Abstract
The current study was designed to assess the effect of different feeding substrates on survival and reproduction of Therophilus javanus (Bhat & Gupta) (Hymenoptera: Braconidae) and Phanerotoma syleptae Zettel (Hymenoptera: Braconidae) in order to optimize their potential as biological control agents against Maruca vitrata Fabricius (Lepidoptera: Crambidae). Experiments were carried out in laboratory with batches of ten adult females exposed to flowers of either Vigna unguiculata (L) Walp., Tephrosia candida (Roxb.) DC, and Sesbania rostrata Bremek & Oberm, pure honey, honey solution, or saccharide solution to assess parasitoid survival. Parasitoid fecundity was evaluated with separate couples of each species using the same substrates. The highest longevity and mean fecundity were obtained for the parasitoids exposed to Tephrosia candida flowers, while there was no difference between control (starved adults) and adults fed using S. rostrata flowers. The putative nutritional compounds of different flowers used in our study affected the biological parameters and performance of these parasitoids differently. Flowers of Tephrosia candida can serve as feeding substrates to both P. syleptae and T. javanus. This study suggests the possibility of using host plants at the flowering stage as a release point for the deployment of the two parasitoids in a classical biological control programme against M. vitrata.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After emergence, adult hymenopteran parasitoids need to acquire food to initiate and maintain oogenesis and for sustaining foraging activities (Jervis and Kidd 1986). Parasitoid longevity and fecundity can be strongly influenced by the composition of adult food substrates (Wäckers 2001; Jervis et al. 2008). Moreover, well-nourished parasitoids are generally more active and more focused in searching for their herbivorous hosts (Wäckers et al. 2005). Often, their primary food source comes from immature stages of the hosts, whereby (Jervis and Kidd 1986). Adult females feed on host body fluids commonly referred to as ‘host-feeding’. Host-feeding can be a critical step for obtaining protein resources for eggs production (Ueno 1999). However, not all parasitoids feed on hosts (Jervis and Kidd 1986). Plant- based substrates such as floral and extra-floral nectars, pollen etc. are also widely exploited for adult feed (Mitsunaga et al. 2004; Jervis et al. 2008).
These plant-based substrates were reported to influence several biological parameters in parasitoid wasps such reproductive capacity or fecundity, longevity, sex-ratio and flight capacity. Thus, females of the larval parasitoid Necrennus tutae Reuter (Hymenoptera: Eulophidae), parasioid of Tuta absoluta Meyrick (Lepidoptera: Gelechidae), significantly increased their egg load when fed using flowers of Fagopyrium esculentum Moench, Lobularia maritiama L. and Ononis natrix L. compared to unfed females (Arnó et al. 2018). Likewise, higher egg load was displayed by females of Bracon nr. nigricans Szépligeta (Hymenoptera: Braconidae) when fed on flowers of O. natrix compared to flowers Tagetes patula L. (Arnó et al. 2018). In field cages experiments using Brussels sprout leaf infested with larvae of Plutella xylostella (Lepidoptera: Plutellidae), Winkler et al. (2006) found that the lifetime fecundity of the parasitoid Diadegma semiclausum Heller (Hymenoptera: Ichneumonidae) was higher when females were fed on flowering F. esculentum plants compared to control plant without flowers. In the endoparasitoid Microplitis croceipes (Cresson), a larval parasitoid of Helicoverpa zea (Boddies) (Lepidoptera: Noctuidae) and Heliothis virescens (L.) (Lepidoptera: Noctuidae), longevity of females was higher with nectary producing cotton plants or honey compared to demineralized water or nectariless cotton plants (Röse et al. 2006). Likewise, longevity of Cotesia vestalis Haliday (Hymenoptera: Braconidae), a larval pararsitoid of P. xylostella increased when fed on flowers of Cosmos sulphureus Cav and Lantana camara L. compared to water or flowers of Zinnia elegans Jacq. (Chau et al. 2019). The sex ratio of M. croceipes was not affected (Röse et al. 2006). Similarly, equal sex ratio was reported in the parasitoid Dolichogenidia tastanica (Cameron) (Hymenoptera: Braconidae) when fed using flowers of Lobularia maritina L. in comparison to water or plants without flowers (Berndt and Wratten 2005). Flight capacity is another biological parameter affecting by adult parasitoid feeding sources. Thus, flight capacity of the braconid M. croceipes was higher when adult wasps were fed on Nectary producing cotton plants compared to nectariless cotton plants (Röse et al. 2006).
In the current study, we assessed the effect of different adult feeding substrates on survival and reproduction of two hymenopteran parasitoids Therophilus javanus (Bhat and Gupta) and Phanerotoma syleptae Zettel (Hymenoptera: Braconidae). They have been imported from the World Vegetable Center in Taiwan to Benin by the International Institute of Tropical Agriculture (IITA) in the framework of a biological control program against the legume pod borer Maruca vitrata Fabricius (Lepidoptera: Crambidae), a key insect pest of cowpea causing 20–80% crop losses in West Africa (Tamò et al. 2003; Srinivasan et al. 2012). This is the first attempt of using flowers of some key M. vitrata host plants to feed adult parasitoid.
Material and methods
Experimental site
All experiments were carried out at the laboratory of the International Institute of Tropical Agriculture in Benin (IITA-Benin), near Cotonou, Benin. The mean temperature was 25.2 ± 0.2 °C and the relative humidity 76.7 ± 8.6% throughout the experiments.
Feeding substrates
Six adult feeding substrates were compared in the present study: pure honey, saccharide solution, honey solution, flowers of Vigna unguiculata (L) Walp.) Sesbania rostrata Bremek & Oberm, Tephrosia Candida (Roxb.) DC. The three plant species selected are all M. vitrata host plants and are abundant in Benin ecosystems (Arodokoun et al. 2003). Open flowers of the cowpea variety “Tawa” and flowers of wild M. vitrata host plants namely S. rostrata, and T. candida were collected at IITA campus. Saccharide and honey were bought from a pharmacy in Cotonou, Benin.
Insect species
Colonies of M. vitrata, P. syleptae and T. javanus were established from pupae obtained from the stock culture at IITA-Benin. Pupae were put in wooden cages (44 cm × 45 cm × 58 cm) with a glass top and provided with sleeves. Emerging adults (males and females) were kept together in the wooden cages for mating during four days. Four-day-old females were transferred in cups (3 com diameter × 3.5 cm height) for 24 h to allow oviposition. The 24-h-old eggs were collected and used in experiments involving the ovo-larval parasitoid P. syleptae. After hatching, larvae were reared using sprouting cowpea seeds as described in detail in Aboubakar Souna et al. (2020). In experiments including the larval parasitoid T. javanus, 3-day-old M. vitrata caterpillars were used.
The egg-larval parasitoid P. syleptae was mass reared by offering to female wasps 24 h M. vitrata eggs for parasitism. Larvae hatching from parasitized eggs were fed with sprouting cowpea seeds till cocoon stage.
The mass rearing of the larval parasitoid T. javanus followed similar procedure as described above. Larvae hatching from M. vitrata eggs were reared for 3 days with sprouting cowpea seeds. Three-day-old larvae were submitted to T. javanus females for parasitism. Parasitized larvae were reared using the above feeding substrate till cocoon stage. Emerged adult wasps of both parasitoid species were used in the different experiments.
Biossays
For the estimation of the parasitoid fecundity (A), treatments were set up to take into account different feeding period. Thus, (A1) consisted of females fed during only the first 48 h of their adult stage; (A2) adults fed daily during their whole adult stage; and (A3) a control consisting of starved adults (not fed). For assessing the parasitoid survival, the effect of oviposition was considered and treatments consisted of (B1) female wasps not allowed to oviposit (naïve females) and fed daily, (B2) female wasps allowed to oviposit when fed using one of the substrates for 48 h after emergence and kept unfed afterwards; (B3) female wasps allowed to oviposit and fed daily.
Effect of different feeding substrates on the fecundity, longevity and sex ratio of P. syleptae
Fecundity: In treatments where female wasps were fed, each of the following feeding substrates were used to feed females lots: pure honey, honey solution at 10% (diluted with distilled water), saccharide solution 10% (diluted with distilled water), flowers of V. unguiculata, T. candida and S. rostrata. Each batch consisted of 10 couples (1 male and 1 female) of P. syleptae. Fecundity was recorded by offering for 24 h to each couple of P. syleptae 30 one-day-old M. vitrata egg, daily. Larvae hatched from parasitized eggs were fed using pre-germinated cowpea seeds till cocoon stage. The number of cocoons was recorded per couple and per treatment. Fecundity was then recorded in terms of number of cocoons Experiments were replicated 4 times.
Longevity: The effect of different adult feeding substrates on the longevity of T. javanus and P. syleptae was assessed using ovipositing and naïve female wasps. A batch of ten (10) couples (1 male and 1 female) were fed using each of the substrates as described above. Ovipositing T. javanus and P. syleptae females were offered for 24 h, 30 three-day-old M. vitrata larvae and 30 one day-old M. vitrata eggs daily for parasitization. Female mortality was checked daily.
Sex ratio: Cocoons of P. syleptae obtained during the experiments on fecundity were kept till emergence. After emergence, the number of individuals belonging to each sex was noted.
Effect of different feeding substrates on the fecundity, longevity and ex ratio of T. javanus
Fecundity: The effect of the five feeding substrates mentioned above on the fecundity of T. javanus was assessed by feeding adult wasps with each of these substrates. Each batch consisted of 10 couples (1 male and 1 female) of T. javanus. Each T. javanus couple was offered for 24 h, 30 three-day-old M. vitrata larvae daily. Parasitized larvae were fed using sprouting cowpea seeds till cocoon stage. The number of cocoons was recorded per couple and per treatment. Fecundity was then recorded in terms of number of cocoons. After emergence, the number of individuals belonging to each sex was noted. Experiments were replicated 4 times.
Longevity: The effect of different adult feeding substrates on the longevity of T. javanus and P. syleptae was assessed using ovipositing and naïve female wasps. A batch of ten (10) couples (1 male and 1 female) were fed using each of the substrates as described above. Ovipositing T. javanus and P. syleptae females were offered 30 three-day-old M. vitrata larvae and 30 one-day old M. vitrata eggs daily for parasitization. Female mortality was checked daily.
Sex ratio: Cocoons of T. javanus obtained during the experiments on fecundity were kept till emergence. After emergence, the number of individuals belonging to each sex was noted.
Data analysis
The mortality rate and parasitoid adult longevity recorded for each parasitoid feeding substrate were analyzed using analysis of variance (ANOVA) with SAS software version 9.2 (SAS Institute Inc 2011). In case of significant differences, the Student–Newman–Keuls (SNK) test was used to separate the means. Data on sex-ratio were analyzed by performing chi-square test. Percent data were Arcsin √ (p) transformed prior to ANOVA.
Results
Effect of adult feeding substrate on the fecundity, longevity and sex ratio of P. syleptae
Fecundity of P. syleptae
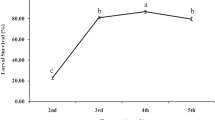
The cumulative fecundity showed an increase in fecundity till it reached a plateau for most substrates, exception for S. rostrata flowers, honey and control (starved parasitoids) where the fecundity was very low (Fig. 1).
Significant differences were observed between parasitoid feeding substrates for P. syleptae fecundity (P < 0.0001; F(6, 201) = 17.12) (Fig. 2). The highest mean fecundity was obtained when adult parasitoids were fed daily or 48 h using saccharide solution and honey followed by flowers of T. candida and honey solution (Fig. 2A). On the other hand, the lowest fecundity was recorded on S. rostrata and control (starved parasitoids).
Mean fecundity of P. syleptae when fed daily (A) or 48 h (B) before oviposition using flowers of cowpea, Sesbania rostrata, Tephrosia candida, pure honey, honey solution, saccharide solution and control (starved wasps). Bars followed by the same letters are not significantly different with ANOVA followed by SNK at 5%. Error bars are standard errors of the means
Longevity of P. syleptae
Longevity of P. syleptae of three feeding modalities is depicted in Table 1. The parasitoid P. syleptae lived longer on saccharide solution, honey solution and cowpea compared to the control and S. rostrata when the female wasps were not allowed to oviposit. But, when female wasps were fed 48 h before oviposition, the highest longevity was recorded on saccharide solution, honey solution and flowers of T. candida while they lived longer when fed daily using saccharide solution compared to the other feeding substrates (P < 0.0001; F(6, 201) = 15.78). Comparison within substrate revealed that females fed 48 h before oviposition lived longer compared to continuously starved females provided with host in the control treatment (starved wasps).
Sex ratio of P. syleptae
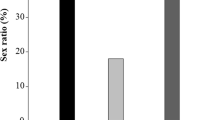
Diets affected the sex ratio of the parasitoid (P given in Table 2 for each substrate). There was a significant difference between proportion of females compared to that of male for the substrates pure honey, saccharide solution and honey, flowers of T. candida (Table 2). When fed 48 h before oviposition, the sex-ratio of P. syleptae was female-biased in all the tested feeding substrates with exception for the control and flowers of S. rostrata. The sex-ratio was not significantly affected by adult feeding substrates when P. syleptae was fed daily, expect pure honey where proportion of female wasps were higher than that of males.
Effect of adult feeding substrate on the fecundity, longevity and sex ratio of T. javanus
Fecundity of T. javanus
The parasitoid T. javanus was able to parasitize the day of emergence. The curve describing the cumulative daily fecundity when females were fed with pure honey was above those obtained when females was fed with saccharide, honey solution or flowers of T. candida (Fig. 3). The lowest cumulative was obtained when female wasps were fed using flowers of S. rostrata or starved females (control).
The substrates used to feed adult parasitoid significantly affect fecundity (P < 0.0001, F(6, 199) = 16.17). The highest fecundity was observed on saccharide solution and flowers of T. candida when T. javanus was fed daily (Fig. 4A). However, the highest fecundity was obtained only when female wasps were fed using saccharide solution and pure honey for 48 h before oviposition (Fig. 4B). The lowest fecundity was recorded on S. rostrata in both cases.
Mean fecundity of T. javanus fed when fed daily (A) or 48 h (B) before oviposition using flowers of cowpea, Sesbania rostrata, Tephrosia candida, pure honey, honey solution, saccharide solution and control (starved wasps). Bars followed by the same letters are not significantly different with ANOVA followed by SNK at 5%. Error bars are standard errors of the means
Longevity of T. javanus
The effect of different sugar sources on the longevity of T. javanus according to the three feeding modes. For all the different observations made during the experiment, treatments with saccharide solution, honey followed by the flowers of V. unguiculata and T. candida, significantly influenced the longevity of T. javanus compared to the control and the flower of S. rostrata (P < 0.0001 F(6, 270) = 10.33) (Table 3). Comparison within each substrate showed that continuously starved females provided with host larvae lived longer than females fed 48 before oviposition.
Sex ratio of T. javanus
Table 4 shows the sex ratio (percentages) of T. javanus when fed 48 h and daily. When fed 48 h before oviposition, the sex-ratio of T. javanus was male-biased in all the tested feeding substrates with exception for honey and flowers of T. candida. The sex-ratio was not significantly affected by adult feeding substrates when T. javanus was fed daily, except honey, flowers of T. candida and control (starved females) with female-biased sex ratio (P given in Table 4 for each substrate).
Discussion
The parasitoid species P. syleptae and T. javanus fed using the different sugar substrates affected both longevity and fecundity. A high proportion of P. syleptae, fed 48 h before oviposition, resulted in high percentage female offsprings. Likewise, when T. javanus adults were fed daily, this resulted in high females” proportion. Parasitoid adult feeding was known to influence their fitness, reproduction and survival (Colinet et al. 2005; Onagbola et al. 2007; Wang et al. 2008). Similar observation has been done by Arnó et al. (2018) in the braconid B. nr nigricans, a parasitoid of T. absoluta with higher lifetime fecundity when female wasps were fed using flowers of O. natrix compared to flowers of T. patula. Natural vegetation is an important source of pollen, nectars or for natural enemies feeding (Arodokoun et al. 2003). It was reported that feeding on wild flowers induced egg maturity in some Hymenopteran species (Jervis et al. 2008). However, not all accepted foods have the same value for reproduction, thus, diets can be distinguished in essential foods that allow development and reproduction, and alternative foods that serve as an energy source, and complementary foods that, when added to essential foods, stimulate reproduction and egg laying (Hodek 1962).
The improvement of fecundity in T. javanus when fed using honey could be related to the amino acids it contained, namely phenylalanine and histidine (Hermosin et al. 2003) known as oviposition stimulants in several insect species including wasps (Barrett and Schmidt 1991). The high sugar content in saccharide diet may also explain the observed differences, as the excess sugar taken by the insect could result in an overload of its carbohydrate metabolism and thereby would affect female reproductive activity (Zera et al. 2016). Röse et al. (2006) reported comparable lifetime fecundity in the parasitoid M. croceipes when fed using nectary producing cotton plants and honey.
The higher number of eggs laid observed in P. syleptae and sometimes less in T. javanus on T. candida may suggest that some flowering plants are suitable for the parasitoid feeding and that within these plants. Sivinski et al. (2011) reported availability in attractiveness and suitability depending on the parasitoid species.
Fecundity of adults fed 48 h before oviposition was lower than that of those fed daily, confirming that when food is far from host sites, switching between resources can become particularly costly, as searching feeding sites limits the time available for host searching and oviposition (Jervis and Kidd 1996; Stapel et al. 1997). The highest longevity of adults fed using saccharide solution is due to the fact that they have a digestive system that allows them to easily absorb simple sugars such as this (Leatemia et al. 1995).
Feeding on T. candida flowers observed in P. syleptae adults could be related to the attractiveness of flowers colour that may also have high sugar content. Differences between plant species might be due to the presence of secondary metabolites in flowers with various properties (Barbosa et al. 1986; Dannon et al. 2010), their morphology (Wäckers et al. 2005) or to the nectar smelling (Zhu et al. 2012). Access to flowers or a similar sugar source increases the longevity of parasitoids (Baggen and Gurr 1998; Winkler et al. 2006, Vattala et al. 2006; Lee et al. 2007). The longevity of T. javanus on honey suggests that T. javanus needs more carbohydrate and water to improve its longevity. Indeed, honey is an excellent food for wasps (Shaw 1997). Temerak (1983) reported that honey provides better nutrition to improve the longevity of adult parasitoids. Increased longevity was observed in the braconid M. croceipes when adult wasps were fed using honey or nectary producing cotton plants (Röse et al. 2006). The braconid C. vestalis was found to live longer when adults were fed on flowers of C. sulphureum or L. camara compared to flowers of Z. elegans or water (Chau et al. 2019). The significant difference between the longevity of P. syleptae females fed 48 h before oviposition and continuously starved females provided with host suggested the absence host feeding in P. syleptae. On the other hand, in T. javanus, the higher longevity observed in the continuously starved females provided with host compared to that of females fed 48 h before oviposition may be explained by the occurrence of host feeding. However, further research work should be carried out to investigate host feeding in both parasitoid species.
Maternal diet was found to significantly affect the sex ratio in both parasitoid species in the current study (Tables 3 and 4). Maternal diet is one of the key factors known to influence sex ratio in parasitoids (King 1987; Berndt and Wratten 2005; Hu et al. 2012; Hopkinson et al. 2013; Benelli et al. 2017). In general, well-fed female wasps may alter progeny sex ratio by given higher proportion of female offspring (Benelli et al. 2017). Thus, in the parasitoid Trichogramma ostriniae (Hymenoptera: Trichogrammatidae), the sex ratio was female-biased when female wasps were provided with honeydew produced by the aphid Rhopalosiphum maidis (Homoptera: Aphididae) (Fuchsberg et al. 2007). Likewise, Berndt and Wratten (2005) reported that the sex ratio was strongly male-biased in the parasitoid Dolichogenidea tasmanica (Hymenoptera: Braconidae), in the absence of Alyssum flowers; but when fed using Alyssum flowers, proportions of males and females were not significantly different. On the other hand, the sex ratio in the braconid D. tasmanica was not affected when fed using flowers of alyssum L. maritina (Berndt and Wratten 2005). Similarly, equal sex ration was observed in M. croceipes when fed on nectary producing cotton plants compared to honey or necatriless cotton plants. Sex ratio was found to be less female-biased in the parasitoid Pachycrepoideus vindemmiae (Rondani) (Hymenoptera: Pteromalidae) when fed using honey compared to tap water (Hu et al. 2012). In the present study, significantly higher proportion of females was observed in P. syleptae when fed 48 h before oviposition using pure honey, honey solution, saccharide solution, flowers of T. candida or when fed daily with pure honey. However, in T. javanus, sex ratio was male-biased when female wasps were fed 48 h before oviposition using honey solution, saccharide solution flowers of S. rostrata, flowers of V. unguiculata or starved females; while the sex ratio was female-biased when female wasps were fed daily using honey and flower of T. candida. Besides, maternal diet, sex ratio may be influenced by many other factors such as maternal age, sperm viability, number of mating and host size and density at oviposition, quality of host feed (Arthur and Wylie 1959; King 1987; Van Alphen and Visser 1990; Dicke 1999; Brodeur and Boivin 2004; Harvey et al. 2004). Such factors could be addressed during further research investigations.
Conclusion
Fecundity and longevity of the parasitoids P. syleptae and T. javanus vary according to different the feeding substrates offered. This study revealed that flowers of T. candida were one of the best adult feeding substrates for both P. syleptae and T. javanus. This host plant could be exploited by the two parasitoid species and may improve their performance after release in Benin ecosystems.
References
Aboubakar Souna D, Bokonon-Ganta AH, Dannon EA, Imorou N, Agui B, Cusumano A, Srinivasan R, Pittendrigh BR, Volkoff AN, Tamò M (2020) Volatiles from Maruca vitrata (Lepidoptera, Crambidae) host plants influence olfactory responses of the parasitoid Therophilus javanus (Hymenoptera, Braconidae, Agathidinae) Biological Control 130: 104–109
Arnó J, Oveja MF, Gabarra R (2018) Selection of flowering plants to enhance the biological control of Tuta absoluta using parasitoids. Biol Control 122:41–50
Arodokoun D, Tamò M, Cloutier C, Adeoti R (2003) Importance of alternative host plants for the annual cycle of the legume pod borer, Maruca vitrata Fabricius (Lepidoptera: Pyralidae) in Southern and Central Benin. Insect Sci & Appl. 23:103–113
Arthur AP, Wylie HG (1959) Effects of host size on sex ratio, development time and size of Pimpla turionellae (L.) (Hymenoptera: Ichneumonidae). Entomophaga 4:297–301
Baggen LR, Gurr GM (1998) Flowers in tri-trophic systems: mechanisms allowing selective exploitation by insect natural enemies for conservation biological control. Entomol Exp Appl 91:155–161
Barbosa P, Saunders JA, Kemper J, Trumbule R, Olechno J, Martinat P (1986) Plant allelochemicals and insect parasitoids: Effect of nicotine on Cotesia congregata (Say) (Hymenoptera: Braconidae) and Hyposoter annulipes (Cresson) (Hymenoptera: Ichneumonidae). J Chem Ecol 12:1319–1328
Barrett M, Schmidt JM (1991) A comparison between the amino acid composition of an egg parasitoid and some of its hosts. Entomol Exp Appl 59:29–41
Benelli G, Giunti G, Tena A, Desneux N, Caselli A, Canale A (2017) The impact of adult diet on parasitoid reproductive performance. J Pest Sci 90(3):807–823
Berndt LA, Wratten SD (2005) Effects of alyssum flowers on the longevity, fecundity, and sex ratio of the leafroller parasitoid Dolichogenidea tasmanica. Biol Control 32:65–69. https://doi.org/10.1016/j.biocontrol.2004.07.014
Brodeur J, Boivin G (2004) Functional ecology of immature parasitoids. Annu Rev Entomol 49:27–49
Chau NNB, Kieu NTP, Dung NVT, Quoc NB, Phuong TK (2019) Effects of floral resources on the longevity and parasitism of Cotesia vestalis Haliday (Hymenoptera: Braconidae) on Plutella xylostella (L.) (Lepidoptera: Plutellidae) in Vietnam. Heliyon 5:e02258
Colinet H, Salin C, Boivin G, Hance Th (2005) Host age and fitness-related traits in a koinobiont aphid parasitoid. Ecol Entomol 30:473–479
Dannon EA, Tamò M, van Huis A, Dicke M (2010) Functional response and life history parameters of Apanteles taragamae, a larval parasitoid of Maruca vitrata. Biocontrol 55:363–378
Dicke M (1999) Direct and indirect effects of plants on the performance of beneficial organisms. In: Ruberson JR (ed) Handbook of Pest Management. Marcel Dekker Inc, New York, USA, pp 105–153
Fuchsberg JR, Yong TH, Losey JE, Carter ME, Hoffmann MP (2007) Evaluation of corn leaf aphid Rhopalosiphum maidis (Homoptera: Aphididae) honeydew as a food source for the egg parasitoid Trichogramma ostriniae (Hymenoptera: Trichogrammatidae). Biol Control 40:230–236. https://doi.org/10.1016/j.biocontrol.2006.10.009
Harvey JA, Bezember TM, Elzinga JA, Strand MR (2004) Development of the solitary endoparasitoid Microplitis demolitor: Host quality does not increase with host age and size. Ecol Entomol 29:35–43
Hermosin I, Chión RM, Cabezudo MD (2003) Free amino acid composition and botanical origin of honey. Food Chem 88:263–268
Hodek I (1962) Essential and alternative food in insects. - Verh. XI Intern. Kongr. Entom., W; en (1960), Z, 698–699
Hopkinson JE, Zalucki MP, Murray DAH (2013) Honeydew as a source of nutrition for Lysiphlebus testaceipes (Cresson) (Hymenoptera: Braconidae): effect of adult diet on lifespan and egg load. Aust J Entomol 52:14–19. https://doi.org/10.1111/j.1440-10076055.2012.00875.x
Hu H-Y, Chen Z-Z, Duan B-S, Zheng J-T, Zhang T-X (2012) Effects of female diet and age on offspring sex ratio of the solitary parasitoid Pachycrepoideus vindemmiae (Rondani) (Hymenoptera, Pteromalidae). Rev Bras Entomol 56:259–262. https://doi.org/10.1590/S0085-56262012005000028
Jervis MA, Kidd NA (1986) Host feeding strategies in hymenopteran parasitoids. Biological Review 61:395–434
Jervis MA, Ellers J, Harvey JA (2008) Resource acquisition, allocation and utilization in parasitoid reproductive strategies. Annu Rev Entomol 53:361–385
Jervis MA, Kidd NAC (1996) Insect natural enemies, practical approaches in their study and evaluation. M. Jervis and N. Kidd. London, Chapman & Hall. 394 p
King BH (1987) Offspring sex ratios in parasitoid wasps. Q Rev Biol 62:367–396
Leatemia JA, Laing JE, Corrigan JE (1995) Effets de la nutrition des adultes sur la
Lee JC, Heimpel GE, Leibee GLS (2007) En comparant les régimes de nectar floral et de miellat des pucerons sur la longévité et les niveaux de nutriments d’une guêpe parasitoïde. Entomol Exp Appl 111:189–199
Mitsunaga T, Shimoda T, Yano E (2004) Influence of diet on the longevity and parasitic capacity of the endoparasitoid, Cotesia plutellae (Hymenoptera: Braconidae). Appl Entomol Zool 39(4):691–697
Onagbola EO, Fadamiro HY, Mbata GN (2007) Longevity, fecundity, and progeny sex ratio of Pteromalus cerealellae in relation to diet, host provision and mating. Biol Control 40:222–229
Röse USR, Lewis J, Tumlinson JH (2006) Extrafloral nectar from cotton (Gossypium hirsutum) as a food source for parasitic wasps. Funct Ecol 20(1):67–74
SAS Institute Inc (2011) Base SAS® 9.2 Procedures Guide. Cary NC, SAS Institute Inc
Shaw SP (1997) Rearing of parasitic Hymenoptera. AES 25:1–46
Sivinski J, Wahl D, Holler T, Al Dobai S, Sivinski R (2011) Conserving natural enemies with flowering plants: estimating floral attractiveness to parasitic Hymenoptera and attraction’s relationship to flower and plant morphology. Biol Control 58:208–214
Srinivasan R, Yule S, Chang JC, Malini P, Lin MY, Hsu YC, Schafleitner R (2012) Towards developing a sustainable management strategy for legume pod borer, Maruca vitrata on yard–long bean in Southeast Asia. In: R Holmer, G Linwattana, P Nath et JDH Keatinge (eds), SEAVEG 2012: Proceedings of the Regional Symposium on High Value Vegetables in Southeast Asia: Production, Supply and Demand, pp 76–82
Stapel JO, Cortesero AM, De Moraes CM, Tumlinson JH (1997) Extrafloral nectar, honeydew and sucrose effects on searching behavior and efficiency of Microplitis croceipes (Hymenoptera: Braconidae) in cotton. Environ Entomol 26:617–623
Tamò M, Ekesi S, Maniania NK, Cherry A (2003) Biological control, a non-obvious component of IPM for cowpea. In: Neuenschwander P, Borgemeister C, Langewald J (eds) Biological control in IPM systems in Africa. CAB International, Wallingford, Oxon, pp 295–309
Temerak SA (1983) Adult longevity of Brevicornis ptarmigan (Hym: Braconidae) influenced by the feeding of artificial and natural foods. Entomophaga 28:145–150. https://doi.org/10.1007/BF02372138
Ueno T (1999) Host-size-dependent sex ratio in a parasitoid wasp. Researches on Population Ecology 41:47–57
Van Alphen JJM, Visser ME (1990) Superparasitism as an adaptive strategy for insect parasitoids. Annu Rev Entomol 35:59–70
Vattala HD, Wratten SD, Phillips CB, Wäckers FL (2006) The influence of flower morphology and nectar quality on the longevity of a parasitoid biological control agent. Biological Cont 39:179–185
Wäckers FL (2001) Une comparaison des sucres de nectar et de miellat en ce qui concerne leur utilisation par le parasitoïde de l’hyménoptère Cotesia glomerata. J Insect Physiol 47:1077–1084
Wäckers FL, Lee JC, Heimp GE, Winkler K, Wagenaar R (2005) Hymenopteran parasitoids synthesize oligosaccharides specific to honeydew. Function School 20:790–798
Wang XY, Yang ZQ, Wu H, Gould JR (2008) Effects of host size on the sex ratio, clutch size, and size of adult Spathius agrili, and ectoparasitoid of emerald ash borer. Biological Cont 44:7–12
Winkler K, Wäckers F, Bukovinszkine-Kiss G, van Lenteren J (2006) Sugar resources are vital for Diadegma semiclausum fecundity under field conditions. Basic Appl Ecol 7:133–140
Zera AJ, Clark R, Behmer ST (2016) Lipogenesis in a wing-polymorphic cricket: Canalization versus morph-specific plasticity as a function of nutritional heterogeneity. J INsect Physiol 95(2016):118–132
Zhu PY, Gurr GM, Lu ZX, Heong KL, Chen GH (2012) Laboratory screening supports the selection of sesame (Sesamum indicum) to enhance Anagrus spp. parasitoids (Hymenoptera: Mymaridae) of rice planthoppers. Biol Control 64:83–89
Acknowledgements
This work was carried out with the support of the CGIAR Research Program on Grain Legumes and Dryland Cereals (GLDC) through the International Institute of Tropical Agriculture (IITA), the Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ), United States Agency for International Development (USAID) and Bill & Melinda Gates Foundation (BMGF) . We also grateful to Mathias Azokpota, François Onikpo, Basile Dato of IITA-Benin for their technical assistance during this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The study did not include humans as subject; therefore, ethical standards were not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dannon, E.A., Azokpota, E.T., Zanzana, K. et al. Effect of adult feeding substrates on survival and reproduction of Therophilus javanus and Phanerotoma syleptae, two parasitoids of Maruca vitrata. Int J Trop Insect Sci 42, 3073–3082 (2022). https://doi.org/10.1007/s42690-022-00844-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-022-00844-0