamide gel electrophoresis differential expression resulted in the identification of peroxidase, Synaptotagmin, and VGSCs as RNAi targets in S. avenae. VGSCs gene was further selected as an RNAi target; essential transmembrane proteins crucial for nerve cell action potentials: and also, a commercial insecticide target. VGSCs were amplified by reverse transcriptase PCR, sequenced, and deposited in Gen Bank. The ID allotted was OR777606. ERNAi tool was utilized to generate 143 small interfering RNA sequences and one double-stranded RNA target. Phylogenetic analysis revealed evolutionary links between the VGSCs gene in S. avenae and related aphid species like Myzus persicae, Acyrthosiphon pisum, and Diuraphis noxia. Quantitative real-time PCR analysis revealed S. avenae mortality, decreased fecundity, and shortened lifespan; attributable to the down-regulation of VGSCs gene expression (35%), and mortality up to 61% among 3rd instar nymphs. Adult S. avenae exposed to dsVGSCs during their 3rd nymph stage exhibit reduced reproducibility and longevity (surviving less than 20 days). The findings suggest VGSCs as a promising RNAi target; having a potential for agricultural pest management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triticum aestivum L. is a crucial staple crop that plays a significant role in ensuring global food security (Albahri et al. 2023). Multiple insect pests have the potential to inflict harm upon this crop, resulting in substantial reductions in yield (Lopes et al. 2016). Sitobion avenae (F.) (Hemiptera: Aphididae), commonly known as the English grain aphid, is a significant pest in the global context, particularly in cereal crops (Winder et al. 2012; Aradottir et al. 2017). Upon infestation, aphids feed on the phloem sap, depleting essential plant nutrients, leading to growth inhibition, reduced crop yield, and plant damage (Tu et al. 2018). Nevertheless, controlling them proves to be a complex task due to the notable high mobility of aphids and their rapid, prolific reproduction, facilitating their swift invasion and harm to plants (Liu et al. 2012). Chemical treatments although employed; had harmful effects along with the development of resistance against aphids (Kaleem Ullah et al. 2023). The transgenic approach is restricted because of the non-targeted approach (Afroz et al. 2022). Along with this Bacillus thuringiensis (Bt) toxin is normally utilized for insect resistance for which the plants had developed the resistance (Zhao et al. 2003). RNA interference (RNAi) emerged as an alternative strategy to suppress essential aphid gene transcripts. Its variable efficiency among insects is attributed to the presence of double-stranded RNA-degrading enzymes in the midgut, and hemolymph. It is also dependent on their recognition of gene-specific exogenous double-stranded RNA (dsRNA) before binding to the target transcript (Ahmad et al. 2024). The utilization of dsRNA as a mechanism for RNAi has arisen as a highly prospective approach for studying gene functionality and has demonstrated significant potential for the genetic management of insect pests (Price and Gatehouse 2008). odorant binding protein 2, salivary protein, laccase, and ecdysone receptor as dsRNA cause effective silencing of M. persicae, S. avenae, and Zeugodacus cucurbitae with mortality ranges from 70, 57, 69, and 68% respectively (Rafique et al. 2023; Mahmood et al. 2022; Ahmad et al. 2023).
RNAi has application in plants by plant transformation (host-induced gene silencing) or spray-induced gene silencing specially to target pests (Sang and Kim 2020; Rafique et al. 2023). Sprayable RNAs were utilized as direct eco-friendly pest management approaches and developmental repressors. Transgenic delivery of RNAs is effective in a way that protects a variety of crops from a specific insect pest. However, in many circumstances, transgenic crops are not a sensible choice; as crops are difficult to transform (Kumar et al. 2021). DsRNA topical delivery displays resistance against specific pests and becomes an alternative to transgenic RNAi.
Proteomics-based differential assays are more informative in comparison to genomics and transcriptomics: as they address the post-translational modifications in addition to expression analysis (Afroz et al. 2011). For large-scale quantification and protein separation, two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) with immobilized pH gradients (IPGs) is an effective approach. Proteins are sorted based on their isoelectric point (pI) in the 1st dimension through isoelectric focusing (IEF), and their molecular weight (MW) in the 2nd dimension through Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE). Proteomics approaches mostly utilize the aphid's secretome. Proteomic analysis of Myzus persicae, Acyrthosiphon pisum, Therioaphis maculate, and Diuraphis noxia revealed glucose dehydrogenase, peroxidase, trehalase, carbonic anhydrase, β-galactosidase, important in glucose metabolism, and defense (Harmel et al. 2008; Carolan et al. 2011; Nicholson et al. 2012; Rao et al. 2013; Cooper et al. 2011). S. avenae secretome proteomics resulted in the identification of glucose dehydrogenase, and oxidoreductases (Rao et al. 2013). No studies focused on the proteomics analysis of S. avenae differential proteins assay pre- and post-feeding in wheat.
Transcriptomic analysis of Monomorium destructor (Shukle et al. 2009), Oncopeltus fasciatus (Francischetti et al. 2007), and Empoasca fabae (Delay et al. 2012) represent lipases, lipid-binding proteins, beta-glucosidase, Angiotensin-converting enzyme-1, Metalloproteases, Odorant binding, and chemosensory proteins. Digestive, detoxifying enzymes, effectors, Ca2+ binding protein, and Glutathione-S-transferase 1 were found in S. avenae salivary glands transcriptomics (Zhang et al. 2017). Odorant binding proteins and chemosensory proteins were found in the salivary glands of Nilaparvata lugens, M. persicae, M. cerasi, and Rhopalosiphum padi (Ji et al. 2013; Thorpe et al. 2016). Lipid-binding proteins and Metalloproteases were found in the saliva of Aphis pisum, Megoura viciae, and M. persicae (Vandermoten et al. 2014).
Odorant-binding, salivary proteins, laccase, and others are proposed as an RNAi target, to silence aphid genes for their enhanced fecundity and mortality (Afroz et al. 2022; Mahmood et al. 2022; Reisenman et al. 2016; Rafique et al. 2023). The most direct approach was to find out proteins expressed pre-, and post-feeding wheat-resistant cultivars in the aphids. The voltage-gated sodium channels (VGSCs) play a crucial role in facilitating the normal propagation of nerve impulses in aphids. Disruption of the action potential caused by insecticides results in paralysis and ultimately leads to insect mortality. The extensive utilization of insecticides including pyrethroids targeting the VGSCs, connected to nervous system signaling was effective until the emergence of insect arthropod resistance; posing a significant challenge for efficient application (Rinkevich et al. 2013). RNAi-mediated knockdown of the VGSCs gene is reported in M. persicae, R. padi, and Tribolium castaneum (Tariq et al. 2019; Zuo et al. 2016; Abd El Halim et al. 2016). Insecticides that specifically act on VGSCs exhibit broad-spectrum effects due to the highly conserved structure; and adverse effects on non-target organisms (Tariq et al. 2019). Therefore, there is a pressing demand for the development of alternative control methods that are species-specific with reduced environmental consequences. RNAi is a highly conserved genetic mechanism occurring at the post-transcriptional level. It enables the suppression of gene expression through the action of small noncoding RNA molecules in nearly all eukaryotic organisms. Over the last decade, RNAi has been harnessed, and refined as a molecular tool for reducing the levels of specific insect’s gene transcripts (Jinek and Doudna 2009; Burand and Hunter 2013).
The simplest and most effective protein extraction method was devised for analyzing differentially expressed proteins of grain aphids. SDS-PAGE and 2D-PAGE were employed to distinguish protein expression in feeding and non-feeding aphids. Artificial feeding assay of dsRNA with VGSCs as RNAi target resulted in substantial mortality among S. avenae. The findings indicate that VGSCs is a promising dsRNA target and can be innovative to be used as a biopesticide against this hemipteran pest.
Materials and methods
Aphid’s rearing on wheat plants
S. avenae was collected from regions in the Gujarat district, and subsequently cultured on T. aestivum cultivar Galaxy 2013 under controlled standard conditions (under white fluorescent light (600 μmol m−2 s−1, 16 h light/8 h dark at 22 °C, and 70% relative humidity). Upon collection, a few S. avenae were preserved in a solution of 70% ethanol, while the remaining aphids were subjected to rearing on potted wheat plants. Subsequently, S. avenae was identified by the Department of Zoology, University of Gujarat Gujrat Pakistan. The S. avenae were then cultured on T. aestivum and classified into various developmental stages for collection.
S. avenae proteome analysis
S. avenae protein extraction approaches for SDS and 2D-PAGE with dye-lysis buffer (0.05 M Tris–HCl (pH 8.0), 0.02% SDS, 5 M Urea, 1% 2-mercaptoethanol, and 0.05% bromophenol-blue). S. avenae pre- and post-infesting wheat cultivars were collected, and stored at -80 °C for further analysis. Non-feeding samples were acquired by transferring them to a sucrose diet for 24 h. S. avenae samples (50 mg) (feeding and non-feeding) were ground in a dye-lysis buffer. The resulting mixture was subjected to centrifugation at 15,000 g for 10 min at 25 °C. The supernatant was retained for SDS and 2D-PAGE. The SDS-PAGE protein sample was denatured for 15 min and run on Mini-Protean II apparatus from (Bio-Rad Laboratories) utilizing 15% polyacrylamide separation gel, and 5% polyacrylamide stacking gel. Following the electrophoretic separation, all gel matrices were subjected to a 12 h Coomassie Brilliant Blue (G-250) staining.
IEF
First-dimensional electrophoresis involves the separation of proteins based on their pI with an IPG strip (3–10 pH). IPG strips were rehydrated for one hour with a Ready-Prep 2-D Starter Kit (163–2105: Bio-Rad Lab). IEF was conducted using the Protean IEF Cell apparatus from Bio-Rad. IEF was carried out at 200 V for 3 h, 1000 V for 1 h, 2000 V for 1 h, 3500 V for 1 h, and 25,000 Vh using the PROTEAN i12 IEF system (Bio-Rad USA: Cat # 164–6000). It was followed by IPG strips equilibration twice in an equilibration buffer (6 M urea, 30% glycerol (v/v), 50 mM Tris–HCl, 2%) SDS for 15 min. The first equilibration was done using 1.2% DTT (w/v) in an equilibration buffer, while in the second equilibration, DTT was replaced by 1.5% iodoacetamide (w/v). SDS-PAGE was performed using 15% polyacrylamide gels at 15◦C using Bio-Rad Midi- Criterion™ Vertical Electrophoresis Cell (Bio-Rad USA: W x L x Thickness = 13.3 × 8.7 cm × 0.1 cm) on 15% separating and 5% stacking gel. Differential proteins in S. avenae post-feeding were identified through densitometry analysis (GS-900 Calibrated Densitometry System Version 5.1: Bio-Rad: 170–7991). Several crucial protein targets, which included Peroxidase, VGSCs gene, and the Synaptotagmin were selected. From the set of proteins, the VGSCs protein was specifically chosen due to its potential to induce elevated mortality rates when its gene is silenced.
RNA isolation and cDNA synthesis
S. avenae RNA extraction pre- and post-feeding was performed using the Pure Link™ RNA Mini Kit, (Cat Number: 12183018A; Thermo Scientific). The Revert Aid First Strand cDNA Synthesis Kit was used for the synthesis of complementary DNA (cDNA) (Thermo Scientific™ K1622).
Reverse transcriptase polymerase chain reaction (RT-PCR)
VGSCs primers were designed using the Primer-BLAST tool and subsequently verified with an oligonucleotide property calculator to ensure the absence of self-complementarity or hairpin formation (Table 1). The PCR reaction mixture consisted of 1.5μL cDNA, 1μL MgCl2, 0.5μL dNTPs mix, 1μL of forward and reverse primers (10 μM), 1μL Taq buffer, and 0.5μL Taq polymerase, with the total volume adjusted to 25 μL. The PCR program included an initial denaturation step lasting 5 min at 95˚C, followed by 40 cycles, each comprising denaturation at 95˚C for 30 s, annealing at 52˚C for 30 s, and extension at 72 °C for 60 s. The process concluded with a final extension step at 72˚C for 5 min. β-actin served as the internal reference gene for normalization in the study. The resulting PCR product was visualized on 1% Agarose gel.
Sequencing and phylogenetic assessment
Following PCR, products were sent to Macrogen Korea for the sequencing process. Subsequently, the sequencing data was analyzed using BLAST. This was followed by a phylogenetic analysis, which was conducted using the phylogene.fr tool. For the specific design of potent small interfering RNA (siRNA) targets against VGSCs gene mRNA sequence, the ERNAi tool was employed, with Aphis pisum as the standard reference, by the protocols established by the Boutros lab, using E-RNAi-Version 3.2 (Supp Table 1).
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
To amplify the target genes via qRT-PCR, SYBR Green super (Thermo Fisher Scientific: Cat #: A25779) was employed. The reaction mixture consisted of 1µL cDNA, 10 mM Tris–HCl (pH 8.5), 50 mM KCl, 2 mM MgCl2, 0.4 µL DMSO, 200 mM dNTPs, 10 pmol/µL of primers, 1U Taq DNA polymerase, and 0.5µL of SYBR GREEN with volume up to 20µL. The PCR conditions were adjusted as follows: an initial denaturation at 94 °C for 10 min, followed by 40 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and a final extension at 72 °C for 45 s, followed by 5 min extension. Each reaction was done in triplicate to ensure consistency and reliability of the results. The relative gene expression levels were determined using the 2-ΔΔCT method (Livak and Schmittgen 2001). The method involves comparing the threshold cycle (Ct) values of the gene of interest to those of the reference gene followed by the calculation of the change in gene expression relative to a control.
dsRNA synthesis
Primer sequences targeting the S. avenae VGSCs were added to a T7 promoter at both forward, and reverse primer, for in vitro dsRNA synthesis. Standard PCR procedures were employed, involving initial cDNA synthesis from total RNA (Table 1). The PCR conditions for gene amplification with T7 promoter-containing primers were consistent, except for the annealing temperature (Tm = 60 °C). The resulting purified gene products were processed with the Ambion MEGA script® kit for in vitro dsRNA synthesis and subsequently stored at -80 °C for use in subsequent dietary experiments.
Statistical analysis
The experiments were conducted in triplicate to calculate the standard error using MS Excel. The statistical analysis of variance (ANOVA) for aphid mortality and mRNA expression was recorded 2-, 4-, 6-, and 8-days post-feeding dsRNA (20 ng/L) was carried out using Minitab. The control groups included a negative control with 20% sucrose and an internal control. In the analysis, different letters and asterisks were employed to indicate the significance levels of the results. Significant and highly significant differences when compared to the control group are indicated by (*), and (**).
Results
S. avenae proteomic profile pre and post-infestation
Differential analysis of S. avenae, protein pre and post-feeding show differential bands. SDS-PAGE of S. avenae revealed Peroxidase (90KDa), Synaptotagmin (67KDa), and VGSCs (36 KDa) proteins appeared in feeding and non-feeding Galaxy 2013 wheat cultivar in comparison to the non-feeding state (Fig. 1). The optical density of peroxidase, Synaptotagmin, and VGSCs increased in feeding and non-feeding S. avenae from 1347, 427, and 331 to 1469, 1130, 1185 respectively (Fig. 1). 2D-PAGE also shows differential protein spots involved in defense peroxidase (Spot no. 1), Synaptotagmin (Spot no. 2), and VGSCs (Spot no. 3) (Fig. 2; Table 2). VGSCs are chosen as a target for the future RNAi. Because Sodium channels hold a pivotal position in physiological mechanisms, facilitating the swift propagation of depolarizing signals within cells and cellular networks. This, in turn, allows for the coordination of a spectrum of complex processes, spanning from locomotion to cognitive functions (Rinkevich et al. 2013).
Two Dimensional Polyacrylamide Gel Electrophoresis (2D-PAGE) of Sitobion avenae, showing differential protein spots from feeding and non-feeding a Feeding S. avenae b Non-Feeding S. avenae (Sucrose diet for 24 h). Differential proteins including Spot no. 1 (Peroxidase), Spot no. 2 (Synaptotagmin), and Spot no. 3 (Voltage-gated sodium channels (VGSCs) appeared in feeding and non-feeding S. avenae. These gels were stained with silver nitrate, and image analysis was conducted using the GS-900 Calibrated Densitometry System
PCR, sequencing, and phylogenetic analysis
RNA, and cDNA after synthesis was run on gel (Fig. 3a, b). Reverse transcriptase PCR shows a larger band of 852 bps with RT-PCR VGSCs primers, and 266 bps for qRT-PCR VGSCs primers (Table 1; Fig. 3b, c). GenBank ID obtained for larger fragment 852 bps was OR777606. The phylogenetic tree of OR77760 indicates 90% similarity with Acyrthosiphon pisum, M. persicae, Aphis gossypii, Aphis glycines, Rhopalosiphum maidis, R. padi, and Diuraphis noxia, covering 95% of the sequence (Fig. 4). S. avenae VGSCs proteins exhibit a substantial 98% resemblance to the VGSCs subunit II of Acyrthosiphon pisum, M. persicae, A. gossypii, A. glycines, R. maidis, R. padi (Fig. 5). VGSCs mRNA siRNA 143 potent siRNA targets and one dsRNA target was designed by ERNAi. These siRNA sequences can be employed for combating grain aphids and related species (Supp Table 1).
a Sitobion avenae RNA extraction by PureLink™ RNA Mini Kit: L1: RNA, L6: DNA Ladder b Revert Aid First Strand cDNA Synthesis Kit was used for the synthesis of S. avenae complementary DNA c Reverse transcriptase polymerase chain reaction (RT-PCR) for Voltage-gated sodium channels (VGSCs) gene with real time primers (L1) 1 Kb ladder, (L2-L5) VGSCs gene (266 bp). d RT-PCR for VGSCs gene (L1) VGSCs (852 bp), (L2) 1 Kb ladder
VGSCs dsRNA effect on aphid growth and mortality
An artificial diet comprising 20% sucrose and supplemented with 20 ng μL−1 of dsRNA was given to S. avenae 1, 4, 6, and 8D post-feeding (Afroz et al. 2022). No significant aphid mortality was observed with the dsGFP, and ds VGSCs treatments after the first day of feeding. However, differences in mortality between these treatments became increasingly evident from the second day of continuous feeding onwards. After the 1-8th days of dsRNA feeding, the average mortality rate of 16–61%, was observed, in comparison to negative (DEPC water), and positive (dsGFP) control (Fig. 6a). The prior dietary delivery of ds VGSCs significantly reduced the longevity and fecundity of S. avenae survivors compared to control treatments (Fig. 6a).
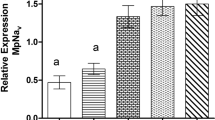
a DsRNA feeding experiment was conducted to assess the effectiveness of the Voltage-gated sodium channels (VGSCs) gene as a RNAi target. Different treatments were administered, including an artificial diet with 20% sucrose, Actin dsRNA at a concentration of 20 ng/µL (-ve control), and VGSCs gene dsRNA (7 µg/µL). The mortality rate was observed after 8 days of dsRNA feeding assay b The mRNA expression levels of the VGSCs gene were quantified using qRT-PCR and compared to both an internal control (Actin) and a negative control (VGSCs without dsRNA feeding assay). These measurements were taken at 2-, 4-, 6-, and 8-D following the feeding. The fold decrease in mRNA expression was determined for each time point compared to the -ve control
Expression analysis of VGSCs gene in response to dsRNA feeding by qRT-PCR
VGSCs expression over 1, 4, 6, and 8 days, following respective dsRNA oral administration was calculated. The subsequent 85–35% expression reduction was observed, 1-8D post-feeding. VGSCs expression 3-8th-day post dsRNA uptake displayed a highly significant decrease compared to control (dsGFP) (P < 0.001) (Fig. 6b).
Discussion
Plants are encountered insect herbivores consistent challenges; which consequently results in agricultural crop production risk (Bharathi et al. 2023; Afroz et al. 2011). In the current study protein differential expression was measured by the number of proteins SDS bands, and spot intensity. Dye-lysis method resulted in a higher number of protein bands, spots and produced reproducible outcomes from S. avenae for SDS-PAGE but also 2D-PAGE. Differential protein expression pre-, and post-feeding was performed to find RNAi targets not documented before in the literature (Figs. 1, 2). RNAi biopesticides are becoming increasingly significant as an alternative to chemical pesticides due to their minimal adverse effects and their precise targeting of specific pest species (Biedenkopf et al. 2020). The potential use of RNAi for pest management in agricultural productivity is significant to prevent residual environmental contamination. It can be done through microinjection, ingestion, or genetic modification (Bharathi et al. 2023; Ahmad et al. 2024).
Rapid degradation of dsRNA by extracellular ribonucleases in the insect's hemolymph and gut is increasingly recognized as a critical factor influencing the efficiency of RNAi across various insect orders (Ahmad et al 2024; Wang et al. 2016). This challenge is particularly pronounced in hemipteran species, as they exhibit extracellular dsRNA salivary degradation; further hindering cellular uptake (Christiaens et al. 2014; Singh et al. 2017; Cao et al. 2018). RNAi is an important and optimal method for controlling biotic, and abiotic stresses; although efficiency varies based on candidate gene selection, target molecule stability along the choice of target crops (Bharathi et al. 2023). The success of RNAi is dependent on the stability of the dsRNA molecules and their efficient uptake by the target species (Joga et al. 2016).
Different RNAi targets including odorant binding proteins, and salivary proteins genes are reported against M. persicae (Mahmood et al. 2022). Ecdysone receptor reported as RNAi plays an important role in molting and reproduction (Ahmad et al. 2023). Laccase gene variable role had been found in the cuticles sclerotization (Rafique et al. 2023). VGSCs is the target of the insecticides that have developed the resistance against them so alternative in form of RNAi assay is required (Rinkevich et al. 2013). VGSCs as RNAi target is identified in M. persicae (Tariq et al 2019). In this study VGSC differential expression is identified by 2D-PAGE and used as RNAi target.
In S. avenae VGSCs dsRNA caused 61% nymphs mortality by the 8th day dsRNA exposure (Fig. 6). In these experiments, silencing the VGSCs gene also resulted in a significant reduction in the longevity of adult aphids, with a decrease of up to 8 days, and a notable decline in their reproductive capacity. Following infestation of the aphids with VGSCs dsRNA: decreased expression at 4, 6, and 8 days post-feeding was observed. qRT-PCR of the VGSCs gene revealed a notable reduction in their expression by up to 35%. Similar results are reported for M. persicae VGSCs silencing (Tariq et al. 2019).
VGSCs are fundamental and widespread contributors to electrical signaling processes in eukaryotic organisms (Rinkevich et al. 2013). In insects, it produces a considerable array of transcriptional edits and splice variants; expressed at different developmental stages during its life cycle (Goldin 2002). RNAi to silence a VGSCs gene through oral administration resulted in larval mortality of up to 51.3%, alongside developmental arrest in the Tribolium castaneum. This larval mortality exhibited a dose-dependent response, notably observed when beetles were consistently exposed to dsRNA over 6 days. Additionally, the emergence of adult beetles was substantially reduced in insects that consumed dsRNA (Abd El Halim et al. 2016). In another research, an RNAi study focused on R. padi, and M. persicae VGSCs; important wheat crop pests resulted in 38.7, and 65% mortality (Zuo et al. 2016; Tariq et al 2019).
In the genome of Acyrthosiphon pisum, two subunit sodium channel genes are located on scaffold 318 with opposite orientations, separated by 23 kB of non-coding DNA sequence. Consequently, the aphids VGSCs is comprised of two-subunit channels that encompass voltage-gated potassium, calcium, and cyclic-nucleotide-gated channels (Amey et al. 2011, 2015). A substantial reduction in the RNA transcripts levels for both H1, and H2 subunits was observed. While in this research work, assessment of S. avenae H2 subunit expression is not included. As this discussed about the response to the administration of H1 dsRNA.
Off-target identification is important before dsRNA application (Bharathi et al. 2023). As in the case of gene sequence similarity to the injected dsRNA in the same or another unintended species will cause nonspecific gene silencing (Lundgren and Duan 2013). The majority of studies support, effective RNAi in insects with 140–500 nucleotides long dsRNA molecules; however, some reports are with 50 bps (Jing and Zhao-Jun 2014). The potential for optimizing dsRNA specificity by utilizing shorter/larger amplified dsRNA fragments, reduce the likelihood of unintended off-target effects.
Conclusion
This research focussed on finding the RNAi target by differential gel-based proteomics of S. avenae. Differential expression of VGSCs resulted in its application as an RNAi target. S. avenae VGSCs silencing led to the mortality of its nymphs as well as aphids. This finding suggests that VGSCs are a promising RNAi target to combat grain aphids by dsRNAs artificial diet assay. VGSC gene suppression led to promising mortality and decreased its expression.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- RNAi:
-
RNA interference
- dsRNA:
-
Double-stranded RNA
- 2D-PAGE:
-
Two-dimensional polyacrylamide gel electrophoresis
- IPGs:
-
Immobilized pH gradients
- pI:
-
Isoelectric point
- IEF:
-
Isoelectric focusing
- MW:
-
Molecular weight
- SDS-PAGE:
-
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
- VGSCs:
-
Voltage-gated sodium channel
- qRT-PCR:
-
Quantitative reverse transcriptase polymerase chain reaction
References
Abd El Halim HM, Alshukri BM, Ahmad MS, Nakasu EY, Awwad MH, Salama EM, Edwards MG (2016) RNAi-mediated knockdown of the voltage-gated sodium ion channel TcNav causes mortality in Tribolium castaneum. Sci Rep 6(1):29301. https://doi.org/10.1038/srep29301
Afroz A, Ali GM, Mir A, Komatsu S (2011) Application of proteomics to investigate stress-induced proteins for improvement in crop protection. Plant Cell Rep 30:745–763. https://doi.org/10.1007/s00299-010-0982-x
Afroz A, Aslam S, Rashid U, Malik MF, Zeeshan N, Khan MR, Shahzad MQ, Shahzad SA (2022) Intron-containing hairpin RNA interference vector for OBP8 shows promising mortality in peach potato aphids. Plant Cell Tissue Organ Cult 148(1):155–166. https://doi.org/10.1007/s11240-021-02174-4
Ahmad S, Jamil M, Jaworski CC, Wu Y, Palma-Onetto V, Lyu B, Luo Y (2023) Knockdown of the ecdysone receptor disrupts development and causes mortality in the melon fly. Zeugodacus Cucurbitae Doi. https://doi.org/10.1111/imb.12867
Ahmad S, Jamil M, Jaworski CC, Luo Y (2024) Double‑stranded RNA degrading nuclease affects RNAi efficiency in the melon fly, Zeugodacus cucurbitae. J Pest Sci 97:397–409. https://doi.org/10.1007/s10340-023-01637-1
Albahri G, Alyamani AA, Badran A, Hijazi A, Nasser M, Maresca M, Baydoun E (2023) Enhancing essential grains yield for sustainable food security and bio-safe agriculture through latest innovative approaches. Agronomy 13(7):1709. https://doi.org/10.3390/agronomy13071709
Amey JS, OReilly AO, Burton MJ, Puinean AM, Mellor IR, Duce IR, Davies TE (2015) An evolutionarily-unique heterodimeric voltage-gated cation channel found in aphids. FEBS lett 589(5):598–607. https://doi.org/10.1016/j.febslet.2015.01.020
Amey JS, Burton MJ, O’Reilly AO, Williamson MS, Mellor IR, Wallace BA, Davies TGE (2011) Molecular characterization of the voltage-gated sodium channel from aphids. Biophys J 100(3):425a. https://doi.org/10.1016/j.bpj.2010.12.2514
Aradottir GI, Martin JL, Clark SJ, Pickett JA, Smart LE (2017) Searching for wheat resistance to aphids and wheat bulb fly in the historical Watkins and Gediflux wheat collections. Ann Appl Biol 170(2):179–188. https://doi.org/10.1111/aab.12326
Bharathi JK, Anandan R, Benjamin LK, Muneer S, Prakash MAS (2023) Recent trends and advances of RNA interference (RNAi) to improve agricultural crops and enhance their resilience to biotic and abiotic stresses. Plant Physiol Biochem 194:600–618. https://doi.org/10.1016/j.plaphy.2022.11.035
Biedenkopf D, Will T, Knauer T, Jelonek L, Furch ACU, Busche T, Koch A (2020) Systemic spreading of exogenous applied RNA biopesticides in the crop plant Hordeum vulgare. ExRNA 2(1):1–10. https://doi.org/10.1186/s41544-020-00052-3
Burand JP, Hunter WB (2013) RNAi: future in insect management. J Invertebr Pathol 112:S68–S74. https://doi.org/10.1016/j.jip
Cao M, Gatehouse JA, Fitches EC (2018) A systematic study of RNAi effects and dsRNA stability in Tribolium castaneum and Acyrthosiphon pisum, following injection and ingestion of analogous dsRNAs. Int J Mol Sci 19. https://doi.org/10.3390/ijms19041079
Carolan JC, Caragea D, Reardon KT, Mutti NS, Dittmer N, Pappan K, Edwards OR (2011) Predicted effector molecules in the salivary secretome of the pea aphid (Acyrthosiphon pisum): a dual transcriptomic/proteomic approach. J Proteome Res 10(4):1505–1518. https://doi.org/10.1021/pr100881q
Christiaens O, Swevers L, Smagghe G (2014) DsRNA degradation in the pea aphid (Acyrthosiphon pisum) associated with lack of response in RNAi feeding and injection assay. Peptides 53:307–314. https://doi.org/10.1016/j.peptides.2013.12.014
Cooper WR, Dillwith JW, Puterka GJ (2011) Comparison of salivary proteins from five aphid (Hemiptera: Aphididae) species. Environ Entomol 40(1):151–156. https://doi.org/10.1603/EN10153
Delay B, Mamidala P, Wijeratne A (2012) Transcriptome analysis of the salivary glands of potato leafopper. Empoasca Fabae J Insect Physiol 58(12):1626–1634. https://doi.org/10.1016/j.jinsphys.2012.10.002
Francischetti IM, Lopes AH, Dias FA, Pham VM, Ribeiro JM (2007) An insight into the sialotranscriptome of the seed feeding bug Oncopeltus fasciatus. Insect Biochem Mol Biol 37:903–910
Goldin AL (2002) Evolution of voltage-gated Na+ channels. J Exp Biol 205:575–584
Harmel N, Letocart E, Cherqui A, Giordanengo P, Mazzucchelli G, Guillonneau F, Francis F (2008) Identification of aphid salivary proteins: a proteomic investigation of Myzus persicae. Insect Mol Biol 17(2):165–174. https://doi.org/10.1111/j.1365-2583.2008.00790.x
Ji R, Yu H, Fe Q, Chen H, Ye W, Li S, Lou Y (2013) Comparative transcriptome analysis of salivary glands of two populations of rice brown planthopper, Nilaparvata lugens, that differ in virulence. PloS one 8(11):10.1371/journal.pone.0079612
Jinek M, Doudna JA (2009) A three-dimensional view of the molecular machinery of RNA interference. Nature 457:405–412. https://doi.org/10.1038/nature07755
Jing Y, Zhao-jun H (2014) Optimization of RNA interference-mediated gene silencing in Helicoverpa armigera. Austral Entomol 53:83–88. https://doi.org/10.1111/aen.12052
Joga MR, Zotti MJ, Smagghe G, Christiaens O (2016) RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: What we know so far. Front Physiol 7:553. https://doi.org/10.3389/fphys.2016.00553
Kaleem Ullah RM, Gao F, Sikandar A, Wu H (2023) Insights into the effects of insecticides on aphids (Hemiptera: Aphididae): Resistance mechanisms and molecular basis. Int J Mol Sci 24(7):6750. https://doi.org/10.3390/ijms24076750
Kumar J, Ramlal A, Mallick D, Mishra V (2021) An overview of some biopesticides and their importance in plant protection for commercial acceptance. Plants (basel) 10(6):1185. https://doi.org/10.3390/plants10061185
Liu D, Jiang HX, Wang ZF, Cao Y, Zhang SE, Zhai GY (2012) The prevention and control of alfalfa aphid. Shandong J Anim Husb Vet 33:94–96
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Lopes T, Hatt S, Xu Q, Chen J, Liu Y, Francis F (2016) Wheat (Triticum aestivum L.)-based intercropping systems for biological pest control. Pest Manag Sci 72:2193–2202. https://doi.org/10.1002/ps.4332
Lundgren JG, Duan JJ (2013) RNAi-based insecticidal crops: potential effects on nontarget species. Biosci 63(8):657–665. https://doi.org/10.1525/bio.2013.63.8.8
Mahmood I, Afroz A, Malik MF, Zeeshan N, Khan MR, Rashid U, Alam S (2022) RNA interference-mediated knockdown of odorant-binding protein 2 and MP58 gene causes mortality in Myzus persicae. Int J Trop Insect Sci 42:315–326. https://doi.org/10.1007/s42690-021-00546-z
Nicholson SJ, Hartson SD, Puterka GJ (2012) Proteomic analysis of secreted saliva from Russian wheat aphid (Diuraphis noxia Kurd.) biotypes that differ in virulence to wheat. J Proteomics 75:2256–2268. https://doi.org/10.1016/j.jprot.2012.01.031
Price DR, Gatehouse JA (2008) RNAi-mediated crop protection against insects. Trends Biotechnol 26(7):393–400. https://doi.org/10.1016/j.tibtech.2008.04.004
Rafique A, Afroz A, Zeeshan N, Rashid U, Khan MAU, Irfan M, Chatha W, Khan MR, Rehman N (2023) Production of Sitobion avenae-resistant Triticum aestivum cvs using laccase as RNAi target and its systemic movement in wheat post dsRNA spray. PLoS ONE 18(5):e0284888. https://doi.org/10.1371/journal.pone.0284888
Rao SA, Carolan JC, Wilkinson TL (2013) Proteomic profiling of cereal aphid saliva reveals both ubiquitous and adaptive secreted proteins. PloS one 8(2):e57413. https://doi.org/10.1371/journal.pone.0057413
Reisenman CE, Lei H, Guerenstein PG (2016) Neuroethology of olfactory-guided behavior and its potential application in the control of harmful insects. Front Physiol 7:271. https://doi.org/10.3389/fphys.2016.00271
Rinkevich FD, Du Y, Dong K (2013) Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pestic Biochem Physiol 106(3):93–100. https://doi.org/10.1016/j.pestbp.2013.02.007
Sang H, Kim JI (2020) Advanced strategies to control plant pathogenic fungi by host-induced gene silencing (HIGS) and spray-induced gene silencing (SIGS). Plant Biotechnol Rep 14:1–8. https://doi.org/10.1007/s11816-019-00588-3
Shukle RH, Mittapalli O, Morton PK, Chen MS (2009) Characterization and expression analysis of a gene encoding a secreted lipase-like protein expressed in the salivary glands of the larval Hessian fy, Mayetiola destructor (Say). J Insect Physiol 55:104–11. https://doi.org/10.1016/j.jinsphys.2008.10.008
Singh IK, Sing S, Mogilicherla K, Shukla JN, Palli SR (2017) Comparative analysis of double-stranded RNA degradation and processing in insects. Sci Rep 7:17059. https://doi.org/10.1038/s41598-017-17134-2
Tamborindeguy C, Monsion B, Brault V, Hunnicutt L, Ju HJ, Nakabachi A, Van Fleet E (2010) A genomic analysis of transcytosis in the pea aphid, Acyrthosiphon pisum, a mechanism involved in virus transmission. Insect Mol Biol 19:259–272. https://doi.org/10.1111/j.1365-2583.2009.00956.x
Tariq K, Ali A, Davies TE, Naz E, Naz L, Sohail S, Ullah F (2019) RNA interference-mediated knockdown of voltage-gated sodium channel (MpNav) gene causes mortality in peach-potato aphid, Myzus persicae. Sci Rep 9(1):5291. https://doi.org/10.1038/s41598-019-41832-8
Thorpe P, Cock PJ, Bos J (2016) Comparative transcriptomics and proteomics of three different aphid species identifies core and diverse effector sets. BMC Genomics 17:172. https://doi.org/10.1186/s12864-016-2496-6
Tu XB, Zhao HL, Zhang ZH (2018) Transcriptome approach to understand the potential mechanisms of resistant and susceptible alfalfa (Medicago sativa L.) cultivars in response to aphid feeding. J Integr Agric 17(11):2518–2527. https://doi.org/10.1016/S2095-3119(17)61843-4
Vandermoten S, Harmel N, Mazzucchelli G, De Pauw E, Haubruge E, Francis F (2014) Comparative analyses of salivary proteins from three aphid species. Insect Mol Biol 23(1):67–77. https://doi.org/10.1111/imb.12061
Wang K, Peng Y, Pu J, Fu W, Wang J, Han Z (2016) Variation in RNAi efficacy among insect species is attributable to dsRNA degradation in vivo. Insect Biochem Mol Biol 77:1–9. https://doi.org/10.1016/j.ibmb.2016.07.007
Winder L, Alexander CJ, Woolley C, Perry JN, Holland JM (2012) The spatial distribution of canopy-resident and ground-resident cereal aphids (Sitobion avenae and Metopolophium dirhodum) in winter wheat. Arthropod-Plant Interact 7:21–32. https://doi.org/10.1007/s11829-012-9216-1
Zhang Y, Fan J, Sun J, Francis F, Chen J (2017) Transcriptome analysis of the salivary glands of the grain aphid, Sitobion avenae. Sci Rep 7:15911. https://doi.org/10.1038/s41598-017-16092-z
Zhao JZ, Cao J, Li Y, Collins HL, Roush RT, Earle ED, Shelton AM (2003) Transgenic plants expressing two Bacillus thuringiensis toxins delay insect resistance evolution. Nat Biotechnol 21(12):1493–1497. https://doi.org/10.1038/nbt907
Zuo Y, Peng X, Wang K, Lin F, Li Y, Chen M (2016) Expression patterns, mutation detection and RNA interference of Rhopalosiphum padi voltage-gated sodium channel genes. Sci Rep 6(1):30166. https://doi.org/10.1038/srep30166
Funding
This study is funded by the National Research Program for Universities (NRPU 6506), Higher Education Commission Islamabad, Pakistan at the Department of Biochemistry and Biotechnology, University of Gujrat, Gujrat Pakistan
Author information
Authors and Affiliations
Contributions
Experimental Design, Conceptualization, Funding source, Draft edition, Supervision, Validation, and Project administration [Amber Afroz]; Data creation, Experimentation, Formal analysis [Javeria Shafqat].
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Corresponding author had taken consent from co-author for submission and publication of the data in International Journal of Tropical Insect Science.
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shafqat, J., Afroz, A. RNA interference of Sitobion avenae voltage-gated sodium channels for improved grain aphid resistance. Int J Trop Insect Sci 44, 1679–1689 (2024). https://doi.org/10.1007/s42690-024-01261-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-024-01261-1