Abstract
Based on the characters of adult’s external morphology and terminalia, a new species of the genus Sarcophaga (s. lat.) (Diptera: Sarcophagidae) is reported from India. Adults of Sarcophaga (Lioproctia) mailansis sp. nov. were collected from Kathua, Jammu and Kashmir, North India. An adult female was captured from decomposing chicken meat that had been used as bait for flesh fly collection. The female larviposit on chicken liver and are reared in ideal conditions to produce adult specimens. The morphological descriptions and illustrations of male, female and immatures of Sarcophaga (Lioproctia) mailansis sp. nov. are provided in this article, that aid in identification of this medically and forensically relevant flesh fly species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Family Sarcophagidae Macquart, 1834 includes several forensically significant flies. There are over 3,000 species of flies in this family, divided into 173 genera with a global distribution (Pape et al. 2011). Sarcophaga Meigen, 1826 is the major genus in the family Sarcophagidae, accounting for about 30% of the family’s species diversity (Partington 1837; Pape 1996; Buenaventura and Pape 2017; Whitmore et al. 2018; Kumar et al. 2021). Sarcophaga species are important for both medicinal and forensic purposes (Sukontason et al. 2014). Many species of Sarcophaga larvae are known to induce myiasis in animals and humans (Das et al. 2010; Priscilla et al. 2018) and have been linked to criminal investigations (Cherix et al. 2012; Sukontason et al. 2014; kumar et al. 2021). The larvae of the fly feed on living or necrotic host tissue, body fluids, or food that has been consumed. Human myiasis most commonly affects the skin, although it can also affect the nasopharynx, digestive tract, ear canal, eyes, and genitourinary tract (Priscilla et al. 2018).
The comparative analysis of these flies from India was compiled and published by Senior-White et al. (1940). Many additional researchers have since documented numerous new records and Sarcophagidae species throughout the country. Furthermore in 2002, Nandi taxonomically documented 163 species gathered from various locations in India and neighboring nations. Chakraborty et al. (2017) published a checklist of Indian Sarcophagidae, tracing a total of 126 species of flesh flies. Among those, the genus Sarcophaga is accounted for over 70% of the species reported and 4 species of the subgenus Lioproctia Enderlein (1928), have been described. Barták et al. (2019) reported fresh data on the oriental Sarcophagidae based on a review of all previous records, listing 138 species that were found in India. Sarcophagidae is also a significant forensic indicator in PMI estimate as they may identify a corpse from a distance of several kilometers within minutes of death (Goff 2000). The majority of Sarcophaginae subfamily species feed on decaying flesh, however a few species also feed on open wounds in humans and animals, causing myiasis (Abd Al Galil and Zambare 2016). Concerns about the identification of these flies are growing as they become involved in medical and forensic investigations. The species features three black longitudinal stripes on the notum, undeveloped subscutellum, and abdomen, as well as a checkered pattern on the surface (De Carvalho and Mello-Patiu 2008). The majority of adults in the subfamily Sarcophaginae look nearly identical (Nandi 2002).
The species of flesh flies are difficult to identify precisely due to their morphological similarities and the lack of identifying keys. Female flesh flies are generally located on decaying bodies to be used as a larviposition site, but due to their similar appearance, they are difficult to distinguish (Vairo et al. 2015). The male is the most diagnostic trait for species identification; hence most identification keys are based on males (Nandi 2002). The objective of this study is to describe both the adult male and female of Sarcophaga (Lioproctia) mailansis sp. nov., as well as to provide authentic identifying characters for species identification that may be significant for medicinal and forensic purposes.
Materials and methods
Study site
Experimental site
The experiment was conducted close to a forest in the rural Maila village (32.50º N to 75.29º E) of district Kathua, Jammu and Kashmir (India). In the winter, the field is mostly utilized to raise wheat crops. The predominant flora of the region includes mango trees (Mangifera indica), neem trees (Azadirachta indica), and curry trees (Murraya Koenigii).
Biological material
The chicken meat 1 kg was used as a bait to collect adult Sarcophagids.
Collection & preservation
The gravid females of Sarcophaga (Lioproctia) mailansis sp. nov. were attracted to decaying chicken meat, used as bait to collect flesh flies. Collected flies were reared in the rearing boxes (1"×1"×1”). The females were fed upon powdered sugar and water with cotton wick in a conical flask. The females were also provided with Chicken liver (10 g) as the oviposition medium. After depositing larvae the first instar larvae were transferred in a 1L glass rearing jar with 20 g of chicken meat as a food source. A piece of muslin cloth was used to seal the glass jar by placing a rubber band to prevent the larval escape. The feeding stages were reared till the prepupation stage (post-feeding stage), when the larvae started leaving the food media, the larvae were transferred to the new jar containing 2 cm of dried soil for pupation and kept till adults emergence. After emergence, the adults were collected and killed by placing them in killing jars having ethyl acetate vapors.
Identification
For species identification, the male abdomen was detached and soaked in a 10% KOH solution for 24 h. The internal genitalia was dissected from the abdomen under a stereo zoom microscope (model no. R1-90-01) and preserved in the clove oil for clearance purposes and later on used for identification and photography. The photographs were taken by Canon EOS 1200D DSLR Camera 18MP/5X optical zoom. All the characters of adults were compared carefully by comparing the morphology and genital structure of all described closely related species and then concluded as a new species.
Terminology given by Nandi (2002); Chaiwong et al. (2009); Zhang et al. (2014) and Kurahashi and Samerjai (2018) is followed for adult characteristics.
Systematics
Genus Sarcophaga Meigen, 1826
Subgenus Lioproctia Enderlein, 1928
Diagnosis
Large-sized, black flies with checkered pattern on abdomen as found in most Sarcophaga. The Subgenus Lioproctia Enderlein includes: a well-developed predorsocentral bristles; a preacrostichal bristle; bare upper part of propleuron, few short setae on parafacial; Male terminalia includes beak shape inner forceps with strong spines; short and lobulated ventralia; juxta longer than the short styli (Nandi 2002).
Distribution: Oriental, Palaearctic, Australasian and Oceanian regions.
Biology: Collected on bushes and breed on various dead animals.
Sarcophaga (Lioproctia) mailansis sp. n.
Type material Holotype male found in Maila Village, Kathua City, Jammu and Kashmir Province, North India, (32.50º N, 75.29º E; 489 m) collected from decomposing chicken meat, collected by Rohit Kumar, 14 May 2020, deposited in the Sarcophagidae Collection, Department of Zoology and Environmental Sciences, Faculty of Forensic Entomology, Punjabi University, Patiala, India. Paratypes include 4 adult females, 4 males with the same collection data as the holotype.
Etymology: The name of this species is the name of the village (Maila) from where this species was collected.
Diagnosis
Large-sized fly, male larger to female; parafacial with 4–6 short setae; female with about 11 frontal bristles; silver-white propleuron; 7 mesopleural bristles; R4 + 5 ventrally with 4–5 short setae; tergite 5 with a row of 12–14 strong median marginal bristle; fifth sternite Y shaped in male with open window; 6th sternite almost rectangular in female; Genital Segment 2/epandrium in male yellowish-brown; Surstulus oval and elongated in male; postgonite grooved laterally in a subapical arc; anterolateral margin of juxta without spine.
Sarcophaga (Lioproctia) mailansis sp. nov., adult male. a male, lateral view. b male head, frontal view. c male, dorsal view. d male thorax, dorsal view. e male thorax, lateral view. f wing, dorsal view. g abdomen, dorsal view. Abbreviations: as- apical scutellar bristle, CS- costal section, dc- dorso-central bristle, ds- discal scutellar bristle, ep- epandrium, hb- humeral bristle, ia- intra-alar bristle, iv- inner vertical bristle, mb- marginal bristle, mp- mesopleural bristle, orb- orbital bristle, ori- frontal bristles, ph- posthumeral bristle, ps- pre-sutural bristle, sa- supra-alar bristle, sp- sternopleuron, syn- syntergosternite 7 + 8, tg- tergite, t- tegula
Male about 1.5 cm; head frontal stripe black, parafrontal and parafacial plate silver-white, parafrontal greyish tinged, having 11–13 frontal bristles, with an orbital bristle (Fig. 1b); ocellar bristles strong, inner vertical bristle double the length of outer vertical bristle (Fig. 1e); antennae long, do not reach vibrissae; postpedicel greyish with brown pollinosity; pedicel dark brown about 1/4 the length of postpedicel; arista long, plumose and dark brown (Fig. 1b); gena silver grey with moderate black and white hairs; postgena with long white hairs (Fig. 1a); parafacial with 4–5 hairs near bottom edge of eye; palpus slender, greyish with brown pollinosity, bearing black bristles; occiput with regular row of postocular bristles (Fig. 1e).
Thorax silver-white with grey pollinosity, having 3 contrasting black longitudinal stripes; propleuron silver-white, upper part bare, lower part with an strong bristle and few hairs; anterior spiracle fuscous brown; posterior spiracle yellowish-brown (Fig. 1d, e); acrostichal 1 + 1 (1 very short); dorsocentral 7 + 5 (posterior 2 post dc strong); humeral 3; posthumeral 2; presutural 1; notopleural 4 (2 strongly developed); intra-alar 1 + 3 ( 3 very short, 1 long); supra-alar 3 (middle strongly developed); postalar 2; scutellum with 4 bristles, discal 1 (short), apical 1 (strong and long) and marginal 2 (1 short + 1 strong and long); sternopleural 1 + 1 + 1; hypopleural 11; mesopleural 7 (7 strong with fine hairs) (Fig. 1d, e).
Wings entirely fuscous hyaline; epaulet blackish with 2 long marginal bristles; basicosta bare and pale yellowish; subcostal sclerite bare and brown; CS1 with 2 long bristles at costaginal break; CS2 with humeral break, incised at SC with spike of spines (Fig. 1e, f); CS1 to CS4 with short spines at anterior margin; CS5 is about 3/4 of CS3; CS5 almost bare, except the base with few spines (Fig. 1f); R1 bare; R4 + 5 with a row of about 9 short setae extends dorsally from the basal node to below halfway of r-m; ventrally 4 setae before the emergence of R4 + 5 and R2 + 3; r4 + 5 closed; bend of m with right angle (Fig. 1f); lower and upper calypter bare and creamy white; halter yellowish-brown.
Legs black with silver-white tinged; coxa silver-grey; fore coxa with 2 rows of fine bristles; trochanter with few setae; fore femur with 2 parallel rows of strong postero-dorsal (pd), antero-dorsal bristles (ad) and a row of long bristles with fine hairs ventrally; fore tibia with a row of 3 short ad bristle on basal one-third and 1 ad with 1 dorsal (d) apically, 1 posterior (p) on apical one-third; both tibia and tarsus black; tarsus with two large pulvilli and elongated divergent claws, with long hairs (Fig. 1a). mid coxa with a row of long bristles; trochanter dark brown with setae; mid femur hairy with 4 short anterior bristle (a) at middle, a row of 5 antero-ventral (av), and a row of 12–14 postero-ventral bristles (pv) present subapically; mid tibia with 3 ad (1 medially + 2 apical), 3 pd (1 medially + 2 apical) and 1 p at middle (Fig. 1a). hind coxa with 2 strong bristles and few fine hairs; hind femur silver-grey with a row of about 6 strong ad, 11 av and 4–6 anterior bristles on middle, hind tibia with a row of about 6 regular ad, 2 apical p and 2 pd bristle (1 medially + 1 sub basal), postero-ventral margin with long hairy fringes (Fig. 1a).
Abdomen slender, black with silver-white assortment, forms a checkered abdominal pattern (Fig. 1g). Tergite (1 + 2) with a pair of lateral marginal bristle; tergite 3 with a median and 2 pairs of lateral marginal bristle; 4th with a pair of median and 3 pairs of lateral marginal bristle; tergite 5 with a row of 12 strong median marginal bristle (Fig. 1g); tergite 6 is undivided; sp 6 is located within the membrane, surrounded by few hairs; sternite 1–4 covered with hairs; fifth sternite Y shaped with open triangular window (Fig. 2c), short sternal arms with short hairs on dorsal and distal surfaces, and a cluster of strong bristles on the inner lateral bud, basal marginal arc is formed by two distinct proximolateral lobes with tiny horns (Fig. 2c); First genital segments (GS1/syntergosternite 7 + 8) yellowish-brown, second genital segments (GS2/epandrium) brown, both with medium size hairs (Fig. 2a); cerci long and curved, slightly divergent at the apex, posteriorly with sharp notch, distal half with scattered short spines, basal half with dense long hairs (Fig. 2a, b), right cercus is slightly shorter than the left (Fig. 2a, b); surstyli oval and elongated, slightly convex at basal and distal end, almost bare, except lateral margin with few long hairs (Fig. 2b); aedeagus large; ejaculatory apodeme fan-shaped (Fig. 2e); hypandrium surrounded by serrated sclerotized membrane (Fig. 2i); pregonite broad, slightly curved, distally blunt with many short hairs, laterally with wide projection at middle (Fig. 2f); postgonite, nearly straight, grooved laterally in a subapical arc and with 1 long hair at base (Fig. 2d and e); phallus wide, tubiform and sclerotized (Fig. 2d, g); juxta translucent, atypical, rounded with blind extremity, antero-laterally, membranous, serrated and spineless, laterally with distinct spine near base (Fig. 2g, h); lateral stylus stout, slender and sclerotized (Fig. 2g, h); vesica wide, tubular and heavily sclerotized with blunt end, ventrally appeared arc-shaped (Fig. 2d, e, h); ventralia translucent, elongated with bifid apex and ventro-laterally stretched (Fig. 2d, h).
Sarcophaga (Lioproctia) mailansis sp. nov., male Terminalia. a last genital segment, lateral view. b cercus and surstylus, caudal view. c fifth sternite, ventral view. d phallus, lateral view. e phallus, ventral view. f basiphallus, postero-lateral view. g phallus, lateral view. h distiphallus, anterolateral view. i serrated sclerotized membrane. Abbreviations: aa- aedeagal apodeme, ce- cercus, ea- ejaculatory apodeme, ep- epandrium, hy- hypandrium, j- juxta, lb- inner lateral bud, ls- lateral stylus, ph- phallus, po- postgonite, pr- pregonite, s- spines, sa- sternal arm, ss- surstylus, sw- sternal window, tg- tergite, ve- vesica, vn- ventralia
Sarcophaga (Lioproctia) mailansis sp. nov., adult female. a head, frontal view. b female, dorsal view. c sternites 1–7, ventral view. d female, lateral view. e terminalia, posterior view. f terminalia, ventral view. g wing, dorsal view. h spermathecae. i terminalia, cerci and hypoproct. Abbreviations: ce- circus, ds- dorsal setae, eprct- epiproct, hprct- hypoproct, iv- inner vertical setae, oc- ocellar bristle, ori- frontal bristle, orb- orbital bristle, ors- fronto-orbital bristle, ov- outer vertical setae, sp- spiracle, SPA- secondary pigmented area, st- sternite, tg- tergite
Female about 1.4 cm; head almost same as male, but with about 11 frontal bristles; 3 short interfrontal bristles; 1orbital and 2 fronto-orbital bristle; 1 ocellar bristle (Fig. 3a); outer vertical bristle 3/4 to inner vertical bristle (Fig. 3d); antennae long, do not reach vibrissae; postpedicel light brown with grey pollinosity, pedicel and arista same as male (Fig. 3a); parafacial with 5–6 hairs near bottom edge of eye; palpus slender and blackish; gena, postgena, and arrangement of bristle and setae on occiput are same as male (Fig. 3a, d).
Thorax almost same as male; anterior spiracle fuscous brown; posterior spiracle light yellowish-brown (Fig. 3b, d); acrostichal 1 + 1 (1 very short); dorsocentral 7 + 5 (posterior 2 post dc stout); humeral 3; posthumeral 2; presutural 1; notopleural 4 (2 strongly developed); intra-alar 1 + 3 ( 3 short, 1 long); supra-alar 3 (middle long); postalar bristle 2; scutellum with 4 bristles, discal 1, apical 1 and marginal 2; sternopleural bristle 3; hypopleural 9; mesopleural 7 (7 strong with fine hairs) (Fig. 3b, d).
Wings hyaline; epaulet and basicosta same as male; costal spines strongly defined, basal costal spine larger to others; CS1 with 2 long bristles at costaginal break; CS2 same as male; R4 + 5 with a row of about 11–14 short setae extend dorsally from basal node to exceedingly halfway of r-m; ventrally with 5 setae before R4 + 5 and R2 + 3 emergence (Fig. 3g); R1 bare; CS5 shorter to CS3; CS5 with spines on basal two-third margin; r4 + 5 closed; lower and upper calypter bare, creamy white; halter yellowish-brown (Fig. 3d).
Legs almost same as male except few characters, fore tibia with a row of 4 ad bristles on base, 1 pd at middle and a row of 4–5 pd apical half, 1 p + 1 ad + 1 pd + 1 pv located apically; both tibia and tarsus black; tarsus with two short pulvilli and less divergent claws, with few short hairs (Fig. 3d). mid femur hairy, with 4 short a (anterior bristle) at middle, two rows of 4 av (antero-ventral) and 6 pv (postero-ventral) bristles and 2 strong pd (postero-dorsal bristle) subapically; mid tibia with a row of 4 p near basal, with 3 ad (2 medially + 1 apical) and 1p at middle, 2 pv and 2 v located apically (Fig. 3d). hind coxa with few bristles and fine hairs; hind femur with a row of about 8 ad, 4 pv and 4 ad medially, 1 d (dorsal), 2 v and p (posterior bristle) subapically; tibia with a row of about 5 regular ad (antero-dorsal bristle), 2 ad and 1 v apically, 2 dorsal and 1 pd medially, 1 sub-basal pd; postero-ventrally without hairy fringes (Fig. 3d).
Abdomen black, oval with silver-white patches forming a checkered pattern. tergite (1 + 2) with 2 pairs of lateral marginal bristle and 2 strong dorsolateral bristle with hairs; tergite 3 with 2 lateral marginal bristle; tergite 4 with a pair of median and 3 lateral marginal bristle; tergite 5 with a row of about 12 to 14 strong marginal; tergite 6 well divided with dense stout bristle on posterior margin; spiracles 6 and 7 within the sclerite; tergite 7 + 8 well-differentiated, transparent without any hair (Fig. 3c, e); sternite 1–6 covered with short hairs (Fig. 3c); sternite 2 with 6 bristles at posterior margin; sternite 3 with 4 bristles; sternite 4 with 8 bristles (middle 2 short); sternite 5 with 2 parallel rows of bristles posteriorly; 6th sternite rectangular and with a row of dense bristles at posterior margin (Fig. 3c); sternite 7 slightly grooved medially, lobulated, almost bare except posterior margin with strong bristles and few hairs; sternite 8 well-differentiated, membranous and without hair (Fig. 3c and f); hypoproct with 12 fine setae (middle 2 short) (Fig. 3f, i); epiproct not apparent (Fig. 3e, f). Cerci triangular shaped with long hairs (Fig. 3e, f); spermathecae knob shaped, divided into an oval head and cylindrical neck with striated segmental constrictions (Fig. 3h).
Variation
The Male body length varies from 1.5 to 1.8 cm. Female body length ranges from 1.3 to 1.6 cm. Female frons wider than male frons. R4 + 5 have a row of about 8–10 short setae dorsally in males, and 11–14 short setae in females. Male abdominal tergites 3–5 are largely black to slightly greyish-brown in coloration. Males have lengthy hairy fringes on their hind tibia, whereas females do not.
Key to male species of Lioproctia
1- Ventralia trilobed; juxta grooved with two projections; pregonite pointed apically .....................................................................................................................................beesoni (Senior-White)
-Ventralia bilobed; juxta without groove and blind; pregonite wide apically.............................................................................................................................................................2
2- Upper part of propleuron black; third tergite without median marginal bristle; GS2/epandrium brownish-black; Surstulus triangular; anterolateral margin of juxta with few spine (Nandi 2002)…………………………………………………………………………....…annandalei (Senior-White 1924)
-Upper part of propleuron silver-white; third tergite with a pair of median marginal bristle; GS2/epandrium yellowish-brown; Surstulus oval and elongated; anterolateral margin of juxta without spine…………..………..………………………………………………………….………………..mailansis sp. nov.
.
Description of immature stages
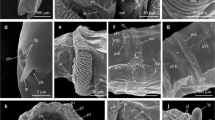
Sarcophaga mailae sp. nov. larvae are nearly cylindrical, muscoid-shaped, vermiform, elongated, tapering at the anterior end, and thickened at the posterior end (Fig. 4a). Mature larvae possess pseudocephalic lobes with antennal complexes and a conspicuous antennal mound on the basal ring (Fig. 4b). On the pseudocephalon, numerous oral ridges can be found ventro-laterally. Some organs, such as the mouth hooks, posterior spiracles, spine bands, and perianal cavity papillae, were clearly visible under a light microscope. Third instar mouthhooks are large at the base and progressively taper, with a blunt and anteriorly slightly curved tip. Anterior spiracles consist of 21 to 23 spiracular lobes. Spines can be seen above the spinose band (Fig. 4b). Spines range in structure from straight to bifurcate. The posterior spiracles are housed within the deep spiracular cavity and are not completely visible from posterior. Pupa rounded and dark black in color (Fig. 4c). All heavily sclerotized protrusions of pupal stage i.e. anterior spiracles are clearly visible by using ultra microscopy (Fig. 4d).
Discussion
According to the morphological identification keys, Sarcophaga (Lioproctia) mailansis sp. nov. belongs to the genus Sarcophaga and shares morphological similarities with the species of the subgenus Lioproctia Enderlein (1928), such as a bare upper propleuron, a few short setae on the parafacial, beak-shaped inner forceps with strong spines, lobulated ventralia, etc. Other identifying characters mentioned by Nandi (2002) for male Sarcophagidae species indicate near striking similarities of new species with the Sarcophaga (Lioproctia) annandalei (Senior-White 1924) which belongs to the same genus and subgenera include bilobed and membranous ventralia, rounded cup-shaped juxta and wide pregonite apically, and so on.
Comparision of male terminalia. a phallus of Sarcophaga (Lioproctia) mailansis sp. nov., lateral view. b phallus of Sarcophaga (Lioproctia) annandalei, lateral view (Sharma et al. 2018). c cercus and surstylus of Sarcophaga (Lioproctia) mailansis sp. nov., caudal view. d cercus and surstylus of Sarcophaga (Lioproctia) annandalei, caudal view (Sharma et al. 2018). Abbreviations: ce- cercus, j- juxta, s- stylus, ss- surstylus, ve- vesica, vn- ventralia
However, chaetotaxy revealed significant differences in bristle number, size, and placement in both species. The male abdominal 4th tergite of Sarcophaga (Lioproctia) mailansis sp. nov. exhibits well-developed median marginal bristles. It has a row of around 7 to 9 setae running from the basal node to below halfway up the r-m, but S. annandalei does not seem to have. R4 + 5 in S. annandalei with a row of 10 short setae dorsally that runs more than half the distance from the basal node to the r-m (Sharma et al. 2018). CS5 in S. annandalei is nearly half the length of CS3, which in described species is about 3/4 the length of CS3 (Nandi 2002). Furthermore, the females of Sarcophaga (Lioproctia) mailansis sp. nov. different from S. annandalei in that they have all scutellar bristles, 11–13 frontal bristles, and three fronto-orbital bristles, rectangular sixth sternite, sternite 8 membranous without hair, and so on (Fig. 3).
The species was also compared to other Sarcophagidae species found in neighbouring countries. Except for a few species that show some resemblance to the new species, the Sarcophagid species of these countries are distinct from the new species. At the posterior view, the terminalia of Sarcophaga (Lioproctia) mailansis sp. nov. are similar to those of Sarcophaga (Lioproctia) annandalei (Senior-White 1924), Sarcophaga (Lioproctia) serratocudo Pape and Kurahashi (2004), and Sarcophaga kiyokoae Chaiwong et al. (2009). However, chaetotaxy and the diverse characters of male terminalia distinguish all of these species from new species (i.e. the shape of fifth sternite, cerci, surstylus, ventralia, and antero-lateral margin of distiphallus) listed in Table 1. Sarcophaga (Lioproctia) mailansis sp. nov., S. annandalei, and S. kiyokoae exhibit a lateral bud on the sternal arm with a cluster of stiff bristles. Sarcophaga (Lioproctia) mailansis sp. nov. exhibits more divergent sternal arms than S. kiyokoae’s fifth sternite. The sternal window in new species is open, whereas the S. kiyokoae species is closed. It has a rounded anterolateral border of the juxta with a blunt end, which is most likely comparable to S. annandalei and S. kiyokoae. Furthermore, the membranous juxta anterolateral margin of distiphallus is identical to that of S. annandalei (Fig. 5a, b) (Nandi 2002). At the rear, the cerci and surstyli of new species resemble those of S. kiyokoae. Sarcophaga (Lioproctia) mailansis sp. nov., on the other hand, has a more divergent apex of cerci than S. kiyokoae. Scrutinizing the shape of surstyli, anterolateral membrane of juxta in Sarcophaga (Lioproctia) mailansis sp. nov. is also different from other described species as listed in Table 1 and illustrated in Fig. 5. In addition, in new species a well-sclerotized distinct spine developed laterally on juxta.
Females of the family Sarcophagidae are extremely difficult to distinguish since lack of keys and similar appearance, creating a barrier during forensic analysis. Mostly gravid females appear on the dead carcass to lay their first instars. As a result, as detailed by several experts, authentic identification of female flesh flies is required for use in forensic investigations (e.g. Bänziger and Pape 2004; Van der Niet et al. 2011; Cherix et al. 2012; Giroux et al. 2010; Zhang et al. 2014). Female flesh flies are frequently difficult to distinguish (Richet et al. 2011; Meiklejohn et al. 2013; Zhang et al. 2014; Vairo et al. 2015). The color of the gena, postgena, orbital and postocellar setae, chaetotaxy of the thorax and abdomen, and the position of the spiracles 6 and 7, might help identify some female Sarcophagidae (Vairo et al. 2015). In most Sarcophagidae cases, tergite 7 is nearly missing, and tergite 8 is mainly present but reduced to bare lateral plate (Shewell 1987). Tergite 7 + 8 is merged and amalgamated in some species e.g. S. nanensis (Kurahashi and Samerjai 2018). Both tergite 7 and 8 are present and well-differentiated in Sarcophaga (Lioproctia) mailansis sp. nov. Tergite 8 is present in a lateral position with a separate bare pigmented plate/secondary pigmented area (Fig. 3c, f). A significant feature of identification is the presence or absence of hypoprocts and epiprocts, as well as whether they are divided or undivided (Vairo et al. 2015). Two strong bristles are also present on the epiproct of some species, such as Sarcophaga (Sarcorohdendorfia) gracilior (Chen 1975) (Zhang et al. 2014). The microstructure of spermatheca differs from genus to genus and species to species, which can aid in the identification of Sarcophagid species. Spermatheca in S. nanensis has an electric bulb-like shape and is divided into a global head and an extended long neck (Kurahashi and Samerjai 2018). Vairo et al. (2015) described the diverse structures of spermatheca, which are elongated and striated in the genus Oxysarcodexia and Sarcophaga, rounded in Peckia (Sarcodexia) lambens and Peckia (Euboettcheria) florencioi, and divided into distal and proximal parts in Peckia (Pattonella) and Microcerella. Sarcophaga (Lioproctia) mailansis sp. nov. spermathecae has two parts: a knobbed head and a long cylindrical neck (Fig. 3h). Considering all distinctive characters, we described both the male and female of Sarcophaga (Lioproctia) mailansis sp. nov. from India, which may aid in their identification.
Conclusion
We described a new species Sarcophaga (Lioproctia) mailansis sp. nov. collected from J and K (India). The diagnosis for the species group was provided, as well as identifying characters for both male and female species.
Data availability
Its available with corresponding author.
References
Abd Al Galil FM, Zambare SP (2016) New species of flesh fly (Diptera: Sarcophagidae) Sarcophaga (Liosarcophaga) geetai in India. J Entomol Zool Stud 4(3):314–318
Bänziger H, Pape T (2004) Flowers, faeces and cadavers: natural feeding and laying habits of flesh flies in Thailand (Diptera: Sarcophagidae, Sarcophaga spp). J Nat Hist 38(13):1677–1694
Barták M, Khrokalo L, Verves Y (2019) New records, synonyms and combinations for oriental Sarcophagidae (Diptera), with updated checklists for Cambodia, India, Taiwan, Thailand and Vietnam. J Asia-Pac Entomol 22(1):44–55
Buenaventura E, Pape T (2017) Multilocus and multiregional phylogeny reconstruction of the genus Sarcophaga (Diptera, Sarcophagidae). Mol Phylogenetics Evol 107:619–629. https://doi.org/10.1016/j.ympev.2016.12.028
Chaiwong T, Sukontason K, Sukontason KL (2009) Two New Species of Sarcophaga s. lat. From Thailand with a key to species (Diptera: Sarcophagidae). J Med Entomol 46(5):986–993
Chakraborty A, Hora G, Parui P, Saha GK, Banerjee D (2017) A biosystematic species inventory of indian Sarcophagidae (Insecta: Diptera: Sarcophagidae). J Entomol Zool Stud 5:465–473
Cherix D, Wyss C, Pape T (2012) Occurrences of flesh flies (Diptera: Sarcophagidae) on human cadavers in Switzerland, and their importance as forensic indicators. Forensic Sci Int 220:158–163. https://doi.org/10.1016/j.forsciint.2012.02.016
Das A, Pandey A, Madan M, Asthana A, Gautam A (2010) Accidental intestinal myiasis caused by genus Sarcophaga. Indian J Med Microbiol 28(2):176
De Carvalho CJB, Mello-Patiu CA (2008) Key to the adults of the most common forensic species of Diptera in South America. Rev Bras Entomol 52:390–406
Giroux M, Pape T, Wheeler TA (2010) Towards a phylogeny of the flesh flies (Diptera: Sarcophagidae): morphology and phylogenetic implications of the acrophallus in the subfamily Sarcophaginae. Zool J Linn Soc 158:740–778. https://doi.org/10.1111/j.1096-3642.2009.00561.x
Goff ML (2000) A fly for the prosecution: insect evidence helps solving crimes. Harvard University Press, London, Cambridge, p 225
Kumar R, Sayed S, Bala M et al (2021) Ultramorphological study of immature stages and male genitalia of forensically significant flesh fly Sarcophaga dux thomson, 1868 (Diptera: Sarchophagidae). J King Saud Univ Sci 33:101460. https://doi.org/10.1016/j.jksus.2021.101460
Kurahashi H, Samerjai C (2018) Revised keys to the flesh flies of Thailand, with the establishment of a new genus (Diptera: Sarcophagidae). Med Entomol Zool 69(2):67–93. https://doi.org/10.7601/mez.69.67
Meiklejohn KA, Dowton M, Pape T, Wallman JF (2013) A key to the australian Sarcophagidae (Diptera) with special emphasis on Sarcophaga (sensu lato). Zootaxa 3680:148–189. https://doi.org/10.11646/zootaxa.3680.1.11
Nandi BC (2002) Fauna of India and the adjacent countries. Diptera (volume x) Sarcophagidae i–xxiv:1–608
Pape T (1996) Catalogue of the Sarcophagidae of the world (Insecta: Diptera). Mem Entomol Int 8:1–558
Pape T, Blagodervo V, Mostovski MB (2011) Order Diptera Linneaus, 1758’ in: animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Zootaxa 3148:222–229
Partington CF (1837) The British cyclopedia of natural history: combining a scientific classification of animals, plants, and minerals; with a popular view of their habits, economy, and structure, vol 3. Rosaceae-Seal, London, pp 577–640
Priscilla L, Adiel A, Tylor M, Martha L, Miguel AS, Grant LH, Miguel MC (2018) Intestinal myiasis caused by Sarchophaga spp. in Cusco, Peru: a case report and review of literature. Case Report in Infectious Disease 2018:1–4
Richet R, Blackith RM, Pape T (2011) Sarcophaga of France. Sarcophagidae) Pensoft Publishers, Diptera, p 327
Senior-White R, Aubertin D, Smart J (1940) Fauna of British India, Diptera. Vol. VI. Family Calliphoridae. Fauna of British India, Diptera. Vol. VI. Family Calliphoridae 6
Sharma M, Singh P, Singh D (2018) Taxonomic studies on Bercaea cruentata, Lioproctia annandalei and sarcosolomania kaushalensis (Diptera: Sarcophagidae) of indian origin. J Entomol Res 42(1):113–119. https://doi.org/10.5958/0974-4576.2018.00020.8
Shewell GE (1987) Sarcophagidae. In: McAlpine JF, Peterson BV, Shewell GE, Teskey HJ, Vockeroth JR, Wood DM (Eds) Manual of Neartic Diptera, volume 2. Monograph 108, Research Branch, Agriculture Canada, Otawa, pp 1159–1186
Sukontason KL, Sanit S, Klong-Klaew T, Tomberlin JK, Sukontason K (2014) Sarcophaga (Liosarcophaga) dux (Diptera: Sarcophagidae): a flesh fly species of medical importance. Biol Res 47(1):1–9
Vairo KP, Moura MO, Mello-Patiu CA (2015) Comparative morphology and identification key for females of nine Sarcophagidae species (Diptera) with forensic importance in Southern Brazil. Rev Bras Entomol 59:177–187
Van der Niet T, Hansen DM, Johnson SD (2011) Carrion mimicry in a south african orchid: flowers attract a narrow subset of the fly assemblage on animal carcasses. Ann Bot 107:981–992. https://doi.org/10.1093/aob/mcr048
Whitmore D, Buenaventura E, Pape T (2018) Odd, outsized, and obscure: Sarcophaga (Hadroxena) karakoncolos sp. n. (Diptera: Sarcophagidae) from Turkey. Zootaxa 4422(3):385–394
Zhang M, Chen J-l, Gao X-Z, Pape T, Zhang D (2014) First description of the female of Sarcophaga (Sarcorohdendorfia) gracilior (Chen, 1975) (Diptera, Sarcophagidae). ZooKeys 396:43–53. https://doi.org/10.3897/zookeys.396.6752
Acknowledgements
We are thankful to the Department of Zoology and Environmental Sciences, Punjabi University, Patiala, India for providing laboratory facilities.
Author information
Authors and Affiliations
Contributions
Designing, conceiving, analysis, and interpretation-MB. Data collection, analysis, interpretation, manuscript writing-RK. Data analysis and interpretation- FM. authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
All the authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, R., Bala, M. & Al Galil, F.M.A. Morphological description of a new species of Genus Sarcophaga s. lat. (Diptera: Sarcophagidae) from Jammu and Kashmir, India. Int J Trop Insect Sci 43, 1659–1668 (2023). https://doi.org/10.1007/s42690-023-01054-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-023-01054-y