Abstract
In this study, we assessed the larvicidal activity of aqueous extracts obtained from the leaves and roots of Plantago major L. and Plantago lagopus L. on fourth instar Culex pipiens L. larvae. Plant material was collected from two regions in Tunisia, Beja and Bizerte. The resulting extracts were prepared at concentrations of 10, 20, 50 and 100 ppm. The toxicity of the extracts was monitored over a 24-hour period. Our findings indicated that leaf extracts from Plantago lagopus L. collected in Bizerte exhibited higher toxicity compared to those collected in Beja up to 12 h, although these differences became insignificant at the 24-hour mark. Furthermore, extracts from the roots of Plantago lagopus L. from Bizerte demonstrated higher toxicity than those from Beja. Statistical analysis revealed no significant differences (P = 0.056) in the toxicity of leaf extracts at 100 ppm between the two species after 24 h of exposure. The root extracts exhibited lower toxicity, resulting in mortality rates ranging from 40 to 70% after 24 h. Specifically, the leaf extracts of Plantago major L. from Bizerte yielded a median lethal concentration (LC50) of 16.068 ppm ± 8.440. Additionally, the quantification of total polyphenols and flavonoids revealed distinct variations between species, plant organs, and geographical origins. Root and leaf extracts from Plantago major L. collected in Beja exhibited higher concentrations compared to those from Bizerte. Conversely, root extracts from Plantago lagopus L. collected in Bizerte displayed the highest concentrations. These preliminary findings provide a foundation for the development of a novel natural biocide for mosquito control, utilizing the distinct larvicidal properties of Plantago species extracts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Plantago genus, belonging to the Plantaginaceae family, encompasses 483 perennial and annual species that are widely distributed across the globe (Tutel et al. 2005). This genus exhibits a notable ecological versatility, thriving in various habitats, including both arable lands and grasslands (Mohsenzadeh et al. 2008; Rosłon et al. 2015). In Tunisia, the Plantago genus is found throughout the region, with numerous species coexisting (Le Floc’h et al. 2010). These species have been documented to possess a substantial content of polyphenols, flavonoids, and condensed tannins, as reported by Samuelsen (2000). These secondary metabolites are important natural substances exclusive to the plant kingdom and contents vary among organs and species. As they are involved in several plant physiological function a great variability in their pattern occurs according to biotic and abiotic stress responses (Achakzai et al. 2009; Isah 2019). Several members of the Plantago genus are used in traditional medicine thanks to the wide span of biological activities provided by the richness in secondary metabolites, especially polyphenols (Gonçalves and Romano 2016). According to different extraction protocol and Plantago spp, most scientific reports have evidenced biological properties connected to human health, among these: antioxidant activity (Hussan et al. 2015); anti-inflammatory and antidiabetic (Vigo et al. 2005; Tinkov et al. 2014); immunomodulatory with reduction of immunosuppressive effects of anticancer drugs (Shepeleva and Nezhinskaya 2008); antiviral (Chiang et al. 2002); anti-candidal (Holetz et al. 2002) and several others such as wound healing, analgesic, anti-ulcerogenic and antihypertensive (Samuelsen 2000; Nyunt et al. 2007). In addition, biological effects on bacteria and fungi have been reported (Orhan et al. 2012; Pensantes-Sangay et al. 2020), while, notwithstanding the increasing interest in insecticidal, deterrent, or repellent activities of botanicals, the outcomes of Plantago spp. on insects have been neglected so far (Isman 2006; Guerra et al. 2020). Almost all research on biological activities of Plantago spp have been carried out on aerial organs (seeds, stems and leaves) and data on root properties are scarce. This background of knowledge on Plantago spp. is argument that root extract toxicity studies on insect vectors of human diseases, such as Culex spp., may open a new scene in the run the achieve additional sustainable ways to control pests.
In this study, the chemical composition and the larvicidal activity on fourth Culex pipiens L. instar has been evaluated according to the geographical origin of Plantago major L. and Plantago lagopus L. roots and leaves extracts.
Materials and methods
Plant material
Plant material was collected in two different geographical areas of Tunisia both classified, as Mediterranean hot summer climate regions according to Köppen (1918). The first place is located in the costal part of the Bizerte Region (37°16’40’’N 9°51’50’’E) and the second in the foothill area of the Beja Region (36°44’N 09°11’E). The designated areas have different mean rainfall values as well as soil characteristics. In both locations, whole Plantago major L. and Plantago lagopus L. plants were collected before flowering (in March 2020). From field to laboratory, plants were kept cooled and at arrival immediately rinsed and dissected separating the roots and leaves and discarding the stems. Then, roots were chopped into small pieces (1–2 cm) and together with the leaves dried in a ventilated room in the shade at 25 ± 2 °C until no weight changes occurred between two subsequent weights. This condition was reached after 15 days for leaves and 25 days for roots. The dried plant organs were finely grinded with a blade mixer, then sieved obtaining a fine uniform sized powder.
Preparation of aqueous extracts
The extracts from roots and leaves were attained by adding 250 mL of distillated water previously boiled and then left to cool into a beaker containing 25 mg (1:10; w:v ratio) of the powdered plant material. Then, the aqueous suspension was subjected to magnetic stirring in the dark at room temperature for one hour. Solid matrixes were separated from the liquid extracts by gravity filtration with Whatman paper (3MM). The filtrate recovered represents the starting stock solution obtained from 25 mg powder extracted with 250 mL water, or a concentration equal to 10%. The resulting solution was concentrated using a rotary evaporator. Different concentrations (10 ppm, 20 ppm, 50 ppm, 100 ppm) were performed for the estimation of the larvicidal activity.
Insect material
The egg, larval, and adult stages of Culex pipiens L. collected from a Salt Lake near the Faculty of Science of Bizerte were identified by Dr. Bejaoui Mustafa and maintained at ambient rearing conditions in the laboratory of Plant Toxicology and Environmental Microbiology. The toxicity experiments were performed employing 4th Culex pipiens L. instar larvae (L4) according to Cachereul (1997).
Toxicity tests
The larvicidal activity of aqueous leave and root extracts was evaluated according to the methodology reported by Ghnimi et al. (2014). Starting from the initial stock extract of roots and leaves (100 ppm / L or 10%) a series of dilutions were performed with the culture water of Culex pipiens L. larvae to attain final concentrations 10 ppm, 20 ppm, 50 ppm and 100 ppm. Then, toxicity tests were carried out in Petri dishes each containing 20 mL of the prepared solution at the given extract concentrations and twenty fourth- instar larvae of C. pipiens L. The same number of larvae was placed in control Petri dish containing 20 mL of larvae water. All toxicity tests have been repeated three times. The number of dead larvae was calculated for each concentration and for each repeat after 1, 2, 4, 6, 12 and 24 h of larval exposure. The lethal concentrations LC50 was calculated according to data recorded after 24 h of exposure using a probit regression analysis regression analysis using SPSS V23 (Mohapatra and Rengarajan 1995).
Extraction and content of phenolic compounds
The determination of the phenolic compounds: total polyphenols (TPP), total flavonoids (TFV) and condensed tannins (CT) present in the different parts of the plants studied was carried out using a UV-visible spectrophotometer ONDA V-10 PLUS of Cary type 50.
Extraction
A series of chemicals have been used to determine the phenolic compounds of the various plant extracts. The Folin-Ciocalteu reagent was supplied by VWR International (France), sodium carbonate (Na2CO3), aluminum trichloride (AlCl3) and sodium hydroxide (NaOH) from Across organics (Belgium), gallic acid, catechin, sodium nitrite (NaNO2) and vanillin are provided by Sigma-Aldrich Chimie (France).
The harvested plant material was dried in the shade, at a temperature of 25 °C. On the other hand, the fine cut of the material (leaves and roots) and its grinding were carried out using a grinder to obtain a powder. 2 g of the powder were weighed, they were mixed with 20 mL of ethanol (80%), filtered on a funnel covered with gauze then the filtrate was centrifuged at 2000 x g for 15 min and finally the extracts obtained (supernatant) were recovered.
Content of total polyphenols
The determination of total polyphenols in leaf and root extracts of the two species studied was carried out according to the Folin-Ciocalteu method (Biozot and Charpentier 2006). The principle of this method was based on the oxidation of phenolic compounds by adding a mixture of phosphotungstic acid (H3PW12O40) and phosphomolybdic acid (H3PMo12O4) of yellow color to each extract, which led to the formation of a new complex of metal oxides of tungsten and blue molybdenum. The intensity of the coloration, with maximum absorption is between 725 and 750 nm and equivalent to the amount of polyphenols present in the plant extracts (Ribéreau-Gayon 1968). In fact, 100 μL of extracts were poured into a test tube containing 6 ml of distilled water, then 500 μl of Folin-Ciocalteu phenolic reagent was added to the mixture and shaken. After 5 min, 100 μL of Na2CO3 solution (7%) was added to the mixture, the tubes were mixed and placed at room temperature for 60 min. The absorbance was then read at 750 nm by a UV / visible spectrophotometer. Quantitative analyzes of total phenols were determined from the linear regression equation of the standard curve, plotted using gallic acid as standard. The values obtained were expressed in mg of gallic acid equivalent per gram of dry matter EGA / g DM.
Content of total flavonoids
For the total flavonoid assay, the method used was developed by Zhishen et al. (1999) with some modifications. In test tubes and successively 1 mL of extracts were diluted with 250 μL of distilled water. At an initial time (0 min) 75 μL of a solution of NaNO2 (5%) were added, after 5 min 75 mL of AlCl3 (10%) were added. 6 min after 500 μL of NaOH (1 N) were added together with 2.5 mL of distilled water. The absorbance of each mixture obtained was directly measured with the UV-visible spectrophotometer at 510 nm. The flavonoid levels of the extracts were calculated from the standard curve, plotted using Catechin as a standard. The results obtained were expressed in mg equivalent of catechin per gram of dry matter (mg EC / g DM).
Content of condensed tannins
To quantify condensed tannins, the vanillin method was used (Schofield et al. 2001) with some modifications. During this reaction, the vanillin will react with the condensed tannins to give colored complexes which absorb at 500 nm. Briefly, 5 g of dry matter of each sample was leached in n-hexane, the resulting residue was dried at room temperature. Then, a mixture of 0.5 g of residue with 15 mL of methanol-HCl solution (1%) was prepared. The mixture obtained was placed in a test tube, vortexed and then placed in a water bath at 35 °C for 20 min. After incubation, the tube was centrifuged at 1532 × g and the supernatant was recovered. 1 mL of the supernatant was mixed with 3 mL of a solution of vanillin included 4 g of vanillin and 100 mL of methanol-HCL (8%). All solutions and whites are prepared under the same conditions. The tubes obtained were again incubated at 35 °C for 20 min. After this second incubation, the absorbance of each solution obtained was directly measured with the UV-visible spectrophotometer at 500 nm. The results obtained were expressed as mg equivalent of vanillin per gram of dry matter (mg EVan / g DM) deducted from a standard curve.
Statistical analysis
The results obtained concerning larvicidal activity, the lethal concentrations (LC50) of the various extracts, content of phenolic compounds and the correlation between larvicidal activity and the content of phenolic compounds were determined using SPSS Statistics 23.0 software. The estimates of LC50 were obtained after 24 h using probit analysis. The results obtained, concerning larvicidal activity, were processed using two-way analysis of variance (ANOVA) which is the origin of the plant material and the concentration of the aqueous extracts. The correlation between larvicidal activity and the content of phenolic compounds was presented by Pearson correlation coefficient. All tests were carried out in triplicate, and the results were expressed as means ± standard deviations. Results were considered statistically significant when P < 0.05.
The percentage of mortality, observed in control and treated 4th instar larvae, is estimated by applying the following formula:
Results
Variation in mortality rates of larvae of the Culex pipiens exposed to aqueous leaf extracts of two species of the genus Plantago
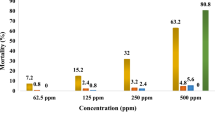
After exposing Culex pipiens larvae at the L4 stage to varying concentrations of aqueous extracts derived from the leaves of P. major and P. lagopus for a duration of 24 h, the resulting mortality rates were computed. It was observed that mortality exhibited fluctuations based on the concentrations, species, and sources. Notably, within the initial hour, no discernible impact on the Culex pipiens larvae was evident from the aqueous extracts of Plantago species sourced from Bizerte. Among the different provenances, the Beja origin exhibited minimal mortality rates, primarily noticeable in the case of P. major. Conversely, the larvicidal efficacy of P. lagopus leaf extracts from Beja was detectable solely at concentrations of 50 and 100 ppm, specifically after a 12 h exposure period. Interestingly, after a 24 h duration, a mortality rate of 40% was recorded even at lower concentrations of 10 and 20 ppm (Fig. 1).
After a 24 h exposure period, the mortality rate reached 100% at a concentration of 100 ppm in Bizerte for both species, P. major and P. lagopus (Fig. 2). In the Beja provenance, the mortality rates were 93.33% for P. major and 100% for P. lagopus. The statistical analysis indicated that there was no significant difference (P = 0.791) between P. major and P. lagopus among the provenances (Table S1 in Supplementary materials). However, notable differences were observed between the concentrations (P < 0.0001). The Tukey test further confirmed a significant distinction (P < 0.0001) among the various concentrations employed (Table S2).
The median lethal concentration (LC50) of the test sample, required to cause mortality in 50% of the 4th instar Culex pipiens larvae, was estimated following a 24 h exposure to aqueous leaf extracts from each species and two provenances (Table 1). The results demonstrated that P. major from Bizerte exhibited the highest toxicity, displaying the lowest LC50 value of 16.068 ppm ± 8.440. In comparison, P. lagopus exhibited lower toxicity. Interestingly, the LC50 values indicated that the lagopus species from Beja was more toxic (LC50 = 17.624 ppm ± 5.56) than its counterpart from Bizerte (LC50 = 28.657 ppm ± 6.998).
Variation in larval mortality rates of Culex pipiens larvae exposed to aqueous root extracts of two Plantago genus species
Following a 24 h exposure of 4th instar Culex pipiens larvae to varying concentrations of aqueous extracts from the roots of P. major and P. lagopus, we assessed the mortality rates. Our findings indicate that during the first, second, and fourth hours of exposure, neither of the two species from the two provenances induced mortality in the Culex pipiens larvae. After a 24 h exposure, a mortality rate of 53.33% was observed at a concentration of 100 ppm for P. lagopus from Bizerte, while the rate was 40% for the Beja provenance (Fig. 3). In the case of the Bizerte origin (Fig. 4), mortality became evident after 12 h of larvae exposure to aqueous root extracts of both species, with a concentration of 20 ppm causing mortality in P. lagopus and 50 ppm in P. major. As for P. major, a low mortality rate (20%) was recorded for both provenances. These results underscore a clear relationship between mortality, exposure duration, concentration levels, and the two Plantago species. Importantly, statistical analyses underscored significant differences in mortality rates between the two species (P < 0.0001), with similar significance noted between the various concentrations utilized (Table S3-S4 in Supplementary materials).
The high toxicity of the aqueous extracts from the roots was evident through the lowest LC50 value recorded, which was 90.515 ppm ± 17.313 for P. lagopus from Bizerte. Additionally, it is noteworthy that the aqueous extracts from the roots of P. lagopus in Beja exhibited a lower LC50 compared to those of P. major from the same origin (Table 1). Moreover, when comparing the mortality results of the larvae exposed to the two types of aqueous extracts, a higher toxicity of the extracts from the leaves was observed in comparison to the extracts from the roots for both species and both origins (Table 1).
Content of total polyphenols, flavonoids and condensed tannins
Content of total polyphenols
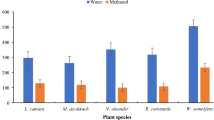
The results presented in Fig. 5 demonstrate that all extracts were notably abundant in total polyphenols (TPP). Notably, the leaves of P. major sourced from Beja exhibited a higher concentration of TPP (52.493 ± 7.015 mg EGA / g DM) compared to those from Bizerte (41.9 ± 0.973 mg EGA / g DM). This trend was similarly observed in the root samples. Conversely, the leaves of P. lagopus originating from Bizerte displayed a greater TPP content (101.687 ± 7.965 mg EGA / g DM) in comparison to those from Beja (34.847 ± 2.311 mg EGA / g DM). Notably, there was a significant disparity in TPP levels between leaves and roots. This distinction was particularly prominent in P. lagopus from Bizerte, with TPP content measured at 101.687 ± 7.965 mg EGA / g DM in leaves and 29.551 ± 0.431 mg EGA / g DM in roots. These TPP values were found to be substantially influenced by both the species’ origin (P < 0.0001) and the specific plant parts (P < 0.0001) (Table S5-S8 in Supplementary materials).
Content of total flavonoids
The results of the total flavonoid (TFV) contents in the leaf and root extracts of two Plantago species were depicted in Fig. 6. The data indicated significant variations in the TFV contents among the different extracts. Specifically, the leaves of P. major from the Beja provenance exhibited higher TFV levels (5.813 ± 0.152 mg ECat / g DM) compared to those from Bizerte (4.86 ± 0.026 mg ECat / g DM). This trend was consistent in the root extracts as well. Conversely, the leaves of P. lagopus from Beja displayed the lowest TFV value (3.493 ± 0.026 mg ECat / g DM) in contrast to the higher content found in those from Bizerte (9.889 ± 0.159 mg ECat / g DM).
Our findings revealed elevated total flavonoid content in both the leaves and roots of P. lagopus sourced from the Bizerte region. Additionally, the diverse TFV values observed exhibited significant correlations with species (P < 0.0001), provenances (P < 0.0001), and plant organs (P < 0.0001) (Table S5-S8 in Supplementary materials).
Content of condensed tannins
The results presented in Fig. 7 indicated that, for both species from the two studied provenances, the leaves exhibited a higher content of CT compared to the roots. In the case of P. major, the leaves from the Bizerte provenance exhibited the highest CT content (2.405 ± 0.004 mg EVan / g DM), while the lowest content (1.525 ± 0.111 mg EVan / g MS) was observed in the Beja provenance. Similarly, for P. lagopus from Bizerte, leaves displayed a notable CT content of 5.202 ± 0.045 mg EVan / g DM. In contrast, the CT content in the roots of both species was relatively consistent.
The ANOVA tests demonstrated a highly significant variance among different regions (F = 201.039; P < 0.0001), species (F = 101.038; P < 0.0001), and organs (F = 260.896; P < 0.0001) concerning TPP, TFV, and CT levels. Furthermore, the Tukey (HSD) test revealed substantial significance not only between the organs but also among both species and provenances (P < 0.0001) (Table S5-S8 in Supplementary materials).
Correlation between larvicidal activity and phenolic compound contents of aqueous extracts of P. major and P. Lagopus
The correlation between larvicidal activity and phenolic compound content was determined using SPSS v23 software (Table S9-S10 in supplementary material). For P. major, a good correlation between larvicidal activity and TPP (r2 = 0.724; P = 0.0001), TFV (r2 = 0.797; P = 0.0001), and CT (r2 = 0.769; P = 0.0001) was observed for the first time. However, for P. lagopus, no significant correlation was found between TPP, TFV, and larvicidal activity. Nevertheless, a significant relationship between CT and larvicidal activity was observed (r2 = 0.795; P = 0.01).
Discussion
Our preliminary findings confirmed larvicidal activity in both species of Plantago. This was also substantiated by Alves et al. (2011) in the case of P. lanceolata. They demonstrated that extracts from P. lanceolata could be utilized in the development of novel products targeting the coffee miner, Leucoptera coffeella. In addition, Guerra et al. (2020) showed that P. lanceolata extracts were effective in controlling Bemisia tabaci whitefly nymphs, which damage various agriculturally important plants directly or indirectly via virus transmission.
Romeh (2014) demonstrated that the leaves and roots of P. major were capable of absorbing cyanophos from water (which is not easily hydrolysed) and can therefore be used for phytoremediation of water contaminated by the insecticide cyanophos.
Nano-encapsulated P. major seed extracts have also proved effective against Tribolium castaneum (Khoshraftar et al. 2020). Aioub et al. (2021) have been recommended the extracts of P. major as a good strategy to protect tomato (Solanum lycopersicum L) plants by absorbing cypermethrin (CYP) residues from the soil, which is one of the most frequently used pyrethroid insecticides against a variety of pests, while being safe for humans and non-target organisms.
Recently, Rahat et al. (2023) demonstrated that P. ovata (Psyllium) has insecticidal activity against Tribolium castaneum.
Moreover, Chiang et al. (2002), Samuelsen (2000), and Nyunt et al. (2007) have elucidated multiple biological activities exhibited by P. major and P. lagopus, including antiviral, wound healing, anti-inflammatory, analgesic, immunomodulatory, anti-ulcerogenic, and antihypertensive effects. Ferreira and Oliveira (2020) presented evidence that P. major possesses antifungal properties, suggesting that this species holds potential for safeguarding essential crops. Bloor (1995) observed that cytotoxicity and antibacterial activity are omnipresent in New Zealand’s P. spathulata and P. triandra. Additionally, our study revealed the insecticidal activity of these two species within the Plantago genus against fourth instar larvae of Culex pipiens.
Comparing our findings with the literature, our results align well with the research conducted by Kobeasy et al. (2011), which demonstrated that leaf extracts of P. major are abundant in total polyphenols (13.05 ± 0.10 mg EGA / g DM), TFV (6.41 ± 0.04 mg ECat / g DM), and CT (5.63 ± 0.06 mg EVan / g DM). Notably, the values of TPP, TFV, and CT in both Tunisian sources are significantly higher than those reported by Kobeasy et al. (2011), likely attributed to differences in the extraction methods employed between our study and theirs. Furthermore, Tunisian P. lagopus leaves exhibited a value of 101.687 mg EGA / g DM, surpassing the measurement declared by Sotek et al. (2019) for P. coronopus leaves from Ukraine. Other investigations have also unveiled substantial content of TPP (79.94 mg / g DM) (Harput et al. 2012), TFV (10.0 ± 0.28 mg / DM), and CT (20.3 ± 0.53 mg / g DM) (Abd El-Gawad et al. 2015). These studies have additionally uncovered the presence of various chemical compounds that our research did not identify, such as verbascoside and calceorioside A (Harput et al. 2012), saponins, alkaloids (Abd El-Gawad et al. 2015), and iridoids (Catalpol, aucubine) isolated from aerial parts of Plantago species (Velázquez-Fiz et al. 2000; Genç et al. 2010). Contrary to our findings, Kapp-Bitter et al. (2020) did not detect traces of CT in P. lanceolata leaves collected during seed maturation, whereas our study highlighted the presence of CT in the two species under investigation. Additionally, Sanna et al. (2022) linked variations in TPP, TFC, and CT content to plant phenological phases, origin, sampling times, and plant parts. Supporting our observations, Harborne and Williams (2000) demonstrated that leaves exhibit the highest phenolic content, further corroborating our results.
Numerous studies have demonstrated strong relationships between the chemical composition of plants and their biological activities. For instance, Ahdiyah et al. (2015) revealed that tannins possess larvicidal potential by inhibiting enzymes such as proteases and amylases, thereby impeding digestion in mosquitoes. Nurhaifah and Sukesi (2015) demonstrated that tannins triggered the death of Aedes aegypti larvae prior to the pupal phase. Regnault et al. (2012) proved the insecticidal activity of phenolic compounds, with this activity often being characterized by an attractive effect accompanied by immobilization. Reynaud (2011) highlighted the toxic effect of polyphenols and alkaloids against insects.
Additionally, results from Baba-Aissa and Badaoui (2016) and from Oulebsir-Mohandkaci et al. (2018) indicated that bio-tests conducted using polyphenolic extracts from the leaves of false fennel and coriander resulted in 100% mortality among 54 Galleria mellonella larvae within 3 to 4 days. Turan and Mammadov (2021) reported that both leaves and roots of Cyclamen cilicium Boiss. & Heldr exhibited potential larvicidal activity against M. domestica and C. pipiens.
The concentration and biological activity of phenolic compounds that inhibit insect growth can be influenced by various factors, including age of the plant, plant part, species type, and place of collection. Indeed, these two endemic species contain higher concentrations of TPP and TFV, as they were collected before their flowering period when polyphenols were more concentrated (Daghbouche et al. 2020). This could justify their biological activity against larvae inhibition. Our findings indicate that leaf extracts from the two species were more effective than roots. This difference can be attributed to the primary site for the synthesis of secondary metabolites, where leaves may contain higher concentrations of bioactive compounds compared to roots.
In our case, there were statistically no significant differences between the two provenances in terms of the percentage of mortality, but there were significant differences between the two provenances in terms of the content of phenolic compounds. However, it’s important to note that the location where the species were collected can influence the concentration and biological activity of phenolic compounds. In some cases, plants from different locations may have different defensive traits due to variations in environmental conditions and other factors (Fei 2016).
Additionally, some compounds may have multiple targets, affecting several cellular processes simultaneously. Studies suggest that phenolic compounds can act as toxins toward larvae feeding and can promote oxidative stress in the larvae digestive tract, leading to death (Pratyusha 2022). Bioactive compounds can also affect glucose and lipid levels in larvae. Studies have shown that after treating Anopheles and Culex larvae with extracts from the antimalarial plant Artemisia, there is a decrease in glucose and lipid levels (Sharma et al. 2011). However, glucose and glycogen are the predominant sources of energy reserves in insects and serve as an energy source for the post-feeding larval and pupal stages (Arrese and Soulages 2010). Therefore, a decrease in the reserved energy level would be detrimental to normal morphology and metabolism of the treated insects. Histological and ultrastructural studies on jujube oil-treated larvae revealed severe damage to the gut, muscles, and cuticle layers, indicating the toxic effect of jujube oil and organic extracts. These substances can serve as agents for controlling Culex pipiens larvae (El Husseiny et al. 2014). For this, understanding the cellular mechanisms targeted by these compounds is crucial for developing effective and environmentally sustainable insecticides.
In this study, maceration, a conventional extraction method widely applied for extracting phenolic compounds from plants, was utilized. It involves soaking the plant material in a solvent (methanol) to allow the compounds to dissolve (Shi et al. 2022). However, it may also co-extract unwanted substances, and the choice of solvent can influence the selectivity and efficiency of extraction. To address this, an alternative method such as Supercritical Fluid Extraction (SFE) can be employed. SFE is a more modern and efficient method, avoiding the use of organic solvents. It provides high selectivity and can be adjusted for different compounds by manipulating pressure and temperature (Jahromi 2019). Since there is no single standard extraction method, as the chemical nature of phenolic compounds, the extraction method employed, sample particle size, storage time and conditions, and the presence of interfering substances can affect the efficiency of the extraction methods (Boeing et al. 2014).
Conclusions
In contemporary times, numerous aromatic and medicinal plants exhibit significant biological properties that have found diverse applications across various sectors, including industry, medicine, pharmacy, and agriculture. In this context, the current research is dedicated to the extraction and chemical identification of phenolic compounds from aqueous extracts of two indigenous Tunisian species, namely P. major and P. lagopus. The study also involves the assessment of their larvicidal activity. The investigation into the toxicity of aqueous extracts from both species on fourth instar larvae of Culex pipiens revealed a pronounced biocidal effect of these extracts against the insect larvae. Notably, the larvicidal efficacy displayed variability based on concentrations, origin, and plant parts. Results indicate that among the leaf extracts, those from P. major of Bizerte origin exhibited the highest larvicidal potential, followed by leaf extracts of P. lagopus from Beja. In the case of root extracts, P. lagopus from Bizerte demonstrated the most effective activity against Culex pipiens larvae. Analysis of total phenolic content (TPP), total flavonoid content (TFV), and condensed tannins (CT) highlighted the abundance of these phenolic compounds in leaves in comparison to roots, which exhibited relatively lower levels. Furthermore, a significant correlation between CT in P. lagopus and larvicidal activity was observed.
Our study unveils, for the first time, the insecticidal activity of these two species within the Plantago genus, underscoring the potential of plant extracts in combatting mosquito vectors responsible for transmitting various pathogens. This work opens promising avenues for utilizing aqueous plant extract powders in the development of biocidal agents. However, it would be valuable to explore other biological activities as well. Indeed, the investigation of in vitro biological activities stands as a crucial stepping-stone, laying the foundation for subsequent in vivo studies on animal models, for instance.
Data availability
All data and analyzes are accessible in the manuscript and supplementary materials.
References
Abd El-Gawad AM, Mashaly IA, AbuZiada ME, Deweeb MR (2015) Phytotoxicity of three Plantago species on germination and seedling growth of hairy beggarticks (Bidenspilosa L.). Egypt. J Basic app Sci 2:303–309
Achakzai AKK, Achakzai P, Masood A, Kayani SA, Tareen RB (2009) Response of plant parts and age on the distribution of secondary metabolites on plants found in Quetta. Pak J Bot 41:2129–2135
Ahdiyah I, Purwan KI (2015) Pengaruh Ekstrak Daun Mangkokan (Nothopanax scutellarium) sebagai Larvasida Nyamuk Culex Sp. Jurnal Sains Dan Seni ITS 4(2):32–36
Aioub AAA, Zuo Y, Aioub AAA, Hu Z (2021) Biochemical and phytoremediation of Plantago major L. to protect tomato plants from the contamination of cypermethrin pesticide. Environ Sci Pollut 28:43992–44001. https://doi.org/10.1007/s11356-021-13853-2
Alves DS, Oliveira DF, Carvalho GA, dos Santos HM Jr, Carvalho DA, Santos MAI et al (2011) Plant extracts as an alternative to control Leucoptera coffeella (Guérin-Mèneville) (Lepidoptera: Lyonetiidae). Neotro Entomol 40:1–12
Arrese EL, Soulages JL (2010) Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol 55:207–225. https://doi.org/10.1146/annurev-ento-112408-085356
Baba-Aissa A, Badaoui S (2016) Etude de l’activité biologique de quelques composés phénoliques, de deux plantes de la famille des ombellifères à l’égard de Galleria mellonella. Mémoire de Mastère 69
Bloor SJ (1995) A survey of extracts of New Zealand indigenous plants for selected biological activities. N Z J Bot 33(4):523–540. https://doi.org/10.1080/0028825X.1995.10410624
Boeing JS, Barizão ÉO, e Silva BC, Montanher PF, Almeida VC, Visentainer JV (2014) Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: application of principal component analysis. Chem Cent J 8:48. https://doi.org/10.1186/s13065-014-0048-1
Boizot N, Charpentier JP (2006) Méthode Rapide d’évaluation Du contenu en composés phénoliques des organes d’un arbre forestier. Cahier Des Techniques d’INRA 79–82
Cachereul A (1997) Les moustiques: cycle de développement, aspects anatomophysiologiques et régulation du cycle ovarien, Thèse de Médecine Vétérinaire, Nantes 117p
Chiang LC, Chiang W, Chang MY, Ng LT, Lin CC (2002) Antiviral activity of Plantago major extracts and related compounds in vitro. Antiviral Res 55:53–62
Daghbouche S, Ammar I, Moalla Rekik D, Djazouli Z, Zebib B, Merah O (2020) Effect of phenological stages on essential oil composition of Cytisus triflorus L’Her. J King Saud Univ Sci 32(4):2383–2387. https://doi.org/10.1016/j.jksus.2020.03.020
El Husseiny IM, El Kholy SE, Othman AA (2014) Laboratory Testing of the toxicity of Jujube (Zizyphus Jujuba) Oil and Leaf extracts against Culex pipiens (Diptera: Culicidae). Afr Entomol 22(4):755–761. https://doi.org/10.4001/003.022.0411
Fei M (2016) The importance of phenology in studies of plant-herbivore-parasitoid interactions. 170 pages. PhD thesis, Wageningen University, Wageningen, NL. ISBN 978-94-6257-655-1
Ferreira C, Oliveira R (2021) Protective antifungal activity of Plantago major extract against the Phytopathogenic Fungi Phytophthora cinnamomi, Diplodia corticola and Colletotrichum species. Proceedings. 70(1):94. https://doi.org/10.3390/foods_2020-07678
Genç Y, Saraçolu I, Nagatsu A, Harput Ü (2010) Iridoid and Megastigman Glucosides from Plantago lagopus L. FABAD. J Pharm Sci 35:29–34
Ghnimi W, Dicko A, Khouja ML, El Ferchichi Ouarda H (2014) Larvicidal activity, phytochemical composition, and antioxidant properties of different parts of five populations of Ricinus communis L. Ind Crops Prod 56:43–51. https://doi.org/10.1016/j.indcrop.2014.02.036
Gonçalves S, Romano A (2016) The medicinal potential of plants from the genus Plantago (Plantaginaceae). Indus. Crops Prod 83:213–226
Guerra JGO, Cerna CE, Ochoa FYM, Landeros FJ, Aguirre ULA, Juárez AH (2020) Insecticidal activity of plant extracts against whitefly nymphs Bemisia tabaci (Hemiptera: Aleyrodidae) in laboratory. J Entomol Zool Stud 8(1):595–599
Harborne JB, Williams CA (2000) Advances in flavonoids research since 1992. Phytochem 55:481–504
Harput US, Genc Y, Saracoglu I (2012) Cytotoxic and antioxidative activities of Plantago lagopus L. and characterization of its bioactive compounds. Food Chem Toxicol 50:1554–1559
Holetz FB, Pessini GI, Sanches NR, Cortez A, Nakamura CV, Filho BP (2002) Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem Inst Oswaldo Cruz 97:1027–1031
Hussan F, Osman BRH, MohdYusof MR, Kamaruddin NA, Othman F (2015) Plantago major treatment enhanced innate antioxidant activity in experimental acetaminophen toxicity. Asian Pac J Trop Biomed 5(9):728–732
Isah T (2019) Stress and defense responses in plant secondary metabolites production. Biol Res 52–39. https://doi.org/10.1186/s40659-019-0246-3
Isman MB (2006) Botanical insecticides, deterrents and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol 51(1):45–66
Jahromi SG (2019) Extraction techniques of phenolic compounds from plants. Plant physiological aspects of phenolic compounds. 120 pp. ISBN: 978-1-78984-034-6. https://doi.org/10.5772/intechopen.84705
Kapp-Bitter AN, Dickhoefer U, Kreuzer M, Leiber F (2020) Mature herbs as supplements to ruminant diets: effects on in vitro ruminal fermentation and ammonia production. Anim Prod Sci 61:470–479
Khoshraftar Z, Shamel A, Safekordi AA, Ardjmand M, Zaefizadeh M (2020) Natural nanopesticides with origin of Plantago major seeds extract for Tribolium castaneum control. J Nanostructure Chem 10:255–264. https://doi.org/10.1007/s40097-020-00346-w
Kobeasy IM, Abdel-Fatah MO, Samiha M, El-Salam A, Zahret El-Ola (2011) Biochemical studies on Plantago major L. and Cyamopsis tetragonoloba L. Int J Biodivers Conserv 3(3):83–91
Köppen W (1918) Klassification Der Klimate Nach Temperatur, Niederschlag and Jahreslauf. Petermanns Geogr Mitt 64:193–203
Le Floc’h E, Loutfy B, Errol V (2010) Catalogue synonymique commenté de la flore de Tunisie. République tunisienne ministère de l’environnement et du développement durable banque nationale de gènes 277–280
Mohapatra C, Rengarajan K (1995) Manual on bioassays in the laboratory and their techniques. Cmfri Spec Publ 64:1–75
Mohsenzadeh S, Nazeri V, Mirtadzadini SM (2008) Chromosome numbers of fifteen species of Plantago L. (Plantaginaceae) from Iran. Iran J Bot 14:47–53
Nurhaifah D, Sukesi TW (2015) Efektifitas Air Perasan Kulit Jeruk Manis Sebagai Larvasida Nyamuk Aedes aegypti. Jurnal Kesehatan Masyarakat Nasional 9(3):2015
Nyunt TM, Lwin KK, Aye TT, Than MA, Chit K, Kyaw T, Hlaing OMT, Wun M, Win NN (2007) Antihypertensive effect of Plantago major Linn. Whole plant (ahkyawpaungtahtaung) on mild to moderate hypertensive patients. Myanmar Health Sci Res J 19:97–102
Orhan DD, Berrin O, Sanem H, Mecit V (2012) Assessment of antioxidant, antibacterial, antimycobacterial, and antifungal activities of some plants used as folk remedies in Turkey against dermatophytes and yeast-like fungi. Turk J Biol 36:672–686TUB. https://doi.org/10.3906/biy-1203-33
Oulebsir-Mohandkaci H, Aissa AB, Badaoui S, Bouyahiaoui H, Kaki SA, Mohammedi A (2018) Comparative study of the toxicity of phenolic compounds of coriander (Coriandrum sativum) and false fennel (Aneth Graveolens) on Galleria mellonella (Lepidoptera, Pyralidae). J Environ Integr 3:1–7
Pesantes-Sangay SJ, Calla-Poma RD, Requena-Mendizabal MF, Alvino-Vales MI, Millones- Gómez PA (2020) Chemical composition and antibacterial effect of Plantago major extract on periodontal pathogens. Pesquisa Brasileria Odontopediatria e Clínica Integrada.20:e0012. 2020. https://doi.org/10.1590/pboci.2020.100
Pratyusha S (2022) Phenolic compounds in the plant development and defense: an overview. Plant Stress Physiology - Perspectives in Agriculture. 224 pp. ISBN: 978-1-83969-867-5. https://doi.org/10.5772/intechopen.102873
Rahat AZ, Anjum S, Zehra FUB, Chishti R (2023) Antibacterial, insecticidal, antifungal and phytochemical screening of Alium Sativum, Nigela sativa and Plantago ovata. Sarhad J Agric 39(2):531–544
Regnault RC, Vincent C, Arnason JT (2012) Essential oils in insect control: low-risk products in a high-stakes world. Annu Rev Entomol 57:405–424
Reynaud J (2011) La flore du pharmacien, édition TEC & DOC, Paris. 256 pp. ISBN-13: 978-2743005290
Ribéreau-Gayon P (1968) Les composés phénoliques des végétaux. Editions Dunod, Paris, p 254
Romeh AA (2014) Phytoremediation of cyanophos insecticide by Plantago major L. in water. J Environ Health Sci Eng 12(38). https://doi.org/10.1186/2052-336X-12-38. http://www.ijehse.com/content/12/1/38
Rosłon W, Gontar Ł, Kosakowska O, Osińska E (2015) Yield and quality of plantain (Plantago major L.) herb in the second year of cultivation. Horticul Landsc Architect 36:21–32
Samuelsen AB (2000) The traditional uses, chemical constituents and biological activities of Plantago major L. a review. J Ethnopharmacol 71:1–21
Sanna F, Piluzza G, Campesi G, Molinu MG, Re GA, Sulas L (2022) Antioxidant contents in a Mediterranean Population of Plantago lanceolata L. Exploited for Quarry reclamation interventions. Plants 11:791
Schofield P, Mbugua DM, Pell AN (2001) Analysis of condensed tannins: a review. Anim Feed Sci Technicol 91:21–40
Sharma P, Mohan L, Dua KK, Srivastava CN (2011) Status of carbohydrate, protein and lipid profile in the mosquito larvae treated with certain phytoextracts. Asian Pac J Trop Med 4(4):301–304. https://doi.org/10.1016/S1995-7645(11)60090-4
Shepeleva VV, Nezhinskaya GI (2008) Immunoprotective activity of medicinal plants preparations infusion in immunodepression caused by cytostatics. Rastite ResurE 44:129–135
Shi L, Zhao W, Yang Z, Subbiah V, Suleria HAR (2022) Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ Sci Pollut Res Int 29(54):81112–81129. https://doi.org/10.1007/s11356-022-23337-6
Sotek Z, Białecka B, Pilarczyk B, Drozd R, Pilarczyk R, Tomza-Marciniak A et al (2019) Antioxidant activity and selenium and polyphenols content from selected medicinal plants natives from various areas abundant in selenium (Poland, Lithuania, and Western Ukraine). Processes 7:878
Tinkov AA, Nemereshina ON, Popova EV, Polyakova VS, Gritsenko VA, Nikonorov AA (2014) Plantago maxima leaves extract inhibits adipogenic action of a high-fat diet in female Wistar rats. Eur J Nutr 53:831–842
Turan M, Mammadov R (2021) Phenolic compounds Screening and potential of Larvicidal Activity of Water Extract of Cyclamen ciliciumBoiss. Heldr Nat Prod Biotechnol 1(1):1–8
Tutel B, Kandemir I, Kus S, Kence A (2005) Classification of Turkish Plantago L. species using numerical taxonomy. Turk J Bot 29:51–61
Velázquez-Fiz MP, Díaz-Lanza AM, Fernández-Matellano L (2000) Iridoids from Plantago Lagopus. Pharm Biol 38(4):268–270
Vigo E, Cepeda A, Gualillo O, Perez-Fernandez R (2005) In vitro anti-inflammatory activity of Pinus sylvestris and Plantago lanceolata extracts: effect on inducible NOS COX-1 COX-2 and their products in J774A.1 murine macrophages. J Pharm Phamacol 57:383–391
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Acknowledgements
We thank Dr. Bejaoui Mustafa (Faculty of Sciences of Bizerte) for helping us to identify the larvae stages of Culex pipiens L.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree to publication.
Competing interests
The authors have declared that no competing interests exist.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bouali, A., D’hallewin, G. & Ouarda, H.E.F. Larvicidal activity on fourth instar Culex pipiens L. and phytochemical characteristics of aqueous extracts from leaves and roots of two species from the genus Plantago. Int J Trop Insect Sci 44, 759–770 (2024). https://doi.org/10.1007/s42690-024-01208-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-024-01208-6